-
PDF
- Split View
-
Views
-
Cite
Cite
Mauro Iafrancesco, Aaron M. Ranasinghe, Martin W. Claridge, Jorge G. Mascaro, Donald J. Adam, Current results of endovascular repair of thoraco-abdominal aneurysms, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 6, December 2014, Pages 981–984, https://doi.org/10.1093/ejcts/ezu090
Close - Share Icon Share
Abstract
Fenestrated and branch endografts represent a totally endovascular solution for high-risk patients with atherosclerotic thoraco-abdominal aortic aneurysms (TAAAs). This study reports the early outcome of endovascular TAAA repair.
Interrogation of a prospective database of consecutive patients who underwent endovascular repair (EVAR) for TAAA between June 2007 and October 2012.
Sixty-two high-risk patients (55 men; median age 72, range 54–84 years) underwent fenestrated (n = 39) or branch (n = 23) EVAR for non-ruptured TAAA [extent I–III (n = 26) and IV (n = 36)]. Twenty patients had undergone 22 previous aortic procedures. A total of 221 target vessels (coeliac 50, superior mesenteric 61, renal 106, left subclavian 1 and hypogastric 3) were preserved with scallops (n = 17), fenestrations (n = 140) or branches (n = 62) and 201 of these vessels were stent-grafted (coeliac 34, superior mesenteric 58, renal 105, left subclavian 1 and hypogastric 3). The 30-day mortality was 1.6% (n = 1) and one further patient died on postoperative day 62 from respiratory complications. Spinal cord injury (SCI) developed in 5 (8%) patients (3 women and 2 men). Two patients required temporary renal replacement therapy and a further two commenced planned postoperative dialysis.
In high-risk patients with TAAA, fenestrated and branch EVAR is associated with low early mortality and requirement for renal support, but the risk of SCI is not insignificant despite the use of cerebrospinal fluid drainage and blood pressure manipulation. Our current practice is to stage the repair of extent I–III aneurysms and this has significantly reduced the incidence of SCI.
INTRODUCTION
In 1953, Charles Rob reported the surgical repair of six abdominal aortic aneurysms (AAAs), using a thoraco-abdominal incision to allow aortic clamping in the chest [1]. This is thought to be the first report of surgical repair of a thoraco-abdominal aortic aneurysm (TAAA). Significant technical advances have resulted in low morbidity and mortality rates for TAAA repair performed in centres of excellence, especially in young patients [2] and those with connective tissue disease (CTD) [3]. For older patients with atherosclerotic TAAA and those operated on outside high-volume centres, the results are less impressive with in-hospital mortality rates in excess of 20% [4]. The first total endovascular TAAA repair was reported in 2001 [5], and this approach has gained popularity as an alternative to open repair in patients with atherosclerotic TAAA. We report our early experience with endovascular treatment of TAAA in high-risk patients unsuitable for open repair.
MATERIALS AND METHODS
Patients
Our prospectively maintained database was interrogated to identify patients who underwent endovascular repair (EVAR) for TAAA between June 2007 and October 2012. All procedures were performed or supervised by the senior author (Donald J. Adam). Sixty-two high-risk patients (55 men; median age 72, range 54–84 years) underwent fenestrated (n = 39) or branch (n = 23) EVAR for non-ruptured TAAA [extent I–III (n = 26) and IV (n = 36)]. Twenty patients had previously undergone 22 aortic procedures. Patients who had fenestrated EVAR for juxtarenal AAA were excluded from analysis. Juxtarenal aneurysms were defined as those commencing immediately below the renal arteries which could be repaired by open surgery using an aortic clamp above one or both renal arteries.
Indications and patients selection
Indication for intervention was based on size (>6 cm in diameter) or growth rate (>1 cm/year) criteria. The cardiothoracic aortic unit in Birmingham has considerable experience in the assessment and management of patients with TAAA. Patients who underwent EVAR were considered unsuitable for open surgery due to significant cardiac and/or respiratory comorbidities or anatomical issues, such as hostile abdomen or chest and previous aortic intervention. All patients in the series had atherosclerotic TAAA and none has associated chronic type B aortic dissection.
Device planning and procedure
Whole aorta CT scan angiography, including the iliac and femoral arteries, was obtained for each patient with a multirow CT scan with an at least 1-mm-thick slice. All procedures were performed using a custom-made endograft based on the Zenith® device (Cook Medical, Brisbane, Australia). Endografts were manufactured with scallops, nitinol-reinforced fenestrations or internal–external cuffs to allow preservation of coeliac axis, superior mesenteric, renal, hypogastric and left subclavian arteries.
All but one procedure was performed under general anaesthesia. Access was achieved transfemorally or using an iliac conduit in selected cases. If target vessels required access from a superior approach, then the proximal brachial artery was exposed in the axilla or (more recently) the left or right axillary artery was exposed below the clavicle. The interface between the fenestrations and branches and their target vessels are secured with bridging stent-grafts. Fenestrations were secured with balloon-expandable Advanta V12 stent-grafts (Maquet), and branches were secured with self-expanding Fluency stent-grafts (Bard) relined with Luminexx (Bard) or, more recently, Zilver (Cook) self-expanding stents.
To reduce the risk of spinal cord injury (SCI), the left subclavian artery (LSA) and one or both internal iliac arteries were preserved whenever possible.
Spinal cord protection strategy
All patients with Crawford extent I–III TAAA underwent cerebrospinal fluid (CSF) drainage perioperatively, which was continued for 48 h to maintain a CSF pressure of 10 mmHg. Blood pressure was targeted to a mean value of 90 mmHg. No patient in this series underwent staged procedure, but this has since become our standard approach for extent I–III TAAA.
Definitions of outcomes
Technical success was defined as complete deployment of all planned aortic stent-grafts and visceral vessels stents. Spinal cord injury (SCI) was defined as new-onset paraplegia or paraparesis. Endoleaks were defined according to reporting standards as recommended by the Society of Vascular Surgery [6]. Myocardial infarction was defined according to the criteria defined by the international Joint Task Force for the universal definition of myocardial infarction [7].
Follow-up
Follow-up involved plain antero-posterior and lateral abdominal and chest X-rays at 1, 6 and 12 months and 6 monthly thereafter with CT angiography at 1 and 12 months and annually thereafter. The mean follow-up was 23 months.
RESULTS
A total of 221 target vessels [coeliac artery (CA) 50, superior mesenteric artery (SMA) 61, renal artery (RA) 106, LSA 1 and hypogastric artery (HA) 3] were preserved with scallops (n = 17), fenestrations (n = 140) or branches (n = 62) and 201 of these vessels were stent-grafted (CA 34, SMA 58, RA 105, LSA 1 and HA 3) (Fig. 1). One target vessel was preserved with a coeliac chimney endograft and another with an ilio-renal bypass. One target vessel (RA) could not be cannulated and occluded intraoperatively.
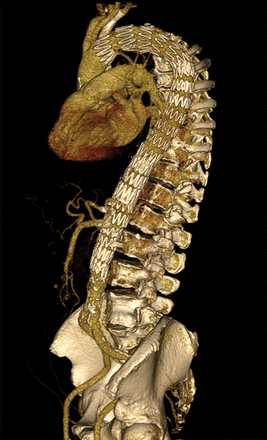
Extent I thoraco-abdominal aortic aneurysm with fenestration for the left subclavian artery and four fenestrations for the visceral and renal arteries.
The 30-day mortality was 1.6% (n = 1) and one further patient died on postoperative day 62 from respiratory complications (Table 1). SCI developed in 5 (8%) patients (3 women and 2 men). Two (5.6%) patients developed SCI after repair of extent IV TAAA; one had bilateral very short common iliac arteries and had undergone external to internal iliac bypass to preserve one internal iliac artery prior to EVAR; the other had previous aorto-bi-femoral bypass for AAA with sacrifice of both internal iliac arteries. Three (11.5%) patients developed SCI after repair of extent I–III TAAA despite the preservation of bilateral internal iliac and left subclavian arteries. Two (3.2%) patients required temporary renal replacement therapy. Two further patients with near end-stage renal failure commenced planned postoperative dialysis: one with a 7-cm Crawford extent III TAAA and renal failure due to glomerulonephritis had an arterio-venous fistula (AVF) created before the TAAA repair with preservation of bilateral RA perfusion; the second had a rapidly expanding 9-cm extent III TAAA and had a tunnelled dialysis line placed before TAAA repair with intentional sacrifice of a single stenosed RA, and an AVF was created postoperatively. One patient developed a stroke manifested as expressive dysphasia, which had recovered at 1 month. One patient had postoperative acute coronary syndrome, which was managed medically.
| Crawford extent . | I–III (n = 26) . | IV (n = 36) . |
|---|---|---|
| Thirty-day mortality | 0 | 1 (3.8%) |
| In-hospital mortality | 1 (3.8%) | 1 (3.8%) |
| Spinal cord injury | 3 (11.5%) | 2 (5.6%) |
| Unplanned permanent dialysis | 0 | 0 |
| Stroke | 0 | 1 (3.8%) |
| Myocardial infarction | 0 | 1 (3.8%) |
| Graft configuration | ||
| Scallops | 1 | 16 |
| Fenestrations | 35 | 105 |
| Branches | 53 | 9 |
| Crawford extent . | I–III (n = 26) . | IV (n = 36) . |
|---|---|---|
| Thirty-day mortality | 0 | 1 (3.8%) |
| In-hospital mortality | 1 (3.8%) | 1 (3.8%) |
| Spinal cord injury | 3 (11.5%) | 2 (5.6%) |
| Unplanned permanent dialysis | 0 | 0 |
| Stroke | 0 | 1 (3.8%) |
| Myocardial infarction | 0 | 1 (3.8%) |
| Graft configuration | ||
| Scallops | 1 | 16 |
| Fenestrations | 35 | 105 |
| Branches | 53 | 9 |
| Crawford extent . | I–III (n = 26) . | IV (n = 36) . |
|---|---|---|
| Thirty-day mortality | 0 | 1 (3.8%) |
| In-hospital mortality | 1 (3.8%) | 1 (3.8%) |
| Spinal cord injury | 3 (11.5%) | 2 (5.6%) |
| Unplanned permanent dialysis | 0 | 0 |
| Stroke | 0 | 1 (3.8%) |
| Myocardial infarction | 0 | 1 (3.8%) |
| Graft configuration | ||
| Scallops | 1 | 16 |
| Fenestrations | 35 | 105 |
| Branches | 53 | 9 |
| Crawford extent . | I–III (n = 26) . | IV (n = 36) . |
|---|---|---|
| Thirty-day mortality | 0 | 1 (3.8%) |
| In-hospital mortality | 1 (3.8%) | 1 (3.8%) |
| Spinal cord injury | 3 (11.5%) | 2 (5.6%) |
| Unplanned permanent dialysis | 0 | 0 |
| Stroke | 0 | 1 (3.8%) |
| Myocardial infarction | 0 | 1 (3.8%) |
| Graft configuration | ||
| Scallops | 1 | 16 |
| Fenestrations | 35 | 105 |
| Branches | 53 | 9 |
Two target vessel stent-grafts occluded during follow-up, both without clinical consequences: one RA at 6 weeks and one SMA at 3 months. Two target vessel stent-grafts have required late reintervention: restent grafting of one RA at 8 months and one SMA at 10 months.
There were no periprocedural type I or type III endoleaks. There was one late (4 years after the procedure) type Ib endoleak, which was successfully treated with a further stent-graft.
DISCUSSION
Since the first endovascular TAAA repair by Chuter et al. [5], there have been a number of large single-centre case series, the largest of which comes from The Cleveland Clinic [8]. This group reported a 5.7% perioperative mortality and 4.3% SCI rate in 633 patients (70% juxtarenal or extent IV TAAA). More recently, Chuter reported on 81 patients (52% pararenal or extent IV TAAA) with a mortality rate of 6.2%, persistent paraplegia in 3.7% and permanent dialysis in 3.7% [9]. Guillou described a series of 89 patients (56% extent IV TAAA) with a mortality rate of 10%, SCI 7.8% and temporary (but no permanent dialysis) in 6.7% [10]. While the mortality rate after endovascular TAAA repair is generally within 10%, the risk of SCI has been reported variably between 3 and 20% with a high patency rate for visceral vessels and a low rate of early endoleaks [11, 12]. The results of the present series, which includes our early learning curve, are similar to those of other European and North American specialist centres with acceptably low rates of perioperative mortality and complications.
SCI remains the true Achilles' heel of every TAAA procedure. The risk is related to the length of the aorta replaced during open surgery [13] or covered during EVAR [14]. This relation between the length of aorta treated and the risk of SCI is a clinical confirmation of the experimental work of Griepp and Griepp [15], who have extensively studied the anatomy of the spinal cord circulation and pointed out the importance of the collateral network blood supply. According to the collateral network theory, the spinal cord is perfused by a network of arterial connections which derive their supply mainly from four sources: thoracic segmental arteries, lumbar segmental arteries, LSA and the internal iliac arteries. The risk of SCI is very low when only one of these sources is interrupted, but it is significantly increased when two or more sources are interrupted simultaneously. Experimental works on pigs have demonstrated that staged procedures with occlusion of thoracic segmental arteries followed by replacement of the thoraco-abdominal aorta carry a significantly lower risk of SCI compared with a single procedure on the entire thoraco-abdominal aorta [16]. Preservation of left subclavian and internal iliac artery perfusion is crucial in TAAA repair. EVAR can be staged in numerous ways, but all approaches aim to cause gradual sac thrombosis and thus gradual segmental thoracic/lumbar artery sacrifice. Between October 2012, when this cohort was completed, and December 2013, we have performed a further 37 fenestrated and branch EVAR procedure for TAAA with no SCI; 19 of these patients had repair of extent I–III aneurysms, which was staged in 18 patients. We believe that staging EVAR for extent I–III TAAA has contributed significantly to the low incidence of SCI in our current practice.
Open TAAA repair has been proved to be a durable option with an acceptable risk for patients with CTD [3] and young, low-risk patients with degenerative TAAA [2]. EVAR for TAAA is reserved for patients with degenerative disease who are high risk for conventional open repair. While EVAR for patients with TAAA and CTD is not currently associated with durable outcomes [17], this is not to say that endografting will not have a role in the future: one possibility would be a combined approach with open arch reconstruction and frozen elephant trunk procedure to create a proximal landing zone for a fenestrated/branch EVAR for the TAAA and a surgical adjunct to secure the distal landing zone in the common iliac arteries. Time will tell whether EVAR will be the best option for the majority of TAAA in 2020 [18].
Conflict of interest: Donald J. Adam is an European preceptor for Cook Medical's fenestrated/branch EVAR device and has received unrestricted research funding from Cook Medical. The other authors report no conflict of interest.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr B Zipfel(Berlin, Germany): Dr Iafrancesco and his colleagues have reported on a large, single-institution series of fenestrated and branched TEVAR focusing on real thoracoabdominal aneurysms, excluding juxta- and para-renal aneurysms with fenestrated procedures. I totally agree that these cases should be viewed separately.
The reported in-hospital mortality of 3.2% and the incidence of spinal cord injury of 8% compares equally to other series published on these extended endovascular procedures. It serves as a reminder that these procedures, although deemed minimally invasive, carry intrinsic risks and are prone to complications.
I have three questions and I would like to have you answer them consecutively. Firstly, the percentage of type IV aneurysms in the series is 58%, and thus higher than in most other series I know. The reported results have to be viewed in this context, and differentiation of the major complications in your two groups of Crawford type I and III, and Crawford IV would be of interest. Specifically, how many of these five spinal cord injuries occurred in the type IV subgroup?
Dr Iafrancesco: That is a very good point. Generally speaking, in the literature, an even higher rate of type IV and pararenal thoracoabdominal aneurysm is reported. In the Cleveland Clinic group series it is about 70%. We did not do a risk analysis because the numbers are still too small. In our series, spinal cord injury occurred in three cases of type I-III and two cases of type IV.
Dr Zipfel: Secondly, in reading the paper, 71% of the target arteries were connected to the stent grafts by fenestrations or scallops. In our practice, we use the fenestrations only if the side branches originate from nearly normal sized aorta in the landing zone of the stent graft. Could this high percentage in your series be explained by predominantly type I aneurysms in the type I-III group or did you use the fenestrations even when there was a gap between the stent graft and the origin of the target vessel?
Dr Iafrancesco: I suspect this is due to a more liberal use of fenestration than in your series, because in the remaining cases of non-type IV are eight type I, eight type II and 10 type III. So it is more or less the same. There is no prevalence of one type over the other.
Dr Zipfel: It is still a very high percentage of fenestrations used.
Dr Iafrancesco: Yes.
Dr Zipfel: So you use the fenestration even if you have gaps?
Dr Iafrancesco: Yes.
Dr Zipfel: The last question is just a technical one. You report four patients who required dialysis postoperatively. Were these events related to some kind of problems with the renal arteries after initially successful stent grafting?
Dr Iafrancesco: No. All patients who had renal problems postop had renal dysfunction preop, and this is very much in line with the previous Cleveland Clinic experience in which they found that actually the preop creatinine is the main factor affecting postoperative renal function. So the two who went to permanent dialysis actually had a creatinine of around 300, and one of them had radial arteriovenous fistula prepared preoperatively, so it was actually a planned postoperative dialysis. The other two had some renal dysfunction but then they recovered to the baseline. So it wasn't related to the occlusion of the renal artery, if that is the question.
Dr M.A.A.M. Schepens(Brugge, Belgium): You limit your experience now to only degenerative aneurysms?
Dr Iafrancesco: Yes.
Dr Schepens: Do you think in the future that you will expand your techniques to post-dissection aneurysms also, or actually will you say that this may be too complicated?
Dr Iafrancesco: The problem with dissection is that the lumen obviously is limited by the flap, so it's very difficult to use a branch in the dissection setting. You could theoretically use fenestration more easily, yes, but I suspect that these are a quite more complicated setting and we really need to build up our experience before starting to do that.
I would like to take the opportunity to say another thing. The problem is, a great proportion of these patients with enlarging distal dissection are usually younger or have connective tissue disease, at least in our experience, rather than an atherosclerotic disease. Patients who are younger with connective tissue disease, should really go to open surgery. What we strongly believe is that there is no one option over the other. We really should try to find out which is the best candidate for every procedure. That's why we think that they should be evaluated in a centre able to offer both procedures, because if you go to a centre where they offer only one procedure, there will always be a bias of selection. But if you are able to offer both, then at this point you are really able to make the best choice for the patient.
Dr K. Minatoya(Osaka, Japan): You showed a really low rate of endoleak in your series, but in reality have you found aneurysms expanding in the follow-up period?
Dr Iafrancesco: No.
Dr Minatoya: Not at all?
Dr Iafrancesco: No.
Dr Minatoya: Amazing.
Dr Iafrancesco: I have to say that, obviously, I can't comment on type II, because the type IV are usually followed up by abdominal X-ray and ultrasound more than CT scan. So that is a limitation of the follow-up, I agree.
Author notes
Presented at the 27th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Vienna, Austria, 5–9 October 2013.
- aortic aneurysm
- stents
- hemodialysis
- aneurysm
- blood pressure
- celiac disease
- objective (goal)
- mesentery
- renal replacement therapy
- rupture
- spinal cord injuries
- abdomen
- dialysis procedure
- kidney
- mortality
- respiratory complication
- thoracic aortic procedures
- study report
- evar trial
- cerebrospinal fluid drainage
- fenestration




