-
PDF
- Split View
-
Views
-
Cite
Cite
Vitaly Barmin, Victor Sadovnichy, Mikhail Sokolov, Oleg Pikin, Ali Amiraliev, An original device for intraoperative detection of small indeterminate nodules, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 6, December 2014, Pages 1027–1031, https://doi.org/10.1093/ejcts/ezu161
Close - Share Icon Share
Abstract
The purpose of this study was to evaluate the efficiency of our newly designed tactile mechanoreceptor in detection of pulmonary lesions during thoracoscopy.
Twenty-seven patients with peripheral undetermined subpleural solitary pulmonary lesions detected on computed tomography were included in a prospective non-randomized trial. All nodules from 7 to 18 mm in diameter were located deep in the lung parenchyma (≥10 mm from the lung surface). All patients underwent thoracoscopic exploration with diagnostic intent. Instrumental palpation with lung forceps was performed first, followed by thorough inspection of lung tissue with the tactile mechanoreceptor. This device is a metal tube 10 mm in diameter, which can be inserted into the pleural cavity via a standard 10-mm port. There is an elastic membrane on its working end, which deforms greatly if the palpated tissue has greater density. Intraoperatively, the surgeon pushed the targeted region of pulmonary tissue with the mechanoreceptor and carried out the measurement. The density of tissue characteristics was displayed with special software using colour change in real time. After detection of a pulmonary nodule, it was resected with endostaplers.
Instrumental palpation was successful in detection of pulmonary lesions in 10 (37%) patients and was confirmed with the tactile mechanoreceptor. In 12 (44%) patients, instrumental palpation failed to locate an intrapulmonary nodule, while the tactile mechanoreceptor facilitated finding the lesion and performing thoracoscopic lung resection in all these patients. Intraoperative histological examination confirmed benign disease in 8, metastatic lesion in 12 and primary lung cancer in 7 patients requiring thoracoscopic lobectomy. In 5 (19%) patients, neither forceps nor the tactile mechanoreceptor was able to detect any pulmonary lesion, necessitating mini-thoracotomy for finger palpation. The overall efficacy of the tactile mechanoreceptor in detection of pulmonary lesions was 81%, and of impalpable nodes 71%.
The tactile mechanoreceptor is an effective tool for detection of impalpable pulmonary lesions during thoracoscopy.
INTRODUCTION
Video-assisted thoracoscopic surgery is used every day both to diagnose and to treat pulmonary nodules. Most of these nodules can be easily detected with an instrumental probe. Nevertheless, there is often no involvement of the visceral pleura in the pathological process during thoracoscopy in patients with peripheral solitary pulmonary lesions. In this case, instrumental palpation is non-diagnostic, because of the deep localization of the lesion in the lung parenchyma and its small size. There are several methods to detect such lesions, with different levels of efficiency, either preoperative or intrathoracoscopic [1]. The simplest method is finger palpation [2]. The others are blue-dye, tracing, hookwire, the intrathoracic stamping method [3] and intraoperative ultrasound. The existing methods, however, have significant disadvantages: invasiveness for the first three of them, a requirement for specific localization of the nodule for the stamping method and a requirement for lung collapse for intraoperative ultrasound.
Therefore, we developed a new device for tactile diagnostics of pulmonary lesions that can overcome these shortcomings. At Lomonosov Moscow State University in the mechanoreception laboratory, we created an original tactile mechanoreceptor (Fig. 1). The aim of the present study was to evaluate the efficiency of this tactile mechanoreceptor for detection of pulmonary lesions during thoracoscopy.
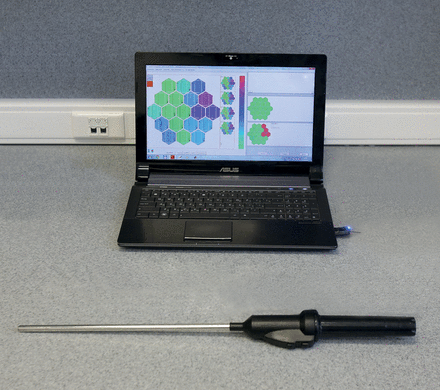
Complex of tactile diagnostics: the tactile mechanoreceptor and the computer.
MATERIALS AND METHODS
From June 2012 to December 2012, 27 patients (20 males and 7 females, ages ranging from 32 to 77 years, average age 59.1 years) with peripheral undetermined subpleural solitary pulmonary lesions detected on CT were included in a prospective non-randomized trial. All nodules with a diameter of 7–18 mm were located deep in the lung parenchyma (the distance from the nearest pleural surface was between 10 and 43 mm). All patients underwent diagnostic thoracoscopic exploration. Three thoracoports were placed in typical positions. At first, we performed instrumental palpation with lung forceps followed by thorough inspection of lung tissue with the tactile mechanoreceptor. When a pulmonary nodule was detected, it was resected with endostaplers. Wedge resection was always performed with at least 1 cm of tissue margin. All specimens underwent frozen section. In the case of primary lung cancer, we placed an additional thoracoport, and after that, a thoracoscopic lobectomy and a lymphadenectomy were also performed.
Description of the tactile mechanoreceptor
This instrument consists of a metal tube with an elastic membrane on its working end and a microelectronic component inside. In the handle of the instrument, there is a Bluetooth transmitter. The elastic membrane has seven independent chambers. It can deform greatly under the pressure of palpated tissue with a higher density (Fig. 2). Recordings of the changing volumes in the chambers are made by discrete piezoelectric pressure sensors. The received analogue signals are then digitized, amplified and sent through a wireless transmission device to the computer. Processing of the received signals with special software permits representation of the tactile information as a visual image and recording of the dynamic change of the signal through time. The size of the instrument corresponds to the size of the surgical instruments of similar type—10 mm in diameter. That size allows it to be inserted into the pleural cavity via the standard 10-mm port during thoracoscopy. This original complex meets all the requirements of the Russian state standards for medical equipment and has been approved for use in the surgical intervention process.
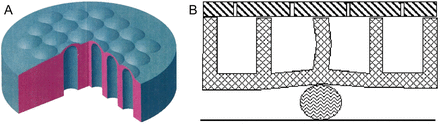
(A) The elastic membrane; (B) mathematical model of the membrane.
Intraoperative manoeuvres
According to the protocol of our study, a surgeon intraoperatively pushes the targeted region of pulmonary tissue with the mechanoreceptor and carries out the measurement. The density of tissue characteristics is displayed using special software by means of colour change in real time: a green light on the diagram reflects lung tissue of normal density, while a red light indicates a lesion in lung tissue (see Supplementary Video S1). With the help of a mathematical algorithm, the surgeon can see if there is a border between normal and high-density tissue in the inspected area (Fig. 3).
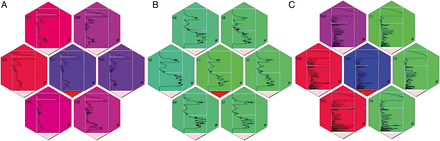
Intraoperative results of palpation with tactile mechanoreceptor. (A) Lesion; (B) intact lung; (C) ‘border’ between the nodule (left side) and normal lung parenchyma (right side).
Statistical analysis
To assess the differences in the efficiency of detection of pulmonary lesions between instrumental palpation and the tactile mechanoreceptor, we used McNemar's test. We performed statistical analysis using Statistica 8.0 (StatSoft, Inc.). The differences were considered statistically significant when the P-level was <0.05.
RESULTS
Of the 27 nodules included in the study, 22 (81%) were successfully detected by means of the electronic device. Instrumental palpation successfully detected pulmonary lesions in 10 (37%) patients, and these results were confirmed with the tactile mechanoreceptor. In 12 (44%) patients, instrumental palpation failed to locate intrapulmonary nodule, while the tactile mechanoreceptor was able to find the lesion and facilitated thoracoscopic lung resection in all of these patients (Table 1). The analysis of the frozen sections revealed primary lung cancer in 7 (26%) cases and secondary lesions in 12 (44%) cases. The remaining 8 cases (30%) were histologically benign.
| Localization (lobe) . | Size (mm) . | Depth from the surface (mm) . | Instrumental palpation detected . | Tactile mechanoreceptor detected . | Finger palpation detected . |
|---|---|---|---|---|---|
| Right upper | 12 | 17 | + | + | |
| Right upper | 15 | 26 | + | + | |
| Right upper | 14 | 33 | − | + | |
| Right upper | 13 | 38 | − | − | + |
| Right middle | 12 | 10 | + | + | |
| Right middle | 17 | 11 | + | + | |
| Right lower | 16 | 17 | + | + | |
| Right lower | 11 | 19 | − | + | |
| Right lower | 12 | 21 | − | + | |
| Right lower | 16 | 23 | − | + | |
| Right lower | 18 | 26 | − | + | |
| Right lower | 8 | 27 | − | − | + |
| Left upper | 15 | 11 | + | + | |
| Left upper | 17 | 15 | + | + | |
| Left upper | 11 | 26 | − | + | |
| Left upper | 14 | 31 | − | + | |
| Left upper | 16 | 33 | − | + | |
| Left upper | 15 | 37 | − | + | |
| Left upper | 11 | 42 | − | − | + |
| Left upper | 7 | 31 | − | − | + |
| Left lower | 14 | 22 | + | + | |
| Left lower | 18 | 25 | + | + | |
| Left lower | 13 | 26 | + | + | |
| Left lower | 16 | 27 | − | + | |
| Left lower | 15 | 34 | − | + | |
| Left lower | 18 | 43 | − | + | |
| Left lower | 10 | 30 | − | − | + |
| Localization (lobe) . | Size (mm) . | Depth from the surface (mm) . | Instrumental palpation detected . | Tactile mechanoreceptor detected . | Finger palpation detected . |
|---|---|---|---|---|---|
| Right upper | 12 | 17 | + | + | |
| Right upper | 15 | 26 | + | + | |
| Right upper | 14 | 33 | − | + | |
| Right upper | 13 | 38 | − | − | + |
| Right middle | 12 | 10 | + | + | |
| Right middle | 17 | 11 | + | + | |
| Right lower | 16 | 17 | + | + | |
| Right lower | 11 | 19 | − | + | |
| Right lower | 12 | 21 | − | + | |
| Right lower | 16 | 23 | − | + | |
| Right lower | 18 | 26 | − | + | |
| Right lower | 8 | 27 | − | − | + |
| Left upper | 15 | 11 | + | + | |
| Left upper | 17 | 15 | + | + | |
| Left upper | 11 | 26 | − | + | |
| Left upper | 14 | 31 | − | + | |
| Left upper | 16 | 33 | − | + | |
| Left upper | 15 | 37 | − | + | |
| Left upper | 11 | 42 | − | − | + |
| Left upper | 7 | 31 | − | − | + |
| Left lower | 14 | 22 | + | + | |
| Left lower | 18 | 25 | + | + | |
| Left lower | 13 | 26 | + | + | |
| Left lower | 16 | 27 | − | + | |
| Left lower | 15 | 34 | − | + | |
| Left lower | 18 | 43 | − | + | |
| Left lower | 10 | 30 | − | − | + |
| Localization (lobe) . | Size (mm) . | Depth from the surface (mm) . | Instrumental palpation detected . | Tactile mechanoreceptor detected . | Finger palpation detected . |
|---|---|---|---|---|---|
| Right upper | 12 | 17 | + | + | |
| Right upper | 15 | 26 | + | + | |
| Right upper | 14 | 33 | − | + | |
| Right upper | 13 | 38 | − | − | + |
| Right middle | 12 | 10 | + | + | |
| Right middle | 17 | 11 | + | + | |
| Right lower | 16 | 17 | + | + | |
| Right lower | 11 | 19 | − | + | |
| Right lower | 12 | 21 | − | + | |
| Right lower | 16 | 23 | − | + | |
| Right lower | 18 | 26 | − | + | |
| Right lower | 8 | 27 | − | − | + |
| Left upper | 15 | 11 | + | + | |
| Left upper | 17 | 15 | + | + | |
| Left upper | 11 | 26 | − | + | |
| Left upper | 14 | 31 | − | + | |
| Left upper | 16 | 33 | − | + | |
| Left upper | 15 | 37 | − | + | |
| Left upper | 11 | 42 | − | − | + |
| Left upper | 7 | 31 | − | − | + |
| Left lower | 14 | 22 | + | + | |
| Left lower | 18 | 25 | + | + | |
| Left lower | 13 | 26 | + | + | |
| Left lower | 16 | 27 | − | + | |
| Left lower | 15 | 34 | − | + | |
| Left lower | 18 | 43 | − | + | |
| Left lower | 10 | 30 | − | − | + |
| Localization (lobe) . | Size (mm) . | Depth from the surface (mm) . | Instrumental palpation detected . | Tactile mechanoreceptor detected . | Finger palpation detected . |
|---|---|---|---|---|---|
| Right upper | 12 | 17 | + | + | |
| Right upper | 15 | 26 | + | + | |
| Right upper | 14 | 33 | − | + | |
| Right upper | 13 | 38 | − | − | + |
| Right middle | 12 | 10 | + | + | |
| Right middle | 17 | 11 | + | + | |
| Right lower | 16 | 17 | + | + | |
| Right lower | 11 | 19 | − | + | |
| Right lower | 12 | 21 | − | + | |
| Right lower | 16 | 23 | − | + | |
| Right lower | 18 | 26 | − | + | |
| Right lower | 8 | 27 | − | − | + |
| Left upper | 15 | 11 | + | + | |
| Left upper | 17 | 15 | + | + | |
| Left upper | 11 | 26 | − | + | |
| Left upper | 14 | 31 | − | + | |
| Left upper | 16 | 33 | − | + | |
| Left upper | 15 | 37 | − | + | |
| Left upper | 11 | 42 | − | − | + |
| Left upper | 7 | 31 | − | − | + |
| Left lower | 14 | 22 | + | + | |
| Left lower | 18 | 25 | + | + | |
| Left lower | 13 | 26 | + | + | |
| Left lower | 16 | 27 | − | + | |
| Left lower | 15 | 34 | − | + | |
| Left lower | 18 | 43 | − | + | |
| Left lower | 10 | 30 | − | − | + |
Thus, there was a statistically significant difference in efficiency for the detection of pulmonary lesions between instrumental palpation and the tactile mechanoreceptor (37 vs 81%; McNemar P = 0.0015). In 5 (19%) of the patients, neither forceps nor the tactile mechanoreceptor was able to detect any pulmonary lesion. These patients required mini-thoracotomy and finger palpation. The reason of failure in 3 cases was small size of lesions: ≤10 mm. In 2 cases, it happened due to the position of lesions near segmental bronchus. The overall efficacy of the tactile mechanoreceptor for the detection of impalpable pulmonary nodes was 71%. In these cases, our device prevented conversion during surgery.
DISCUSSION
The problem of detection of peripheral solitary pulmonary lesions has been discussed in many publications [4]. Various studies have shown that the results of different diagnostic methods for the identification of such lesions approach futility. Even the specificity and sensitivity levels of positron emission tomography are not satisfactory in cases of nodules with a diameter of <10 mm [5]. In such lesions, video-assisted thoracoscopic surgery seems to be the best tool for diagnosis and treatment. On the other hand, lesions of that kind can be difficult to locate during thoracoscopy because of the size of the lesion and its depth. Some authors report that they cannot localize nodules at the time of the procedure in 7.5–11% of cases [6–8].
Pre- and intraoperative techniques of pulmonary nodule localization have been extensively studied, and their pros and cons are well understood. Instrumental palpation is the most accessible method, but usually it does not work if the lesion is located deep in the lung parenchyma. Finger palpation is the simplest method. After mini-thoracotomy has been done, two fingers inserted into pleural cavity palpate the lung tissue. The efficiency of this method is about 25–40% [9, 10]. Hookwire is another method for localization of pulmonary lesions. Before the operation, transthoracic puncture under computer tomography (CT) is performed with a special 20-G needle with a hook on its end. The distal end with the hook is inserted into the lesion, while the proximal end is fixed on the skin. The efficiency is rather high and reaches about 84–92% [10, 11]. The needle-wire technique is, however, associated with several complications, such as dislodgement of the needle, pneumothorax, pleuritic pain, lung haemorrhages or haematoma and pleural bleeding [12–14]. Some surgeons inject methylene blue into the lesion or close to it in the lung parenchyma. The blue colour of the visceral pleura helps to detect the lesion during thoracoscopy. One of the major problems is the time elapsed between the location of the nodule and the surgical intervention: the longer the time interval, the greater the diffusion of contrast medium into the pulmonary parenchyma surrounding the nodule. The method also has a high rate of complications such as pneumothorax, pleuritic pain, pulmonary bleeding and fits of coughing [15–17]. A failure rate of around 13% has been reported for methylene blue injection [18]. The next method is the gamma probe. Transthoracic puncture under CT is performed before the operation with a 22-G needle. Radiopharmaceutical medium with non-ionic contrast (which allows a following radiological control) is injected into the lesion or close to it in the lung parenchyma. After that, thoracoscopy is performed and followed by thorough inspection of lung tissue with a gamma detector. The efficiency of the method is 80–96% [4, 19–21]. A marking technique that avoids pleural puncture—the intrathoracic stamping method—is another way to localize the pulmonary lesions. A thread inserted into the pleural cavity is withdrawn from another surgical port, and a small dye-containing gauze ball is tied to the thread. The gauze ball is pulled back into the pleural cavity and tugged towards the internal surface of the thoracic wall. When the lung is re-expanded, the dye from the gauze ball stamps the surface of the lung. The contraindication is the localization of the nodules in the apical portion, on the mediastinal and diaphragmatic surfaces, behind the scapulae etc., which cannot be reached from the body surface by the shortest way. This technique was successfully performed on 13 lesions in 12 patients [3]. Intraoperative ultrasound is also used: an ultrasonic sensor is inserted into the pleural cavity and pressed to the collapsed lung, and it carries out the measurement. The efficiency of this method is also 80–96% [22]. Despite the lack of complications and the high sensitivity and specificity of ultrasound [23], it is known to present some limitations in localizing inflammatory nodules [24].
Currently, there are no data in the literature about the use of endoscopic tactile devices that can record the dynamic change of a tactile signal through time during inspection of the lung parenchyma. The tactile complex is fully compatible with the surgical endoscopic stands used today. There are no contraindications for the use of the tactile mechanoreceptor during thoracoscopy. According to our results, we can suppose that the size of the lesion <10 mm and the position near segmental bronchus are limitations of the method.
The device can operate both in real time and through recording. Recorded information can be presented in an amplified or reduced form and correlated with the visual image captured during the surgical intervention. This allows not only analysis of the data but also demonstration of the tactile characteristics of the object in order to carry out training of students or doctors.
Our data demonstrated the high efficiency (81%) of this device in detection of pulmonary lesions. The results that we obtained allow us not only to avoid conversion in several cases but also to reduce the period of the surgical intervention and anaesthesia. The tactile mechanoreceptor is an effective and easy-to-use tool for the detection of impalpable pulmonary lesions during thoracoscopy.
CONCLUSION
In our study, we developed an original diagnostic instrument for the detection of pulmonary lesions. We demonstrated that the tactile mechanoreceptor facilitates the detection of pulmonary lesions and decreases the conversion rate during surgery. We can conclude that this device could be routinely used during thoracoscopy for the detection of impalpable pulmonary nodules.
SUPPLEMENTARY MATERIAL
Supplementary material (Video 1) is available at EJCTS online.
Video S1: The use of the device in the operating theatre. (Left upper quadrant) Video from thoracoscope; (Right upper quadrant) Video from operation theatre's camera; (Left lower quadrant) Video from the computer. All video fragments were recorded from the same point of time. A 14 mm nodule was found in the right upper lobe. The red color of the histogram proves it.
Funding
This work was supported by the Russian Government decree ‘On measures of state funding for the development of cooperation of Russian institutions of higher education and organizations implementing complex projects on high-tech production’ [Contracts # 13.G36.31.002, 02.G25.31.0030].
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We thank Barry Alpher for his editorial correction of the English style of the manuscript.
REFERENCES
Author notes
Presented at the 21st European Conference on General Thoracic Surgery, Birmingham, UK, 26–29 May 2013.
- computed tomography
- lung
- color
- objective (goal)
- intraoperative care
- mechanoreceptors
- tissue membrane
- metals
- palpation
- software
- thoracoscopy
- diagnosis
- fingers
- pleura
- lung volume reduction
- lung cancer
- minithoracotomy
- pulmonary nodule
- lobectomy
- lung parenchyma
- medical devices
- inspection
- diameter
- forceps




