-
PDF
- Split View
-
Views
-
Cite
Cite
Edouard Sage, Sacha Mussot, Grégoire Trebbia, Philippe Puyo, Marc Stern, Philippe Dartevelle, Alain Chapelier, Marc Fischler, Lung transplantation from initially rejected donors after ex vivo lung reconditioning: the French experience, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 5, November 2014, Pages 794–799, https://doi.org/10.1093/ejcts/ezu245
Close - Share Icon Share
Only 15% of brain death donors are considered suitable for lung transplantation (LTx). The normothermic ex vivo lung perfusion technique is used to potentially increase the availability of high-risk lung donors. We report our experience of LTx with initially rejected donors after ex vivo lung reconditioning (EVLR).
From April 2011 to May 2013, we performed EVLR for 32 pairs of donor lungs deemed unsuitable for transplantation and rejected by the 11 French lung transplant teams. After EVLR, lungs with acceptable function were transplanted. During the same period, 81 double-lung transplantations (DLTx) were used as controls.
During EVLR, 31 of 32 donor lungs recovered physiological function with a median PO2/FiO2 ratio increasing from 274 (range 162–404) mmHg to 511 (378–668) mmHg at the end of EVLR (P < 0.0001). Thirty-one DLTx were performed. The incidence of primary graft dysfunction 72 h after LTx was 9.5% in the EVLR group and 8.5% in the control group (P = 1). The median time of extubation, intensive care unit and hospital lengths of stay were 1, 9 and 37 days in the EVLR group and 1 (P = 0.17), 6 (P = 0.06) and 28 days (P = 0.09) in the control group, respectively. Thirty-day mortality rates were 3.3% (n = 1) in the EVLR group and 3.7% (n = 3) in the control group (P = 0.69). One-year survival rates were 93% in the EVLR group and 91% in the control group.
EVLR is a reliable and repeatable technique that offers a significant increase of available donors. The results of LTx with EVLR lungs are similar to those obtained with conventional donors.
INTRODUCTION
Lung transplantation (LTx) is often the only treatment for patients with end-stage lung disease, but donor lung shortage is still a major limit for this therapeutic option. Only 15–20% of lungs from multiorgan donors are suitable for transplantation [1, 2] as a result of damage due to the development of neurogenic oedema and pro-inflammatory changes caused by brain death [3]. Thus, we deplore a substantial waiting list mortality rate [4], which is 16% per year in France [2].
To improve this situation, several strategies have been developed to alleviate donor lung shortage such as living lobar transplantation [5], cadaveric lobar transplantation [6] and non-heart-beating donor [7]. More recently, to increase the availability of high-risk lung donors, the group from Toronto performed a clinical trial with normothermic ex vivo lung perfusion (EVLP), demonstrating the safety of the procedure [8, 9].
After several years of animal experiments to develop the technique, Steen et al. [10] successfully performed the first human LTx of an initially rejected donor lung, after reconditioning ex vivo in 2005. Since then, ex vivo lung reconditioning (EVLR) of clearly unacceptable or rejected lungs has been reported in a few cases [11–14]. We designed a prospective non-randomized clinical trial to evaluate the capacity of EVLR to improve the function of donor lungs deemed unsuitable for transplantation and rejected by the 11 French lung transplant teams.
MATERIALS AND METHODS
Study design
We designed a prospective non-randomized trial. From April 2011 to May 2013, the clinical outcome of patients undergoing LTx after EVLP was compared with those who underwent conventional LTx (control group) during the same period. The study was registered and approved by a French ethics committee (CPP 2010/36), and all patients gave their written informed consent to participate in the study when listed for transplantation. They were informed that an EVLP procedure would be performed before LTx if the donor lungs were deemed unsuitable for transplantation and rejected by the 11 French lung transplant teams.
Recipient and donor inclusion criteria
Planned lobar transplantation and retransplantation were not considered to be exclusion criteria. Only patients who had high-emergency LTx were excluded from this study. Recipients were selected on the basis of compatible blood type, size of the organ and urgency status.
Donor lung allocation is made by the French National Biomedicine Agency (FNBA) that regulates the distribution of grafts. The donor lung assessment is made from a donor score (0–18) [15] based on history (age and smoking status), arterial blood gas measurement, bronchoscopic examination and chest radiographic findings. French donors are divided into three categories, given in Table 1.
| . | Optimal donor . | Extended criteria donor . | Marginal donor . |
|---|---|---|---|
| Age (years) | <56 | 56–70 | >70 |
| PO2/FiO2 | (and) >400 mmHg | (and/or) 200–400 mmHg | (and/or) <200 mmHg |
| Chest X-ray | (and) Normal | (and/or) Abnormal | |
| Smoking history | (and) No | (and/or) Yes | |
| Gastric aspiration | (and) No | (and/or) Yes |
| . | Optimal donor . | Extended criteria donor . | Marginal donor . |
|---|---|---|---|
| Age (years) | <56 | 56–70 | >70 |
| PO2/FiO2 | (and) >400 mmHg | (and/or) 200–400 mmHg | (and/or) <200 mmHg |
| Chest X-ray | (and) Normal | (and/or) Abnormal | |
| Smoking history | (and) No | (and/or) Yes | |
| Gastric aspiration | (and) No | (and/or) Yes |
| . | Optimal donor . | Extended criteria donor . | Marginal donor . |
|---|---|---|---|
| Age (years) | <56 | 56–70 | >70 |
| PO2/FiO2 | (and) >400 mmHg | (and/or) 200–400 mmHg | (and/or) <200 mmHg |
| Chest X-ray | (and) Normal | (and/or) Abnormal | |
| Smoking history | (and) No | (and/or) Yes | |
| Gastric aspiration | (and) No | (and/or) Yes |
| . | Optimal donor . | Extended criteria donor . | Marginal donor . |
|---|---|---|---|
| Age (years) | <56 | 56–70 | >70 |
| PO2/FiO2 | (and) >400 mmHg | (and/or) 200–400 mmHg | (and/or) <200 mmHg |
| Chest X-ray | (and) Normal | (and/or) Abnormal | |
| Smoking history | (and) No | (and/or) Yes | |
| Gastric aspiration | (and) No | (and/or) Yes |
The extended criteria donor lungs initially considered unsuitable for transplantation and rejected were proposed by the FNBA for the study. The selection criteria for EVLR were the same as for regular transplantations: donors with non-reversible criteria such as advanced age, long smoking history, established pneumonia or severe mechanical parenchymal traumatic injury were excluded. Once selected for EVLR, neither blood gas analysis nor bronchoscopy was performed. After opening the chest, if inspection of the lungs revealed no contraindication, the donor lungs were accepted for EVLR. Then, the lungs were harvested with anterograde Perfadex® (XVIVO Perfusion, Göteborg, Sweden) flushed and stored on ice during transport.
Ex vivo lung reconditioning technique
The ex vivo technique used was the protocol previously described by the Toronto group [16, 17]. First, the lungs were transferred to the XVIVO chamber (XVIVO Perfusion); the pulmonary artery and the funnel-shaped cannula sewn to the left atrium were connected. During the gradual increase in temperature, and before the initiation of ventilation, a bronchoscopy with broncho-alveolar lavage was performed to clear out secretions. Then, when 31°C was reached, ventilation with 3 ml/kg of donor body weight at positive end expiratory pressure (PEEP) 5 and FiO2 21% was started and increased to obtain 7 ml/kg at 37°C. At the same time, the perfusate flow rate of Steen® (XVIVO Perfusion) solution was gradually increased with a target of 40% of the estimated donor cardiac output. Functional assessment (PO2/FiO2 in the pulmonary artery and vein, lung dynamic compliance and peak airway pressures) was performed hourly after 15 min of FiO2 100% and 5 min of lung recruitment with PEEP 10.
When the left atrial PO2/FiO2 was more than 400 mmHg and the other functional parameters were stable or improving after a minimum of 2 h of EVLP, the lungs were considered suitable for transplantation. If the criteria for acceptance were not reached after 2 h of EVLP, the lungs were considered unsuitable for transplantation and sent to pathology. In the case of acceptance, the lungs remained on the perfusion system until explantation of the first lung onto the recipient. Then, the lung block was cooled down in the circuit to 15°C in a 10-min period. The first lung was immediately transplanted and the second was statically preserved at 4°C until transplantation.
For the EVLR group, the total ischaemic time was divided into three periods: the first cold ischaemia time (1CIT), from aortic cross-clamp to initiation of EVLR, the EVLR time and finally the second cold ischaemia time (2CIT) from the end of EVLR to reperfusion of the second lung on the recipient.
Study endpoints
The primary endpoint was grade 3 primary graft dysfunction (PGD) at 72 h post LTx. The secondary endpoints were: length of intubation, intensive care unit (ICU) and hospital lengths of stay, and 30-day mortality.
Statistical analysis
All data were analysed with GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Continuous data are presented as median and ranges. The Mann–Whitney test was used to compare numerical data and a Fischer's exact test was performed for categorical variables. Two-way analysis of variance was used for the comparison of ex vivo PO2/FiO2 between the two groups. Kaplan–Meier curves were used for survival plots. P-values <0.05 were considered to denote statistical significance.
RESULTS
During the study period, 131 LTx were performed at the Foch Hospital using only brain-dead donors. Among them, 19 LTx were done in high emergency and therefore excluded from this study. During the same period, 53 extended criteria donor lungs deemed unsuitable for transplantation and rejected by the 11 French lung transplant teams were proposed by the FNBA for the study. Thirty-two of these lungs were selected for EVLR. Thirty-one LTx were performed after EVLR and 81 conventional LTx were used as control.
Recipient characteristics
The recipient characteristics are summarized in Table 2. There were no significant differences between the two groups in age and demography.
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Age (years) | 40 (21–60) | 41 (17–65) | 0.91 |
| Male/female | 11/20 | 39/42 | 0.22 |
| Indication, n (%) | |||
| Cystic fibrosis | 15 (48) | 40 (49) | 0.92 |
| COPD | 9 (29) | 16 (20) | 0.29 |
| Pulmonary fibrosis | 3 (10) | 12 (15) | 0.47 |
| Other | 4 (13) | 13 (16) | 0.67 |
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Age (years) | 40 (21–60) | 41 (17–65) | 0.91 |
| Male/female | 11/20 | 39/42 | 0.22 |
| Indication, n (%) | |||
| Cystic fibrosis | 15 (48) | 40 (49) | 0.92 |
| COPD | 9 (29) | 16 (20) | 0.29 |
| Pulmonary fibrosis | 3 (10) | 12 (15) | 0.47 |
| Other | 4 (13) | 13 (16) | 0.67 |
EVLR: ex vivo lung reconditioning; COPD: chronic obstructive pulmonary disease.
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Age (years) | 40 (21–60) | 41 (17–65) | 0.91 |
| Male/female | 11/20 | 39/42 | 0.22 |
| Indication, n (%) | |||
| Cystic fibrosis | 15 (48) | 40 (49) | 0.92 |
| COPD | 9 (29) | 16 (20) | 0.29 |
| Pulmonary fibrosis | 3 (10) | 12 (15) | 0.47 |
| Other | 4 (13) | 13 (16) | 0.67 |
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Age (years) | 40 (21–60) | 41 (17–65) | 0.91 |
| Male/female | 11/20 | 39/42 | 0.22 |
| Indication, n (%) | |||
| Cystic fibrosis | 15 (48) | 40 (49) | 0.92 |
| COPD | 9 (29) | 16 (20) | 0.29 |
| Pulmonary fibrosis | 3 (10) | 12 (15) | 0.47 |
| Other | 4 (13) | 13 (16) | 0.67 |
EVLR: ex vivo lung reconditioning; COPD: chronic obstructive pulmonary disease.
Donor characteristics
Donor age, height and weight were similar in the two groups. Gas exchange was significantly worse in the EVLR group [median donor PO2/FiO2 274 (range 162–404) mmHg] than in the control group [392 (range 21–585) mmHg] (P < 0.0001 and P = 3 × 10−11), and the donor score was significantly higher in the EVLR group [median donor score 10 (range 7–15) vs 6 (range 0–12)] than in the control group (P = 0.03) (Table 3).
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Height (cm) | 172 (154–193) | 170 (150–200) | 0.66 |
| Weight (kg) | 80 (57–100) | 72 (45–111) | 0.07 |
| Donor score | 10 (7–15) | 6 (0–12) | 0.03 |
| Age (years) | 48 (21–67) | 51 (14–70) | 0.72 |
| Tobacco >20 pack-years | 9 | 17 | 0.37 |
| Chest X-ray opacity | 22 | 16 | <0.001 3 × 10−7 |
| Purulent bronchial secretions | 20 | 34 | 0.03 |
| PO2/FiO2 (mmHg) | 274 (162–404) | 392 (221–585) | <0.001 3 × 10−11 |
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Height (cm) | 172 (154–193) | 170 (150–200) | 0.66 |
| Weight (kg) | 80 (57–100) | 72 (45–111) | 0.07 |
| Donor score | 10 (7–15) | 6 (0–12) | 0.03 |
| Age (years) | 48 (21–67) | 51 (14–70) | 0.72 |
| Tobacco >20 pack-years | 9 | 17 | 0.37 |
| Chest X-ray opacity | 22 | 16 | <0.001 3 × 10−7 |
| Purulent bronchial secretions | 20 | 34 | 0.03 |
| PO2/FiO2 (mmHg) | 274 (162–404) | 392 (221–585) | <0.001 3 × 10−11 |
EVLR: ex vivo lung reconditioning.
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Height (cm) | 172 (154–193) | 170 (150–200) | 0.66 |
| Weight (kg) | 80 (57–100) | 72 (45–111) | 0.07 |
| Donor score | 10 (7–15) | 6 (0–12) | 0.03 |
| Age (years) | 48 (21–67) | 51 (14–70) | 0.72 |
| Tobacco >20 pack-years | 9 | 17 | 0.37 |
| Chest X-ray opacity | 22 | 16 | <0.001 3 × 10−7 |
| Purulent bronchial secretions | 20 | 34 | 0.03 |
| PO2/FiO2 (mmHg) | 274 (162–404) | 392 (221–585) | <0.001 3 × 10−11 |
| . | EVLR (n = 31) . | Control (n = 81) . | P-value . |
|---|---|---|---|
| Height (cm) | 172 (154–193) | 170 (150–200) | 0.66 |
| Weight (kg) | 80 (57–100) | 72 (45–111) | 0.07 |
| Donor score | 10 (7–15) | 6 (0–12) | 0.03 |
| Age (years) | 48 (21–67) | 51 (14–70) | 0.72 |
| Tobacco >20 pack-years | 9 | 17 | 0.37 |
| Chest X-ray opacity | 22 | 16 | <0.001 3 × 10−7 |
| Purulent bronchial secretions | 20 | 34 | 0.03 |
| PO2/FiO2 (mmHg) | 274 (162–404) | 392 (221–585) | <0.001 3 × 10−11 |
EVLR: ex vivo lung reconditioning.
Ex vivo lung reconditioning procedure
During the EVLR procedure, only one pair of lungs among the 32 was rejected for clear signs of progressive oedema and decreasing PO2/FiO2 to less than 400 after 2 h of the procedure. For the 31 lungs used for transplantation, the PO2/FiO2 ratio increased from 274 (range 162–404) mmHg to 511 (range 378–668) mmHg after the first hour and remained stable until the end of the procedure (P < 0.0001 and P = 7 × 10−19).
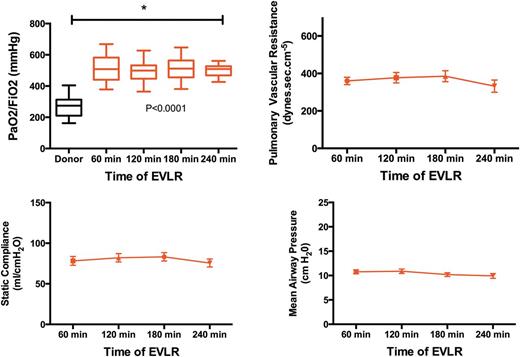
Lung function parameters during ex vivo lung reconditioning (EVLR).
Clinical outcome
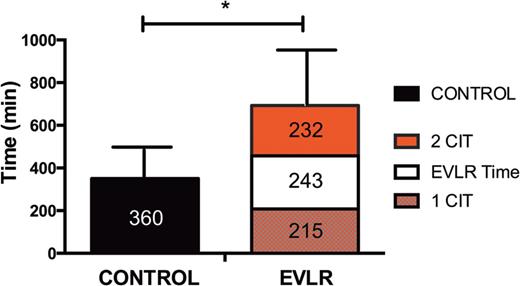
Ischaemic time before the second-lung implantation after EVLR (P < 0.0001). 1CIT: first cold ischaemia time from aortic cross-clamp to initiation of EVLR, EVLR time; 2CIT: second cold ischaemia time from the end of EVLR to reperfusion of the second lung.
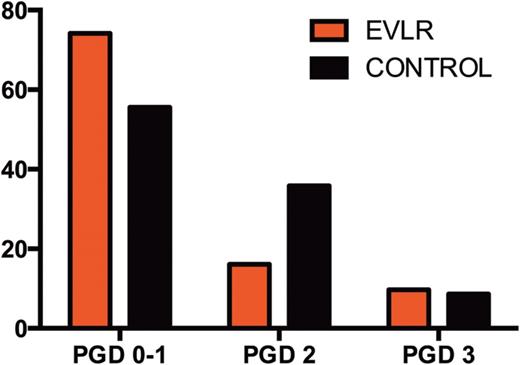
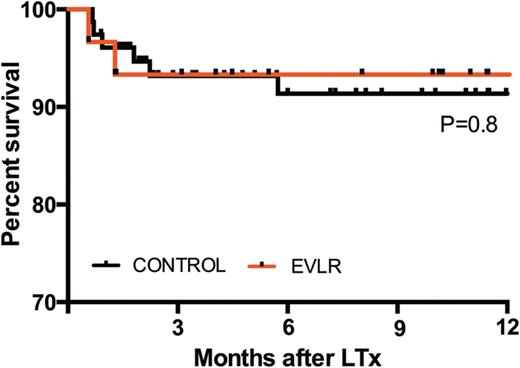
Overall survival established with the Kaplan–Meier method. In red survival of the EVLR group and in black survival of the control group. LTx: lung transplantation.
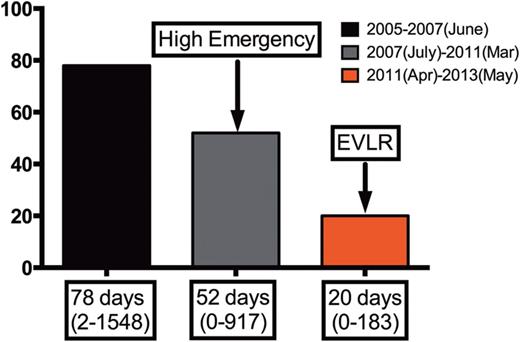
Impact of EVLR on the waiting time on the list at the Foch Hospital.
DISCUSSION
Our study describes the use of EVLR for initially rejected donor lungs. With this study design, we avoided variations in the definition of high-risk donor lungs between the conservative and the aggressive lung transplant centres. Indeed, even though the acceptability criteria for lung donors were reviewed in a report from the International Society for Heart and Lung Transplantation (ISHLT) in 2003 [18], the definition of ‘marginal donors’ is unclear. The individual donor will have several factors that may affect post-transplant lung function such as cause of death, duration of mechanical ventilation, radiographic findings, microbial organisms, arterial blood gas or smoking exposure [19]. Each lung transplant team combines these factors to classify their ideal, marginal or unusable donors. However, marginal donors remain rarely used because of contrasting outcome. Some studies have reported equivalent outcome after transplantation [20], even if others have reported a significant difference with impaired outcome of LTx from marginal donors [21]. Thus, the majority of clinicians tend to be highly conservative when selecting donors or starting an EVLP programme [8, 11, 13, 14, 22] to increase available donor lungs.
The conversion rate from EVLP to transplantation varies between 46 and 87% in the literature [8, 11, 13, 14, 22]. The highest rate was obtained by the Toronto group with 87% [8]. This high conversion rate is probably related to their previously described technique: a combination of reduction of inflammatory cytokines by an acellular perfusate together with a hyperoncotic composition of the Steen solution® (XVIVO Perfusion). Using the same technique in our study, we could obtain a slightly higher conversion rate at 96%. Indeed, by starting our EVLP programme later, we benefited from the experience of the Toronto group to reduce our learning curve. By combining their technique with several other factors, we could explain these results as follows. First, strict exclusion of donor lungs with established pneumonia or severe mechanical lung injury defined by extensive contusions, confirmed by the donor's history, CT scan pictures and/or bacteriological samples. The cause of lung dysfunction was thus expected to be reversible. Secondly, after inclusion of donor lungs in the EVLP protocol, no active donor management was done at the donor site. The graft was quickly harvested to avoid the damage due to the development of neurogenic oedema and pro-inflammatory changes caused by brain death. Thirdly, a thorough bronchoscopy with bronchial lavage was performed to clear out secretions during the first hour of gradual increase of temperature, and before the initiation of ventilation. These ‘in-house’ operating conditions of EVLR with repeated bronchial toilets are difficult to perform at the donor site. So these controlled reperfusions, protective lung ventilation strategies and extensive recruitment may have played a key role in the improvement in the conversion rate in our study.
The optimal time to keep a lung on the EVLP system remains to be determined. In their clinical study, the Toronto group performed EVLP for 4 h in all patients [8, 9]. During EVLR, no further significant improvement in PO2 was observed beyond the second hour. Therefore, if the criteria for acceptance were not reached after 2 h of procedure, the lungs were considered unsuitable for transplantation and sent to pathology. When the PO2/FiO2 ratio reached 400 mmHg, we accepted the lungs for transplantation. However, the grafts were kept on the perfusion system until the first lung was explanted onto the recipient. Indeed, if no further significant improvement in was observed if longer perfusion was made, the protocol could be safely continued for up to 12 h in animal models [16]. We confirmed these experimental data in our study with three procedures continuing over 7 h with perfect lung function stability. This possibility to extend the time lapse between donor cross-clamping and recipient reperfusion was an attractive positive feature of EVLP. During this additional time, we were able to rearrange the organization of the operating rooms around the recipient before the transplant procedure.
In our study, about 30% of initially rejected lungs could potentially have been considered suitable for transplantation. This observation, already reported in the literature [23], highlights the difficulties of lung assessment without physical examination of the pulmonary graft at the donor hospital. The policy of going to the donor hospital in order to evaluate the lung in situ as far as possible seems to be the best strategy to increase lung acceptance. By using this method, the Leuven lung transplant team increased their lung acceptance rate to 41% of all effective organ donors [19]. However, this policy requires a high number of surgeons. Nevertheless, in our study design, the lungs accepted for the protocol were to be reconditioned ex vivo even if they seemed directly suitable for transplantation. Two principal steps have reduced the waiting time on the list in the Foch Hospital. The first was related to the national allocation system with the introduction of the high-emergency LTx programme (HELTx) in July 2007 [24]. Indeed, this programme provides urgent LTx in patients with the highest short-term risk of death. Only three different groups of disease were notified by the French board of experts giving patients national priority for access to LTx: cystic fibrosis and other causes of diffuse bronchiectasis, pulmonary fibrosis and pulmonary vascular diseases. This emergency procedure based on strict criteria resulted in a median delay of 3 days before LTx [24]. Considering that our lung transplant programme includes a majority of cystic fibrosis, the HELTx procedure has significantly decreased the waiting time on our list. The second step was due to the fact that the EVLR programme started in our hospital in April 2011 using lungs rejected by all French lung transplant teams. EVLR has represented about 25% of the number of LTx in our centre for the last 2 years. This programme has dramatically decreased the waiting time on our list. Currently, the waiting time is shorter than 3 weeks. Furthermore, during this period no patient died on the Foch waiting list.
The limitation of our study is that it does not demonstrate that the lungs accepted for EVLR would have performed well if they had been directly transplanted. A randomized clinical trial between EVLR and directly transplanting lungs rejected by all French lung transplant teams would be ethically difficult to establish.
To conclude, the Toronto technique of EVLP is a reliable and repeatable technique that offers a significant increase in available donors to alleviate organ shortage. With the use of EVLR, even in the case of a marginal donor, the results of LTx are similar to those obtained with conventional donors.
ACKNOWLEDGEMENTS
We thank ‘Fondation Foch’, ‘Vaincre la Mucoviscidose’ and ‘Gregory Lemarchal Association’ for their support.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 21st Meeting of the European Society of Thoracic Surgeons, Birmingham, UK, 26–29 May 2013.
Surgery: P. Bonnette, D. Mitilian, P. Puyo, N. Salley, E. Sage, A. Chapelier; Pneumology: S. De Miranda, D. Grenet, A. Hamid, C. Picard, A. Roux, M. Stern; Anesthesiology: J. Bresson, V. Dumans-Nizard, J.L. Dumoulin, S. Ghiglione, S. Jacqmin, M. Le Guen, L. Ley, N. Liu, J.-Y. Marandon, M. Michel-Cherqui, O. Pruszkowski, B. Rives, B. Szekely, B. Vandenbunder, N. Verroust, M. Fischler; Intensive care: J. Devaquet, F. Parquin, A.-G. Si Larbi, G. Trebbia, C. Cerf.




