-
PDF
- Split View
-
Views
-
Cite
Cite
Bastien Orsini, Edouard Sage, Anne Olland, Emmanuel Cochet, Mayeul Tabutin, Matthieu Thumerel, Florent Charot, Alain Chapelier, Gilbert Massard, Pierre Yves Brichon, Francois Tronc, Jacques Jougon, Marcel Dahan, Xavier Benoit D'Journo, Martine Reynaud-Gaubert, Delphine Trousse, Christophe Doddoli, Pascal Alexandre Thomas, High-emergency waiting list for lung transplantation: early results of a nation-based study, European Journal of Cardio-Thoracic Surgery, Volume 46, Issue 3, September 2014, Pages e41–e47, https://doi.org/10.1093/ejcts/ezu259
Close - Share Icon Share
The high mortality rate observed on the regular waiting list (RWL) before lung transplantation (LTx) prompted the French organ transplantation authorities to set up in 2007 a dedicated graft allocation strategy, the so-called ‘high-emergency waiting list’ (HEWL), for patients with an abrupt worsening of their respiratory function. This study reports on the early results of this new allocation system.
Among 11 active French LTx programmes, 7 were able to provide full outcome data by 31 December 2011. The medical records of 101 patients who were listed on the HEWL from July 2007 to December 2011 were reviewed for an intention-to-treat analysis.
Ninety-five patients received LTx within a median waiting time on the HEWL of 4 days (range 1–26), and 6 died before transplantation. Conditions were cystic fibrosis (65.2%), pulmonary fibrosis (24.8%), emphysema (5%) and miscellaneous (5%). The median age of the recipient was 30 years (range 16–66). Patients listed on the HEWL came from the RWL in 48.5% of the cases and were new patients in 51.5%. Forty-nine were placed under invasive ventilation and, in 26 cases, extracorporeal membrane oxygenation (ECMO) prior to transplantation was necessary as a complementary treatment. ECMO for non-intubated patients was performed in 6 cases. Eighty-one bilateral and 14 single LTx were performed, with an overall in-hospital mortality rate of 29.4%. One- and 3-year survival rates were 67.5 and 59%, respectively. Multivariate analysis shows that the use of ECMO prior to transplantation was the sole independent mortality risk factor (hazard ratio = 2.77 [95% CI 1.26–6.11]).
The new allocation system aimed at lowering mortality on the RWL, but also offered an access to LTx for new patients with end-stage respiratory failure. The HEWL increased the likelihood of mortality after LTx, but permitted acceptable mid-term survival rates. The high mortality associated with the use of ECMO should be interpreted cautiously.
INTRODUCTION
Lung transplantation (LTx) is the standard treatment for end-stage respiratory diseases, but lung graft shortage is a real obstacle. In France, in 2006 for one lung graft available, 1.9% patients were potentially eligible. As a consequence, 7.3, 8.6 and 8.3% of patients died on the regular waiting list (RWL) in 2005, 2006 and 2007, respectively. This problem of lung graft shortage is recognized in all European institutions having a LTx programme and experiencing death on the RWL. For instance, death rate on the RWL in 2010 was 10% in UK, 11% in Italy and 5.2% in Belgium. Long waiting time and, consequently, death on the RWL is still a matter of concern and has led the French transplantation authorities to set up a dedicated emergency procedure, the so-called high-emergency waiting list (HEWL) in 2007, giving priority after an external expertise, to selected patients registered on the RWL who present with acute respiratory failure. At the end of 2011, in France, the median time on the RWL was almost 4 months (range 3.4–4.1) [1]. However, high risks for postoperative mortality and organ wastage in this population are significant problems as described by some studies [2–4]. The aim of this multicentre study is to investigate the early results of this new allocation system and find potential risk factors of death.
MATERIALS AND METHODS
Criteria for the high-emergency waiting list
The HEWL offers national priority for critically ill patients who have very limited life expectancy if they do not receive LTx. To be listed in the HEWL, patients have to fulfil strict pharmacological, clinical and biological criteria established by the French Biomedicine Agency in 2007, as given in Table 1. Patients requiring mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO) are automatically listed on the HEWL. A request is sent to French transplantation authorities by mail or fax and evaluated by two different experts. National priority is valuable for 8 days with only one possible renewal. Unlimited derogation can be accorded in selected cases. Inclusion on the RWL for a minimum delay of 2 days is a mandatory prerequisite to be listed on the HEWL.
Criteria for inclusion on the waiting list for high-emergency lung transplantationa
| Disease criteria . | Description . |
|---|---|
| Cystic fibrosis and other causes of diffuse bronchiectasis | 1. Invasive MV |
| 2. Risk of imminent invasive MV defined as PaCO2 >80 mmHg, despite non-invasive MV for >18 h/day and >3 days, in the absence of any identified reversible cause | |
| 3. Respiratory assistance, such as ECMO | |
| Pulmonary fibrosis (idiopathic or not) | 1. Invasive MV |
| 2. Risk of imminent invasive MV, defined as SaO2 <90% under continuous high-concentration oxygen therapy via a facemask, despite maximal medical treatment for acute exacerbation of pulmonary fibrosis (methylprednisolone bolus etc.), in the absence of any identified reversible cause | |
| Pulmonary vascular diseases | 1. Severe PH requiring admission to the intensive care unit and not improving after >72 h of maximal medical treatment, including specific treatment for PH. Severe PH is defined as Class IV on the NYHA classification, cardiac index <2 l/min/m2 and pulmonary vascular resistance >1200 dyn s cm–5 |
| Disease criteria . | Description . |
|---|---|
| Cystic fibrosis and other causes of diffuse bronchiectasis | 1. Invasive MV |
| 2. Risk of imminent invasive MV defined as PaCO2 >80 mmHg, despite non-invasive MV for >18 h/day and >3 days, in the absence of any identified reversible cause | |
| 3. Respiratory assistance, such as ECMO | |
| Pulmonary fibrosis (idiopathic or not) | 1. Invasive MV |
| 2. Risk of imminent invasive MV, defined as SaO2 <90% under continuous high-concentration oxygen therapy via a facemask, despite maximal medical treatment for acute exacerbation of pulmonary fibrosis (methylprednisolone bolus etc.), in the absence of any identified reversible cause | |
| Pulmonary vascular diseases | 1. Severe PH requiring admission to the intensive care unit and not improving after >72 h of maximal medical treatment, including specific treatment for PH. Severe PH is defined as Class IV on the NYHA classification, cardiac index <2 l/min/m2 and pulmonary vascular resistance >1200 dyn s cm–5 |
ECMO: extracorporeal membrane oxygenation; MV: mechanical ventilation; NYHA: New York Heart Association; PaCO2: partial pressure of arterial carbon dioxide; PH: pulmonary hypertension; SaO2: arterial oxygen saturation.
aFor all the above indications, the following exclusion criteria must be absent: acute failure of an extrathoracic organ or multiorgan failure and systemic infection with or without bacteraemia.
Criteria for inclusion on the waiting list for high-emergency lung transplantationa
| Disease criteria . | Description . |
|---|---|
| Cystic fibrosis and other causes of diffuse bronchiectasis | 1. Invasive MV |
| 2. Risk of imminent invasive MV defined as PaCO2 >80 mmHg, despite non-invasive MV for >18 h/day and >3 days, in the absence of any identified reversible cause | |
| 3. Respiratory assistance, such as ECMO | |
| Pulmonary fibrosis (idiopathic or not) | 1. Invasive MV |
| 2. Risk of imminent invasive MV, defined as SaO2 <90% under continuous high-concentration oxygen therapy via a facemask, despite maximal medical treatment for acute exacerbation of pulmonary fibrosis (methylprednisolone bolus etc.), in the absence of any identified reversible cause | |
| Pulmonary vascular diseases | 1. Severe PH requiring admission to the intensive care unit and not improving after >72 h of maximal medical treatment, including specific treatment for PH. Severe PH is defined as Class IV on the NYHA classification, cardiac index <2 l/min/m2 and pulmonary vascular resistance >1200 dyn s cm–5 |
| Disease criteria . | Description . |
|---|---|
| Cystic fibrosis and other causes of diffuse bronchiectasis | 1. Invasive MV |
| 2. Risk of imminent invasive MV defined as PaCO2 >80 mmHg, despite non-invasive MV for >18 h/day and >3 days, in the absence of any identified reversible cause | |
| 3. Respiratory assistance, such as ECMO | |
| Pulmonary fibrosis (idiopathic or not) | 1. Invasive MV |
| 2. Risk of imminent invasive MV, defined as SaO2 <90% under continuous high-concentration oxygen therapy via a facemask, despite maximal medical treatment for acute exacerbation of pulmonary fibrosis (methylprednisolone bolus etc.), in the absence of any identified reversible cause | |
| Pulmonary vascular diseases | 1. Severe PH requiring admission to the intensive care unit and not improving after >72 h of maximal medical treatment, including specific treatment for PH. Severe PH is defined as Class IV on the NYHA classification, cardiac index <2 l/min/m2 and pulmonary vascular resistance >1200 dyn s cm–5 |
ECMO: extracorporeal membrane oxygenation; MV: mechanical ventilation; NYHA: New York Heart Association; PaCO2: partial pressure of arterial carbon dioxide; PH: pulmonary hypertension; SaO2: arterial oxygen saturation.
aFor all the above indications, the following exclusion criteria must be absent: acute failure of an extrathoracic organ or multiorgan failure and systemic infection with or without bacteraemia.
Patients
From 1 July 2007 to 31 December 2011, 1031 LTx were performed in France. Among 11 active French LTx programmes, 8 were able to provide full outcome data by 31 December 2011, but data from one of these reference centres were not included as it dealt specifically with heart–LTxs. During the study period, the seven centres participating in the study performed 684 LTxs, consisting of 66.3% of all procedures. One hundred and three patients were listed on the HEWL during this period in the seven centres. Among these 103 patients, 2 received heart–LTxs and were excluded from the present study. Medical records of the remaining 101 patients who were listed on the HEWL from July 2007 to December 2011 for double or single LTx were reviewed with an intention-to-treat analysis.
Design and studied variables
The Institutional Review Board (IRB) of the French Thoracic and Cardiovascular Surgery Society assessed the design of this study. Because of its retrospective nature, the IRB waived the need for patient consent. From a prospective database, we retrospectively reviewed the following parameters: age; gender; diagnosis; waiting time on the RWL; waiting time on the HEWL; MV; necessity of ECMO as bridge to LTx, during or after LTx; type of LTx; haemodialysis; preoperative FEV1; postoperative FEV1 at 6 months and 1 year; length of stay in the intensive care unit (ICU); length of hospital stay; in-hospital mortality; and survival.
Statistical analysis
Analyses were performed using the PASW Statistics software (PASW v17.0; SPSS, Inc., Chicago, IL, USA). Categorical data were summarized by frequency and percentage, and continuous variables by mean ± standard deviation or by median with minimum and maximum values. Student's t-test was used for comparison of two means, and the non-parametric Mann–Whitney test for comparison of two medians. Comparisons of percentages were performed using χ2 test or Fischer's exact test, when appropriate. The survival rate was calculated using a Kaplan–Meier method and to compare survival curves. All factors with P < 0.2 at univariate analysis and any relevant clinical variables were included for the multivariate Cox regression model analysis. Hazard ratios (HRs) were produced with their 95% confidence interval. The time origin for survival analyses was the time the patient was first included on the HEWL. Statistical significance was defined as P < 0.05.
RESULTS
Indications and demography
Among the 101 patients listed on the HEWL, the median age was 30 years (range 16–66) and 52 males (51.5%) vs 49 females (48.5%) were included. Indications for LTx were cystic fibrosis in 66 (65.2%) patients, emphysema in 5 (5%), pulmonary fibrosis in 25 (24.8%) and miscellaneous in 5 (5%), among them being 3 redo LTx (Table 2). Fifty-two (51.5%) patients were not listed on the RWL at the time of referral. The median waiting time on the RWL was 183 days (range 21–917) for those 49 previously listed patients, and 4 days (range 1–26) on the HEWL for all patients. Forty-nine patients were under MV (48.5%) before LTx. Six (5.9%) patients died on the HEWL before LTx, despite ECMO implementation in 4 cases (66.6%). Ninety-five (94%) patients were successfully bridged to transplantation.
| Variable . | N = 101 . |
|---|---|
| Male | 52 (51.5%) |
| Female | 49 (48.5%) |
| Age (years) (median and range) | 30 (6–66) |
| Diagnosis | |
| Cystic fibrosis | 66 (65.2%) |
| Pulmonary fibrosis | 25 (24.8%) |
| Emphysema | 5 (5%) |
| Miscellaneous | 5 (5%) |
| Listed before acute lung failure | 49 (48.5%) |
| Non-listed before acute lung failure | 52 (51.5%) |
| Mechanical ventilation | 49 (48.5%) |
| Waiting time on the RWL (median and range, in days) | 183 (21–197) |
| Waiting time on HEWL (median and range, in days) | 4 (1–26) |
| Death on the HEWL | 6 (5.9%) |
| Surgical | |
| Bilateral LTx | 81 (85.3%) |
| Single LTx | 14 (14.7%) |
| Ischaemic time (median and range) | 360 (190–540) |
| Preoperative FEV1 (% of predicted median and range) | 25 (9–83) |
| Variable . | N = 101 . |
|---|---|
| Male | 52 (51.5%) |
| Female | 49 (48.5%) |
| Age (years) (median and range) | 30 (6–66) |
| Diagnosis | |
| Cystic fibrosis | 66 (65.2%) |
| Pulmonary fibrosis | 25 (24.8%) |
| Emphysema | 5 (5%) |
| Miscellaneous | 5 (5%) |
| Listed before acute lung failure | 49 (48.5%) |
| Non-listed before acute lung failure | 52 (51.5%) |
| Mechanical ventilation | 49 (48.5%) |
| Waiting time on the RWL (median and range, in days) | 183 (21–197) |
| Waiting time on HEWL (median and range, in days) | 4 (1–26) |
| Death on the HEWL | 6 (5.9%) |
| Surgical | |
| Bilateral LTx | 81 (85.3%) |
| Single LTx | 14 (14.7%) |
| Ischaemic time (median and range) | 360 (190–540) |
| Preoperative FEV1 (% of predicted median and range) | 25 (9–83) |
RWL: regular waiting list; HEWL: high-emergency waiting list; LTx: lung transplantation.
| Variable . | N = 101 . |
|---|---|
| Male | 52 (51.5%) |
| Female | 49 (48.5%) |
| Age (years) (median and range) | 30 (6–66) |
| Diagnosis | |
| Cystic fibrosis | 66 (65.2%) |
| Pulmonary fibrosis | 25 (24.8%) |
| Emphysema | 5 (5%) |
| Miscellaneous | 5 (5%) |
| Listed before acute lung failure | 49 (48.5%) |
| Non-listed before acute lung failure | 52 (51.5%) |
| Mechanical ventilation | 49 (48.5%) |
| Waiting time on the RWL (median and range, in days) | 183 (21–197) |
| Waiting time on HEWL (median and range, in days) | 4 (1–26) |
| Death on the HEWL | 6 (5.9%) |
| Surgical | |
| Bilateral LTx | 81 (85.3%) |
| Single LTx | 14 (14.7%) |
| Ischaemic time (median and range) | 360 (190–540) |
| Preoperative FEV1 (% of predicted median and range) | 25 (9–83) |
| Variable . | N = 101 . |
|---|---|
| Male | 52 (51.5%) |
| Female | 49 (48.5%) |
| Age (years) (median and range) | 30 (6–66) |
| Diagnosis | |
| Cystic fibrosis | 66 (65.2%) |
| Pulmonary fibrosis | 25 (24.8%) |
| Emphysema | 5 (5%) |
| Miscellaneous | 5 (5%) |
| Listed before acute lung failure | 49 (48.5%) |
| Non-listed before acute lung failure | 52 (51.5%) |
| Mechanical ventilation | 49 (48.5%) |
| Waiting time on the RWL (median and range, in days) | 183 (21–197) |
| Waiting time on HEWL (median and range, in days) | 4 (1–26) |
| Death on the HEWL | 6 (5.9%) |
| Surgical | |
| Bilateral LTx | 81 (85.3%) |
| Single LTx | 14 (14.7%) |
| Ischaemic time (median and range) | 360 (190–540) |
| Preoperative FEV1 (% of predicted median and range) | 25 (9–83) |
RWL: regular waiting list; HEWL: high-emergency waiting list; LTx: lung transplantation.
Extracorporeal membrane oxygenation use prior to lung transplantation
ECMO was used as a bridge to LTx for 32 (31.7%) patients (Table 3). Twenty-two (75%) patients suffered from cystic fibrosis, 6 (18.6%) from pulmonary fibrosis, 2 (6.2%) from emphysema and 2 (6.2%) from secondary bronchiolitis obliterans. A pump ECMO assistance was used in 31 cases, veno-venous (V-V) in 27 (84.4%) and veno-arterial (V-A) in 4 (12.5%) with systematic limb reperfusion. Among those patients with a V-V ECMO, a bicaval dual lumen cannula (Avalon®) was used in awake non-intubated patients in 6 (18.7%) cases. One (3%) pumpless arterio-venous lung assistance was performed (Novalung®). A cannulation technique, either open or percutaneous, was decided depending on the experience of each centre. The median duration of preoperative ECMO was 72 h (range 6–240). Twenty-eight (87.5%) patients were successfully bridged to transplantation, but 59.4% died in the postoperative period. All 6 awake patients with ECMO were successfully bridged to LTx, but 4 died during the first postoperative year.
| Variable . | . |
|---|---|
| Extracorporeal assistance in bridge to transplantation | 32 (31.7%) |
| ECMO V-V | 27 (84.4%) |
| Awake patients | 6 (5.9%) |
| ECMO V-A | 4 (12.5%) |
| Pumpless assistance | 1 (3.1%) |
| Preoperative ECMO duration (h) (median and range) | 72 (6–240) |
| Intraoperative extracorporeal assistance | 70 (73.7%) |
| Cardiopulmonary bypass | 34 (48.5%) |
| ECMO V-A | 18 (53%) |
| ECMO V-V | 16 (47%) |
| ECMO post-LTx | 35 (36.8%) |
| ECMO weaning | 18 (80%) |
| Postoperative ECMO duration (h) (median and range) | 120 (5–464) |
| Variable . | . |
|---|---|
| Extracorporeal assistance in bridge to transplantation | 32 (31.7%) |
| ECMO V-V | 27 (84.4%) |
| Awake patients | 6 (5.9%) |
| ECMO V-A | 4 (12.5%) |
| Pumpless assistance | 1 (3.1%) |
| Preoperative ECMO duration (h) (median and range) | 72 (6–240) |
| Intraoperative extracorporeal assistance | 70 (73.7%) |
| Cardiopulmonary bypass | 34 (48.5%) |
| ECMO V-A | 18 (53%) |
| ECMO V-V | 16 (47%) |
| ECMO post-LTx | 35 (36.8%) |
| ECMO weaning | 18 (80%) |
| Postoperative ECMO duration (h) (median and range) | 120 (5–464) |
ECMO V-V: extracorporeal membrane oxygenation veno-venous; V-A: veno-arterial; LTx: lung transplantation.
| Variable . | . |
|---|---|
| Extracorporeal assistance in bridge to transplantation | 32 (31.7%) |
| ECMO V-V | 27 (84.4%) |
| Awake patients | 6 (5.9%) |
| ECMO V-A | 4 (12.5%) |
| Pumpless assistance | 1 (3.1%) |
| Preoperative ECMO duration (h) (median and range) | 72 (6–240) |
| Intraoperative extracorporeal assistance | 70 (73.7%) |
| Cardiopulmonary bypass | 34 (48.5%) |
| ECMO V-A | 18 (53%) |
| ECMO V-V | 16 (47%) |
| ECMO post-LTx | 35 (36.8%) |
| ECMO weaning | 18 (80%) |
| Postoperative ECMO duration (h) (median and range) | 120 (5–464) |
| Variable . | . |
|---|---|
| Extracorporeal assistance in bridge to transplantation | 32 (31.7%) |
| ECMO V-V | 27 (84.4%) |
| Awake patients | 6 (5.9%) |
| ECMO V-A | 4 (12.5%) |
| Pumpless assistance | 1 (3.1%) |
| Preoperative ECMO duration (h) (median and range) | 72 (6–240) |
| Intraoperative extracorporeal assistance | 70 (73.7%) |
| Cardiopulmonary bypass | 34 (48.5%) |
| ECMO V-A | 18 (53%) |
| ECMO V-V | 16 (47%) |
| ECMO post-LTx | 35 (36.8%) |
| ECMO weaning | 18 (80%) |
| Postoperative ECMO duration (h) (median and range) | 120 (5–464) |
ECMO V-V: extracorporeal membrane oxygenation veno-venous; V-A: veno-arterial; LTx: lung transplantation.
Lung transplantation and early outcome
Eighty-one (85.3%) bilateral and 14 (14.7%) single LTx were performed. Thirteen single LTx were performed for lung fibrosis. In one case, because of haemodynamic instability, an attempted bilateral LTx was switched to a single LTx. The median ischaemic time was 360 min (range 190–540). During the LTx procedure, 70 (73.6%) patients required a formal cardiopulmonary bypass (CPB; 48.5%) or a V-V/V-A-ECMO (51.5%). V-V-ECMO implanted as a bridge to LTx transplantation was switched in 11 cases in CPB (39.3%) and in 1 case in a VA-ECMO. The single patient with the pumpless lung assistance died before LTx. Thirty-five (36.8%) patients required ECMO support during the postoperative period. Seven (20%) died under ECMO, but 28 were successfully weaned (80%). The median postoperative ECMO duration was 120 h (range 5–464).
Patients listed versus patients not listed on the regular waiting list prior to the high-emergency waiting list
Forty-nine (48.5%) patients were previously listed on the RWL, whereas 52 (51.5%) were not due to a late referral to the transplant centre (Table 4). In the listed group, 19 (38.7%) required MV and among them 9 (18.4%) required an additional ECMO while, in the non-listed group, 30 (57.7%) were bridged to LTx under MV with 17 (33.3%) under additional ECMO. Six (11.7%) patients, not previously listed on the RWL, were bridged to LTx with awake ECMO. Five of them died on the HEWL (83.3%). The in-hospital mortality rate was 28.5% in the listed group and 38.5% in the non-listed group.
Indications and demography between listed and non-listed patients prior to their listing on the HEWL
| Variable . | Listed on the RWL . | Non-listed on the RWL . | P-value . |
|---|---|---|---|
| N | 49 | 52 | |
| Male | 24 (49%) | 28 (53.8%) | 0.625 |
| Female | 25 (51%) | 24 (46.2%) | |
| Age (median and range, in years) | 30 (17–66) | 30 (16–66) | 0.812 |
| Diagnosis | |||
| Cystic fibrosis | 32 (65.3%) | 34 (65.4%) | 0.993 |
| Pulmonary fibrosis | 12 (24.4%) | 13 (25%) | 0.953 |
| Emphysema | 2 (4.1%) | 3 (5.7%) | 1.000 |
| Miscellaneous | 3 (6.1%) | 2 (3.8%) | 0.672 |
| Mechanical ventilation | 19 (38.7%) | 30 (57.7%) | 0.057 |
| ECMO pre-LTx | 9 (18.4%) | 23 (44.2%) | 0.005 |
| Waiting time on the HEWL (median and range, in days) | 4 (8.2%) | 4 (7.7%) | 1.000 |
| Death on the HEWL | 1 (2%) | 5 (9.6%) | 0.105 |
| Surgical | |||
| Bilateral LTx | 39 (81.3%) | 42 (89.4%) | 0.265 |
| Single LTx | 9 (18.8%) | 5 (10.6%) | |
| Ischaemic time (median and range, in minutes) | 375 (190–540) | 344 (205–510) | 0.218 |
| Preoperative FEV1 (% of predicted median and range) | 23.5 (9–83) | 27 (12–78) | 0.026 |
| In-hospital mortality | 14 (28.5%) | 20 (38.5%) | 0.293 |
| Estimate of cumulative proportion surviving at | |||
| 30 days | 77.1% ± 0.12 | 85.0% ± 0.10 | 0.398 |
| 1 year | 70.6% ± 0.13 | 63.9% ± 0.14 | |
| 3 years | 65.9% ± 0.15 | 51.4% ± 0.17 | |
| Variable . | Listed on the RWL . | Non-listed on the RWL . | P-value . |
|---|---|---|---|
| N | 49 | 52 | |
| Male | 24 (49%) | 28 (53.8%) | 0.625 |
| Female | 25 (51%) | 24 (46.2%) | |
| Age (median and range, in years) | 30 (17–66) | 30 (16–66) | 0.812 |
| Diagnosis | |||
| Cystic fibrosis | 32 (65.3%) | 34 (65.4%) | 0.993 |
| Pulmonary fibrosis | 12 (24.4%) | 13 (25%) | 0.953 |
| Emphysema | 2 (4.1%) | 3 (5.7%) | 1.000 |
| Miscellaneous | 3 (6.1%) | 2 (3.8%) | 0.672 |
| Mechanical ventilation | 19 (38.7%) | 30 (57.7%) | 0.057 |
| ECMO pre-LTx | 9 (18.4%) | 23 (44.2%) | 0.005 |
| Waiting time on the HEWL (median and range, in days) | 4 (8.2%) | 4 (7.7%) | 1.000 |
| Death on the HEWL | 1 (2%) | 5 (9.6%) | 0.105 |
| Surgical | |||
| Bilateral LTx | 39 (81.3%) | 42 (89.4%) | 0.265 |
| Single LTx | 9 (18.8%) | 5 (10.6%) | |
| Ischaemic time (median and range, in minutes) | 375 (190–540) | 344 (205–510) | 0.218 |
| Preoperative FEV1 (% of predicted median and range) | 23.5 (9–83) | 27 (12–78) | 0.026 |
| In-hospital mortality | 14 (28.5%) | 20 (38.5%) | 0.293 |
| Estimate of cumulative proportion surviving at | |||
| 30 days | 77.1% ± 0.12 | 85.0% ± 0.10 | 0.398 |
| 1 year | 70.6% ± 0.13 | 63.9% ± 0.14 | |
| 3 years | 65.9% ± 0.15 | 51.4% ± 0.17 | |
ECMO: extracorporeal membrane oxygenation; LTx: lung transplantation; HWEL: high-emergency waiting list.
Indications and demography between listed and non-listed patients prior to their listing on the HEWL
| Variable . | Listed on the RWL . | Non-listed on the RWL . | P-value . |
|---|---|---|---|
| N | 49 | 52 | |
| Male | 24 (49%) | 28 (53.8%) | 0.625 |
| Female | 25 (51%) | 24 (46.2%) | |
| Age (median and range, in years) | 30 (17–66) | 30 (16–66) | 0.812 |
| Diagnosis | |||
| Cystic fibrosis | 32 (65.3%) | 34 (65.4%) | 0.993 |
| Pulmonary fibrosis | 12 (24.4%) | 13 (25%) | 0.953 |
| Emphysema | 2 (4.1%) | 3 (5.7%) | 1.000 |
| Miscellaneous | 3 (6.1%) | 2 (3.8%) | 0.672 |
| Mechanical ventilation | 19 (38.7%) | 30 (57.7%) | 0.057 |
| ECMO pre-LTx | 9 (18.4%) | 23 (44.2%) | 0.005 |
| Waiting time on the HEWL (median and range, in days) | 4 (8.2%) | 4 (7.7%) | 1.000 |
| Death on the HEWL | 1 (2%) | 5 (9.6%) | 0.105 |
| Surgical | |||
| Bilateral LTx | 39 (81.3%) | 42 (89.4%) | 0.265 |
| Single LTx | 9 (18.8%) | 5 (10.6%) | |
| Ischaemic time (median and range, in minutes) | 375 (190–540) | 344 (205–510) | 0.218 |
| Preoperative FEV1 (% of predicted median and range) | 23.5 (9–83) | 27 (12–78) | 0.026 |
| In-hospital mortality | 14 (28.5%) | 20 (38.5%) | 0.293 |
| Estimate of cumulative proportion surviving at | |||
| 30 days | 77.1% ± 0.12 | 85.0% ± 0.10 | 0.398 |
| 1 year | 70.6% ± 0.13 | 63.9% ± 0.14 | |
| 3 years | 65.9% ± 0.15 | 51.4% ± 0.17 | |
| Variable . | Listed on the RWL . | Non-listed on the RWL . | P-value . |
|---|---|---|---|
| N | 49 | 52 | |
| Male | 24 (49%) | 28 (53.8%) | 0.625 |
| Female | 25 (51%) | 24 (46.2%) | |
| Age (median and range, in years) | 30 (17–66) | 30 (16–66) | 0.812 |
| Diagnosis | |||
| Cystic fibrosis | 32 (65.3%) | 34 (65.4%) | 0.993 |
| Pulmonary fibrosis | 12 (24.4%) | 13 (25%) | 0.953 |
| Emphysema | 2 (4.1%) | 3 (5.7%) | 1.000 |
| Miscellaneous | 3 (6.1%) | 2 (3.8%) | 0.672 |
| Mechanical ventilation | 19 (38.7%) | 30 (57.7%) | 0.057 |
| ECMO pre-LTx | 9 (18.4%) | 23 (44.2%) | 0.005 |
| Waiting time on the HEWL (median and range, in days) | 4 (8.2%) | 4 (7.7%) | 1.000 |
| Death on the HEWL | 1 (2%) | 5 (9.6%) | 0.105 |
| Surgical | |||
| Bilateral LTx | 39 (81.3%) | 42 (89.4%) | 0.265 |
| Single LTx | 9 (18.8%) | 5 (10.6%) | |
| Ischaemic time (median and range, in minutes) | 375 (190–540) | 344 (205–510) | 0.218 |
| Preoperative FEV1 (% of predicted median and range) | 23.5 (9–83) | 27 (12–78) | 0.026 |
| In-hospital mortality | 14 (28.5%) | 20 (38.5%) | 0.293 |
| Estimate of cumulative proportion surviving at | |||
| 30 days | 77.1% ± 0.12 | 85.0% ± 0.10 | 0.398 |
| 1 year | 70.6% ± 0.13 | 63.9% ± 0.14 | |
| 3 years | 65.9% ± 0.15 | 51.4% ± 0.17 | |
ECMO: extracorporeal membrane oxygenation; LTx: lung transplantation; HWEL: high-emergency waiting list.
Outcome
Forty (39.6%) patients died during the study period and 61 (60.4%) were still alive at the date of data collection (Table 5). The median follow-up was 360 days (range 0–1589). Eight intraoperative deaths occurred (8.4%). Haemodialysis was necessary postoperatively in 22 (25.2%) patients. Median postoperative FEV1 values at 6 months and 1 year were 63% of predicted (range 32–155) and 75% of predicted (range 39–113), respectively. The median length of stay in the ICU was 20.5 days (range 3–568). The median hospital stay was 55 days (range 4–580). The in-hospital mortality rate was 35.8% (n = 34). Overall, 30-day, 1-year and 3-year survival rates were 81, 67.5 and 59.4%, respectively. Overall, 30-day, 1-year and 3-year survival rates were 85.1, 64 and 56.1% for listed and 77.1, 70.6 and 62.1% for non-listed patients, respectively.
| Intraoperative death | 8 (8.4%) |
| Postoperative FEV1 (% of predicted) | |
| 6 months (median and range) | 63 (32–155) |
| 1 year (median and range) | 75 (39–13) |
| ICU stay (median and range, in days) | 20.5 (3–568) |
| Hospital stay (median and range, in days) | 55 (4–580) |
| In-hospital mortality rate | 34 (35.8%) |
| Survival rate | |
| 30 days | 81% |
| 1 year | 67.5% |
| 3 years | 59.4% |
| Follow-up (median and range, in days) | 360 (0–1589) |
| Intraoperative death | 8 (8.4%) |
| Postoperative FEV1 (% of predicted) | |
| 6 months (median and range) | 63 (32–155) |
| 1 year (median and range) | 75 (39–13) |
| ICU stay (median and range, in days) | 20.5 (3–568) |
| Hospital stay (median and range, in days) | 55 (4–580) |
| In-hospital mortality rate | 34 (35.8%) |
| Survival rate | |
| 30 days | 81% |
| 1 year | 67.5% |
| 3 years | 59.4% |
| Follow-up (median and range, in days) | 360 (0–1589) |
| Intraoperative death | 8 (8.4%) |
| Postoperative FEV1 (% of predicted) | |
| 6 months (median and range) | 63 (32–155) |
| 1 year (median and range) | 75 (39–13) |
| ICU stay (median and range, in days) | 20.5 (3–568) |
| Hospital stay (median and range, in days) | 55 (4–580) |
| In-hospital mortality rate | 34 (35.8%) |
| Survival rate | |
| 30 days | 81% |
| 1 year | 67.5% |
| 3 years | 59.4% |
| Follow-up (median and range, in days) | 360 (0–1589) |
| Intraoperative death | 8 (8.4%) |
| Postoperative FEV1 (% of predicted) | |
| 6 months (median and range) | 63 (32–155) |
| 1 year (median and range) | 75 (39–13) |
| ICU stay (median and range, in days) | 20.5 (3–568) |
| Hospital stay (median and range, in days) | 55 (4–580) |
| In-hospital mortality rate | 34 (35.8%) |
| Survival rate | |
| 30 days | 81% |
| 1 year | 67.5% |
| 3 years | 59.4% |
| Follow-up (median and range, in days) | 360 (0–1589) |
Risk factors of death
| Factor . | Death . | Alive . | P-value . |
|---|---|---|---|
| Age (median and range) | 33 (17–66) | 29.5 (16–66) | 0.786 |
| Cystic fibrosis | 23 (34.8%) | 43 (65.2%) | 0.11 |
| Pulmonary fibrosis | 12 (48%) | 13 (52%) | 0.2 |
| Chronic obstructive pulmonary disease | 2 (40%) | 3 (60%) | 0.97 |
| Miscellaneous | 3 (60%) | 2 (40%) | 0.26 |
| Male | 18 (34.6%) | 34 (65.4%) | 0.37 |
| Female | 22 (44.9%) | 27 (55.1%) | 0.37 |
| Non-listed on RWL | 23 (46.9%) | 26 (53.1%) | 0.17 |
| Mechanical ventilation | 23 (46.9%) | 26 (53.1%) | 0.13 |
| Extracorporeal assistance in bridge to LTx | 19 (59.4%) | 13 (40.6%) | 0.002 |
| Factor . | Death . | Alive . | P-value . |
|---|---|---|---|
| Age (median and range) | 33 (17–66) | 29.5 (16–66) | 0.786 |
| Cystic fibrosis | 23 (34.8%) | 43 (65.2%) | 0.11 |
| Pulmonary fibrosis | 12 (48%) | 13 (52%) | 0.2 |
| Chronic obstructive pulmonary disease | 2 (40%) | 3 (60%) | 0.97 |
| Miscellaneous | 3 (60%) | 2 (40%) | 0.26 |
| Male | 18 (34.6%) | 34 (65.4%) | 0.37 |
| Female | 22 (44.9%) | 27 (55.1%) | 0.37 |
| Non-listed on RWL | 23 (46.9%) | 26 (53.1%) | 0.17 |
| Mechanical ventilation | 23 (46.9%) | 26 (53.1%) | 0.13 |
| Extracorporeal assistance in bridge to LTx | 19 (59.4%) | 13 (40.6%) | 0.002 |
LTx: lung transplantation; RWL: regular waiting list.
| Factor . | Death . | Alive . | P-value . |
|---|---|---|---|
| Age (median and range) | 33 (17–66) | 29.5 (16–66) | 0.786 |
| Cystic fibrosis | 23 (34.8%) | 43 (65.2%) | 0.11 |
| Pulmonary fibrosis | 12 (48%) | 13 (52%) | 0.2 |
| Chronic obstructive pulmonary disease | 2 (40%) | 3 (60%) | 0.97 |
| Miscellaneous | 3 (60%) | 2 (40%) | 0.26 |
| Male | 18 (34.6%) | 34 (65.4%) | 0.37 |
| Female | 22 (44.9%) | 27 (55.1%) | 0.37 |
| Non-listed on RWL | 23 (46.9%) | 26 (53.1%) | 0.17 |
| Mechanical ventilation | 23 (46.9%) | 26 (53.1%) | 0.13 |
| Extracorporeal assistance in bridge to LTx | 19 (59.4%) | 13 (40.6%) | 0.002 |
| Factor . | Death . | Alive . | P-value . |
|---|---|---|---|
| Age (median and range) | 33 (17–66) | 29.5 (16–66) | 0.786 |
| Cystic fibrosis | 23 (34.8%) | 43 (65.2%) | 0.11 |
| Pulmonary fibrosis | 12 (48%) | 13 (52%) | 0.2 |
| Chronic obstructive pulmonary disease | 2 (40%) | 3 (60%) | 0.97 |
| Miscellaneous | 3 (60%) | 2 (40%) | 0.26 |
| Male | 18 (34.6%) | 34 (65.4%) | 0.37 |
| Female | 22 (44.9%) | 27 (55.1%) | 0.37 |
| Non-listed on RWL | 23 (46.9%) | 26 (53.1%) | 0.17 |
| Mechanical ventilation | 23 (46.9%) | 26 (53.1%) | 0.13 |
| Extracorporeal assistance in bridge to LTx | 19 (59.4%) | 13 (40.6%) | 0.002 |
LTx: lung transplantation; RWL: regular waiting list.
| Factor . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 0.99 | 0.952–1.031 | 0.659 |
| Cystic fibrosis | 0.25 | 0.06–1.05 | 0.06 |
| Pulmonary fibrosis | 0.68 | 0.18–2.59 | 0.57 |
| Chronic obstructive pulmonary disease | 0.53 | 0.09–3.33 | 0.50 |
| Miscellaneous | 1 | ||
| Male | 1 | 0.329 | |
| Female | 1.39 | 0.72–2.70 | |
| Non-listed on the RWL | 1.14 | 0.59–2.23 | 0.696 |
| Mechanical ventilation | 0.99 | 0.47–2.09 | 0.983 |
| Extracorporeal assistance in bridge to LTx | 2.77 | 1.26–6.11 | 0.01 |
| Factor . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 0.99 | 0.952–1.031 | 0.659 |
| Cystic fibrosis | 0.25 | 0.06–1.05 | 0.06 |
| Pulmonary fibrosis | 0.68 | 0.18–2.59 | 0.57 |
| Chronic obstructive pulmonary disease | 0.53 | 0.09–3.33 | 0.50 |
| Miscellaneous | 1 | ||
| Male | 1 | 0.329 | |
| Female | 1.39 | 0.72–2.70 | |
| Non-listed on the RWL | 1.14 | 0.59–2.23 | 0.696 |
| Mechanical ventilation | 0.99 | 0.47–2.09 | 0.983 |
| Extracorporeal assistance in bridge to LTx | 2.77 | 1.26–6.11 | 0.01 |
LTx: lung transplantation; RWL: regular waiting list.
| Factor . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 0.99 | 0.952–1.031 | 0.659 |
| Cystic fibrosis | 0.25 | 0.06–1.05 | 0.06 |
| Pulmonary fibrosis | 0.68 | 0.18–2.59 | 0.57 |
| Chronic obstructive pulmonary disease | 0.53 | 0.09–3.33 | 0.50 |
| Miscellaneous | 1 | ||
| Male | 1 | 0.329 | |
| Female | 1.39 | 0.72–2.70 | |
| Non-listed on the RWL | 1.14 | 0.59–2.23 | 0.696 |
| Mechanical ventilation | 0.99 | 0.47–2.09 | 0.983 |
| Extracorporeal assistance in bridge to LTx | 2.77 | 1.26–6.11 | 0.01 |
| Factor . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Age | 0.99 | 0.952–1.031 | 0.659 |
| Cystic fibrosis | 0.25 | 0.06–1.05 | 0.06 |
| Pulmonary fibrosis | 0.68 | 0.18–2.59 | 0.57 |
| Chronic obstructive pulmonary disease | 0.53 | 0.09–3.33 | 0.50 |
| Miscellaneous | 1 | ||
| Male | 1 | 0.329 | |
| Female | 1.39 | 0.72–2.70 | |
| Non-listed on the RWL | 1.14 | 0.59–2.23 | 0.696 |
| Mechanical ventilation | 0.99 | 0.47–2.09 | 0.983 |
| Extracorporeal assistance in bridge to LTx | 2.77 | 1.26–6.11 | 0.01 |
LTx: lung transplantation; RWL: regular waiting list.
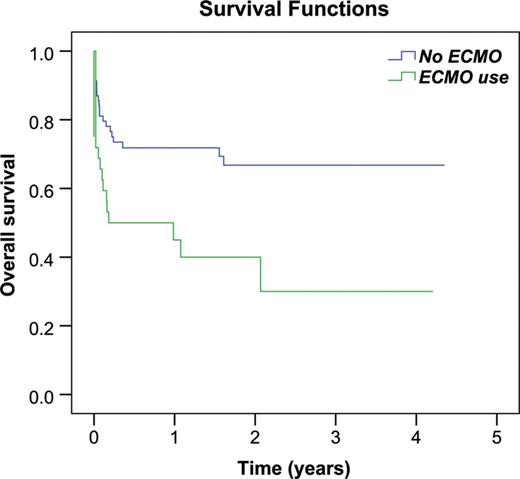
Survival curves comparing ECMO use before LTx. ECMO: extracorporeal membrane oxygenation; LTx: lung transplantation.
DISCUSSION
Worldwide, lung graft shortage has led several countries to establish various LTx allocation systems [5, 6]. For instance, American authorities set up a lung allocation score (LAS) system in May 2005 [7]. This LAS system allows selecting patients based on a score calculated to reflect the risk of death and expected outcome of each patient. With this score, a patient with emphysema and aged over 50 years has a lower priority than a 20-year-old patient with cystic fibrosis. This new allocation system was also introduced in Germany by Eurotransplant Foundation since December 2011 [8]. Recent American or European studies have tended to show a real benefit with reduced waiting times on the RWL and some survival benefit for specific patients' categories such as cystic fibrosis [9]. In the French system, any patient can be listed on the HEWL as long as they have been listed on the RWL for at least 2 days and if they fulfilled specific criteria. To date, only one French monocentric study and one prospective multicentric study within a 1-year study period have reported the early results of such a strategy [10, 11]. The present study exposes the results of the dedicated French emergency procedure since its creation in July 2007, in seven institutions representing 67% of the national LTx activity in 2011 [1]. The noteworthy results of the present study can be summarized in three points as follows: (i) the HEWL has allowed to decrease substantially death on the regular WL. However, the potential benefit of this emergency procedure was balanced with a higher postoperative mortality. (ii) The HEWL has offered a fast-track pathway to LTx for those patients referred too late to lung transplant teams, who previously would have died before having any chance to be transplanted. The strategy proved to be efficient as it resulted in LTx in 94% of the cases. (iii) Short- and mid-term survival rates seem acceptable when they are considered from the perspective of critically ill situations where patients are under MV or under ECMO.
Since the creation of the HEWL in France, the mortality rate on the RWL fell from 8.6% in 2006 to 3.5% in 2011. However, these impressive results have to be toned down. In 2006, 182 LTx were performed in France as opposed to 312 in 2011. LTx activity was almost doubled in only 5 years and, in addition to this phenomenon, patient registration did not increase dramatically, with 257 new registrations on the RWL in 2006 vs 325 in 2011 (+26%). In addition, for one lung graft available, 1.9% patients were eligible for LTx in 2006 vs 1.6 in 2011 [1]. Obviously, this dramatic increase of lung graft availability was the main source of improvement. The new allocation system was likely to have a significant impact on mortality prior to transplantation but not for the anticipated population. Before the creation of the HEWL, patients who were admitted in the ICU for acute respiratory failure and who required MV or ECMO had no chance of access to LTx. A large majority of these patients died without being listed and, consequently, without being incorporated in French authorities' statistics. This represents an important bias considering that national data on this specific population are not available prior to July 2007. The French allocation system allows patients to be listed on the HEWL after being registered on the RWL for at least 2 days. Actually, these patients are at the same time the most severely affected and the least evaluated candidates owing to lack of time. However, this study shows that these patients had similar outcomes as those of patients registered for a longer time on the waiting list. In the majority of the cases, patients were registered on the HEWL after MV/ECMO, based on whether interviews with their family and relatives led to their presumed consent for LTx. This fact raises ethical concerns as the process sustained for patients on the RWL is more complex and includes more substantial information on the benefit/risk ratio of the procedure [12]. In that way, avoiding MV and favouring awake ECMO may partially alleviate those ethical issues [13–15].
For many years, thoracic surgeons and pulmonologists were reluctant to perform LTx on patients under MV and even more under ECMO. Many studies and the report of International Society of Heart and Lung Transplantation [16] confirmed that preoperative MV and ECMO were strong risk factors for mortality after LTx. Using an American national database, Singer et al. found that LTx for adult patients who were dependent on MV was associated with a poorer 6-month survival compared with non-ventilated adult patients [2–4].
When traditional respiratory support is inefficient, ECMO is the next therapeutic intervention. Since the first successful clinical series of lung transplants, reported in 1987 [17], sporadic cases have been published using ECMO as a bridge to primary or redo lung transplant, with mixed results [18, 19]. ECMO, as a bridge to rehabilitation, supposes that the cause of the worsening can be reversible. It also supposes that side effects and complications of ECMO are not crippling. Side effects of ECMO include haemolysis, bleeding complications, severe infection and renal insufficiency. Nowadays, centrifugal pumps are commonly used to reduce haemolysis, and heparin-coated circuits have led to reduced platelet activation, reduced complement activation, reduced granulocyte activation and greatly reduced heparin requirements. New devices like pumpless extracorporeal lung assist or bi-caval dual lumen cannula have been developed. With these technical improvements combined with the creation of the HEWL, ECMO was revisited [20, 21]. In our experience, 87.5% of patients under assistance were successfully bridged to LTx; however, 59.4% of these patients died intra- or postoperatively. As a result, ECMO implementation as a bridge to transplantation had a 3-fold increased risk of death after LTx. Mason et al. [2], with a cohort of 51 patients under ECMO before LTx, also demonstrated worse survival rates compared with non-supported patients with 50% of survival at 1 year post-transplantation. These results were confirmed by the French study by Lafarge et al. [22], which shares part of the material presented herein. The intent-to-treat survival rate with ECMO was 55.4% at 1 year and 50.4% at 2 years, ranging from 21% for pulmonary fibrosis patients to 71% for cystic fibrosis patients. Fischer et al. [23] report a high in-hospital mortality rate for ECMO post-LTx (58%) for all patients. Based on these results, thoracic surgeons and pulmonologists initiated reflection on better patient selection, technical improvement and more suitable strategies. Thus, in the last few years, results of ECMO implementation as a bridge to LTx in awake and non-intubated patients have been published [13–15]. Recently, Fuehner et al. [24] reported on an 80% 6-month survival rate in awake ECMO patients versus 50% in patients receiving invasive MV. Because of the small number of patients (n = 6), our study was not powered enough to find any advantage nor disadvantage using this new approach.
From previous studies, patients listed on the HEWL seem to have worse survival rates after LTx than those who are transplanted on the RWL [1–7, 10–11]. This worse survival relates to the very critical conditions in which patients are transplanted. Indeed, pre-transplant deconditioning has a significant impact on outcomes for all lung transplant patients and is likely a major contributor to increased mortality in critically ill lung transplant recipients. Rehder et al. [25] described a series of patients bridged to LTx with ECMO and report on the beneficial impact of active rehabilitation and ambulation during pre-transplant ECMO with 100% one-year survival.
To conclude, despite several limitations, this study is the first attempt to evaluate the French dedicated emergency allocation process among seven institutions which represented two-thirds of the overall national LTx activity. The new organ-sharing procedure aimed at lowering mortality on the RWL, but this goal was primarily reached for new patients with end-stage respiratory failure referred very late. The HEWL increased the likelihood of perioperative mortality after LTx, but permitted acceptable mid-term survival rates. The high mortality associated with the use of ECMO should be interpreted cautiously in the context of the most critical conditions in which it was used. Finally, the HEWL disclosed a new public health need for those patients referred very late which has not been addressed so far. The related high mortality and the concern to avoid any graft wastage emphasize the need to increase the pool of donors. In this regard, it should be reminded that lung harvesting in non-heart-beating donors is not permitted by French regulations to date.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr G.A. Patterson(St. Louis, MO, USA): This is an important piece of work. It is another outstanding contribution from the Marseille group. I would like to make a couple of comments and then ask a question at the end.
In actual fact, this mirrors in many ways the recent experience in the US. Prior to 2005 in the United States, waiting list priority for potential transplant recipients was determined entirely by time on the waiting list, and that favoured the emphysema patients, who will survive forever if they just use their oxygen, but it disadvantages the CF patients and the patients with idiopathic pulmonary fibrosis, who have a much higher waiting time mortality. It also resulted in a large number of patients being listed. The stimulus for the programmes was to list as many patients as possible, even listing patients who didn't necessarily need a transplant at that current time.
Since 2005, we have had an allocation system that is calculated based on the likelihood of death on the waiting list, the likelihood of survival following transplant, and I don't think that factor, survival following transplant, is considered in your current algorithm. In other words, there are patients who can become so sick prior to transplant that their score actually falls. The goal is to make a more efficient use of available donor lungs. But since we adopted that allocation score in 2005, it has completely changed the patients that we transplant. We now transplant patients who are much sicker, much higher acuity patients. It has increased the number of transplants because it makes transplant allocation much more efficient. In other words, the organ procurement teams don't have to consider so many potential recipients because there are only high-risk patients at the top of the list.
In summary, it has been a very good system, and it has accomplished all of those positive goals without a significant increase in mortality. In our own experience, our perioperative mortality rate went from 5% to 6%, and nationally it is well under 10%, but your mortality rate in France with this system is much higher than that in this experience. And it seems to me that there might be two possible causes for this unacceptably high mortality rate, and I think it is something you want to think about and try and correct.
So I would like to ask you whether you think this high mortality rate is a result of the transplant team's inexperience with complicated cases or is it a problem of poor recipient selection? And this brings to mind your ECMO experience, and I applaud your efforts to transplant patients using ECMO and other means of support, but those ECMO patients had a very high mortality rate. So I would appreciate your comments on those two potential causes of this excessive mortality rate.
Dr Orsini: First, in France we have no lung allocation score. It is not like in the USA. The recipient and the donor choice is made by the team. About the high mortality rate of our study, that is true, a 34.5% in-hospital mortality rate is very high. In fact, there are maybe two causes. The first one is the inexperience. In our study, for a centre we have only nine patients on the high emergency waiting list, and I think that can explain this high mortality rate, the inexperience of the team.
And the second cause that is true is poor recipient selection with patients under ECMO, and that is a very interesting thing. But without the high emergency waiting list and without ECMO prior to lung transplantation, this category of patient died before creation of this list. So I think, yes, we have a high mortality rate, but we can offer access to lung transplantation for these new patients which will give a chance for survival after lung transplantation.
In your series I saw a lot of studies about ECMO prior to lung transplantation in the USA, and you have identified ECMO prior to lung transplantation as a potential risk factor for mortality like us, but we have to propose something for this category of patients.
Dr M. Nosotti(Milano, Italy): Tomorrow afternoon in the oral session I have a presentation on the same item, especially on the ECMO. Could I have your comment on this particular situation?
Dr Orsini: In our study, we had six patients without intubation under ECMO. All the ECMO were performed with the mono cannula, the Avalon cannula, and you saw in my presentation from the Marseille team that we performed our first case in January, so the patient is excluded from our study, by a veno-venous femoro-femoral approach without intubation. This new approach of ECMO is very interesting, because you can keep a social relation with the patient and you provide overall inflammatory response. So I think it will be a good way to study ECMO without intubation prior to lung transplantation. In our study, all the patients under ECMO without intubation were successfully bridged, 100%, six out of the six, but in the postoperative period, 66% of the patients died after lung transplantation. Thus, it is an efficient procedure to bridge patients.
Dr D. Van Raemdonck(Leuven, Belgium): Can you tell us a little bit more about the system in France, how patients are accepted on the high emergency waiting list? Are the data reviewed by a committee? Is there an audit? How many auditors are there and how is the decision made to put a patient on the high emergency waiting list?
Dr Orsini: To be listed on the high emergency waiting list, the patient, as you can see from my presentation, had to fulfill some criteria, and after fulfilling these criteria, the request is sent to a college of experts, two French experts. They analyse the request and they give consent or not to be listed on the high emergency waiting list. When we have a patient under ECMO or under mechanical ventilation, the patient is placed automatically on the high emergency waiting list. Patients must have only lung failure and no multiorgan failure.
Author notes
Presented at the 20th European Conference on General Thoracic Surgery, Essen, Germany, 10–13 June 2012.




