-
PDF
- Split View
-
Views
-
Cite
Cite
Maximilian Luehr, Christian D. Etz, Lukas Lehmkuhl, Andrej Schmidt, Martin Misfeld, Michael A. Borger, Friedrich-Wilhelm Mohr, Surgical management of delayed retrograde type A aortic dissection following complete supra-aortic de-branching and stent-grafting of the transverse arch, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 5, November 2013, Pages 958–963, https://doi.org/10.1093/ejcts/ezt180
Close - Share Icon Share
Abstract
Hybrid endovascular procedures are rapidly evolving and have recently been adopted for high-risk patients deemed unsuitable for conventional aortic arch surgery. We describe here our initial experience with this technique, including the management of 2 patients who developed a retrograde type A aortic dissection post-de-branching.
Between May 2010 and October 2012, 109 patients underwent conventional aortic arch repair at our institution. A further 9 high-risk patients with complex aortic arch pathology (median logistic EuroSCORE: 26, range: 11–41) were deemed unsuitable for conventional total aortic arch replacement and therefore underwent hybrid aortic arch repair. Complete supra-aortic de-branching, followed by endovascular stent-grafting (TEVAR) of the transverse arch and descending aorta, was performed in these high-risk patients.
In-hospital mortality was zero and no patient developed paraplegia/paraparesis due to spinal cord ischaemia. However, 2 patients (22%) developed retrograde type A aortic dissection on Days 10 and 12 post-TEVAR. Both patients had a dilated ascending aorta and received a stent graft containing bare metal springs at the proximal end. Emergency ascending aortic replacement was performed during moderate-to-mild hypothermia (28–34°C) and bilateral antegrade cerebral perfusion via cannulation of the de-branching prosthesis. A Hemashield prosthetic graft was anastomosed to the proximal stent graft in an elephant trunk technique. Both patients suffered from minor non-debilitating stroke, with 1 being discharged home and 1 transferred to a neurological rehabilitation centre 2 and 3 weeks after reoperation, respectively.
Retrograde type A aortic dissection after hybrid endovascular treatment of the aortic arch represents a new—most likely under-reported—pathology that may be successfully treated with open surgical repair. The use of stent grafts with protruding proximal bare springs and the implementation of oversizing and post-deployment ballooning should be avoided in patients undergoing hybrid arch procedures, particularly if the ascending aorta is dilated.
INTRODUCTION
Conventional open ‘two-stage’ surgery for extensive thoracic aortic pathologies—including the aortic arch and the descending aorta—remains challenging, with an associated morbidity and mortality in the range of 13–36% [1–4]. Hybrid endovascular procedures are evolving and have recently been adopted for high-risk patients deemed to be unsuitable for conventional aortic arch surgery. Such procedures have been used to treat a wide range of aortic disease involving the transverse aortic arch and the descending thoracic aorta.
New less-invasive surgical strategies—involving off-pump partial or complete surgical re-routing of the supra-aortic branches followed by thoracic endovascular aortic repair (TEVAR)—have recently been introduced into clinical practice to treat aortic arch pathologies in high-risk patients with promising early results [5–7]. The main concept of ‘supra-aortic de-branching’ is the creation of a proximal landing zone (Zones 0, 1, 2) to allow for safe second-stage endovascular stent graft deployment to completely exclude transverse and distal arch pathologies, e.g. aneurysms, penetrating ulcers and dissections [8]. Although TEVAR has been developed as a less-invasive approach for the treatment of aortic pathologies, it is also associated with a dangerous and formerly unknown complication: retrograde type A aortic dissection (rAAD) [5, 9].
The risk of rAAD during and after hybrid de-branching procedures in the management of aortic arch pathologies remains unknown. However, the occurrence of rAAD during TEVAR seems to be multifactorial rather than directly related to intraoperative manipulation alone, particularly in the course of delayed rAAD [5].
We describe here our early experience with aortic de-branching procedures, with a particular focus on the diagnosis and management of patients who developed postoperative rAAD.
PATIENTS AND METHODS
Between May 2010 and October 2012, 109 patients underwent conventional aortic arch surgery at our institution. A further 9 high-risk patients with pathology of the aortic arch and descending aorta were deemed unsuitable for conventional surgery due to significant comorbidities (median logistic EuroSCORE: 26, range: 11–41), and were therefore admitted for hybrid endovascular aortic repair. Preoperative patient demographics and comorbidities are shown in Table 1.
| Age ± SD, range | 76.4 ± 4.8 (67–82) years |
| Male, n (%) | 9 (100) |
| Hypertension, n (%) | 9 (100) |
| Obesity, n (%) | 2 (22) |
| Hyperlipidaemia, n (%) | 6 (67) |
| Coronary artery disease, n (%) | 4 (44) |
| Previous myocardial infarction, n (%) | 1 (11) |
| Chronic obstructive pulmonary disease, n (%) | 2 (22) |
| Diabetes mellitus, n (%) | 4 (44) |
| Chronic renal failure, n (%) | 1 (11) |
| Chronic pancreatitis, n (%) | 1 (11) |
| Liver cirrhosis, n (%) | 1 (11) |
| Previous cardiac operation, n (%) | 2 (22) |
| Previous major surgery (non-cardiac), n (%) | 6 (67) |
| Age ± SD, range | 76.4 ± 4.8 (67–82) years |
| Male, n (%) | 9 (100) |
| Hypertension, n (%) | 9 (100) |
| Obesity, n (%) | 2 (22) |
| Hyperlipidaemia, n (%) | 6 (67) |
| Coronary artery disease, n (%) | 4 (44) |
| Previous myocardial infarction, n (%) | 1 (11) |
| Chronic obstructive pulmonary disease, n (%) | 2 (22) |
| Diabetes mellitus, n (%) | 4 (44) |
| Chronic renal failure, n (%) | 1 (11) |
| Chronic pancreatitis, n (%) | 1 (11) |
| Liver cirrhosis, n (%) | 1 (11) |
| Previous cardiac operation, n (%) | 2 (22) |
| Previous major surgery (non-cardiac), n (%) | 6 (67) |
| Age ± SD, range | 76.4 ± 4.8 (67–82) years |
| Male, n (%) | 9 (100) |
| Hypertension, n (%) | 9 (100) |
| Obesity, n (%) | 2 (22) |
| Hyperlipidaemia, n (%) | 6 (67) |
| Coronary artery disease, n (%) | 4 (44) |
| Previous myocardial infarction, n (%) | 1 (11) |
| Chronic obstructive pulmonary disease, n (%) | 2 (22) |
| Diabetes mellitus, n (%) | 4 (44) |
| Chronic renal failure, n (%) | 1 (11) |
| Chronic pancreatitis, n (%) | 1 (11) |
| Liver cirrhosis, n (%) | 1 (11) |
| Previous cardiac operation, n (%) | 2 (22) |
| Previous major surgery (non-cardiac), n (%) | 6 (67) |
| Age ± SD, range | 76.4 ± 4.8 (67–82) years |
| Male, n (%) | 9 (100) |
| Hypertension, n (%) | 9 (100) |
| Obesity, n (%) | 2 (22) |
| Hyperlipidaemia, n (%) | 6 (67) |
| Coronary artery disease, n (%) | 4 (44) |
| Previous myocardial infarction, n (%) | 1 (11) |
| Chronic obstructive pulmonary disease, n (%) | 2 (22) |
| Diabetes mellitus, n (%) | 4 (44) |
| Chronic renal failure, n (%) | 1 (11) |
| Chronic pancreatitis, n (%) | 1 (11) |
| Liver cirrhosis, n (%) | 1 (11) |
| Previous cardiac operation, n (%) | 2 (22) |
| Previous major surgery (non-cardiac), n (%) | 6 (67) |
Indications for hybrid treatment comprised extensive atherosclerotic arch aneurysm (n = 7) and arch aneurysm due to an aberrant subclavian artery (n = 2). Preoperative multislice computed tomography (CT), with subsequent 3D reconstruction of the entire aorta, was performed in order to identify an adequate proximal landing zone (Ishimaru classification) for endovascular stent-grafting and to exclude any major occlusive vessel disease of the supra-aortic and thoracoabdominal aortic branches [8]. To allow for complete stent-grafting of the transverse arch, a proximal landing zone of at least 2.0 cm in the distal ascending aorta (Zone 0) was chosen in all patients. Preoperative coronary angiography and echocardiography were performed in all patients.
Six operations were performed in a hybrid operation theatre equipped with an angiographic C-arm system allowing for concomitant TEVAR, while the other 3 patients underwent a staged procedure. The de-branching procedures were performed without cardiopulmonary bypass (CPB) in 6 patients, and with CPB in 1 patient who had a patent LIMA-LAD bypass graft post-CABG. Concomitant off-pump coronary artery bypass grafting (OPCAB) was performed in 2 patients. Later in the series, 2 patients with a dilated ascending aorta (diameter >40 mm) underwent supracoronary ascending aortic replacement followed by supra-aortic de-branching using a four-branched prosthetic graft (Lupiae graft, Vascutek Terumo, Scotland, UK). The ascending aorta was replaced in these patients during CPB in order to achieve an adequate proximal landing zone and to avoid the risk of rAAD.
Endovascular stent-grafting of the distal ascending aorta, transverse arch and proximal descending aorta was successfully performed retrogradely via the femoral artery under fluoroscopy followed by completion angiography in all cases. The TEVAR procedure was performed during the same operation as the de-branching procedure in 6 patients, and as a second-stage completion repair 5–8 days later in 3 patients. A postoperative routine follow-up CT scan was performed 1 week post-TEVAR in all patients.
RESULTS
Open supra-aortic de-branching, with (n = 3) or without (n = 6) the use of CPB was successfully performed in all patients. Concomitant OPCAB surgery was performed in 2 patients. No patient required intraoperative conversion to conventional open repair with deep hypothermic circulatory arrest.
Retrograde stent graft deployment via the femoral artery was successful in all 9 patients. Stent graft quantity depended on the individual extend of aortic pathology to allow for complete exclusion: 6 patients received three stent grafts (Valiant®, Medtronic Vascular, Santa Rosa, CA, USA)—covering the transverse arch and descending aorta up to the celiac trunk—and 2 were treated with one stent graft (Valiant®, Medtronic Vascular, Santa Rosa, CA, USA). Only 1 patient underwent TEVAR with two Zenith® (Cook, Inc., Bloomington, IN, USA) stent grafts.
Intraprocedural balloon dilatation of the aortic stent grafts was necessary in 4 cases and depended on individual aortic arch pathology and the occurrence of endoleaks immediately after stent graft deployment. No endoleaks were detected during the following clinical course.
Morbidity and mortality after hybrid arch repair
The median intensive care unit (ICU) stay and hospital stay were 11.3 ± 8.5 days (range 2–29 days) and 19.5 ± 12.1 days (range 7–42 days), respectively. In-hospital mortality was zero.
Perioperative stroke and transient postoperative delirium occurred in 2 and 3 patients, respectively. No postoperative transient or permanent spinal cord ischaemia (i.e. paraplegia/paraparesis) occurred, even in patients with coverage of the entire descending aorta to the celiac axis.
Five patients developed respiratory insufficiency postoperatively, and 3—one with chronic obstructive pulmonary disease—required percutaneous tracheostomy in order to be weaned from the ventilator. One patient with chronic renal insufficiency required temporary dialysis during the early postoperative course. Reoperation was required in 3 patients because of postoperative bleeding, acute innominate artery bypass occlusion and sternum instability in 1 patient each.
Two patients developed retrograde type A dissection post-TEVAR and are described in more detail below.
Case 1
At the beginning of the series, an 80-year old male patient presented with progressive back pain due to a large aneurysm of the distal aortic arch and descending aorta (max. diameter 60 mm) post-type B aortic dissection. The patient also suffered from several comorbidities including dyspnea at rest, obesity, atrial fibrillation, arterial hypertension, symptomatic hyperthyroidism and acute gastroenteritis. With regard to the medical history and the actual condition, this patient was deemed frail and high risk for open surgery.
He underwent off-pump supra-aortic de-branching and TEVAR of the entire descending aorta using three stent grafts. TEVAR coverage extended distally to the celiac axis, with good perfusion of the celiac trunk on completion angiography.
The procedure was successful and the patient recovered uneventfully. On the 10th postoperative day, however, he developed chest pain and acute cardiac tamponade requiring cardiopulmonary resuscitation. Emergency chest CT showed acute rAAD in close proximity to the proximal end of the stent graft and the anastomosed ‘de-branching’ graft (Fig. 1A and B). Replacement of the supracoronary ascending aorta was performed as an open emergency operation. Bilateral antegrade selective cerebral perfusion (800 ml/min) was performed via cannulation of the de-branching graft, during moderate hypothermia (28°C) and distal circulatory arrest. Intraoperatively, a dissection entry due to bare spring perforation of the enlarged ascending aorta (diameter 42 mm) was confirmed.
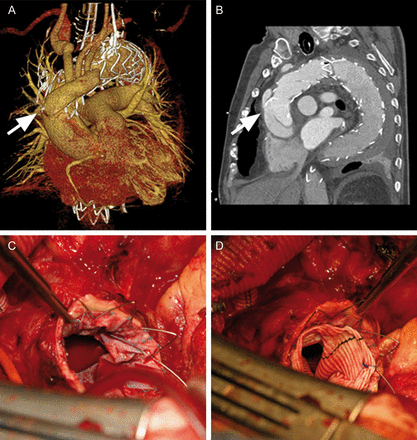
CT 3D reconstruction of the thoracic aorta after supra-aortic de-branching and thoracic endovascular stent graft repair showing retrograde aortic type A dissection (rAAD) due to proximal bare spring perforation (A and B). Intraoperative view of the proximal portion of the deployed stent graft after resection of the ascending aorta (C). An ‘elephant trunk procedure’ was performed by suturing a Hemashield graft to the proximal end of the stent graft (D); the bare springs can be cut off before or after completion of this anastomosis.
A Hemashield graft (32 mm) was anastomosed to the proximal end of the stent graft in an elephant trunk technique achieving good hemostasis (Fig. 1C and D). The right coronary sinus was dissected but the aortic valve was still competent. In view of the patient's age and comorbidities, the decision was made to glue together the layers of the right coronary sinus with fibrin glue, and the proximal anastomosis was completed thereafter. After finishing the proximal anastomosis, the de-branching graft was anastomosed end-to-side to the new ascending aortic graft. Circulatory arrest time was 35 min, aortic cross-clamp time was 82 min and CPB time was 158 min.
A postoperative cranial CT scan showed multiple infarction syndrome. Two weeks after reoperation for rAAD, the patient was responsive and able to weakly move all extremities, and was discharged to a neurological rehabilitation clinic.
Case 2
The second patient (age 67) had been admitted for an aberrant right subclavian artery aneurysm with involvement of the distal aortic arch, as well as significant left main coronary artery disease. The diameter of his native ascending aorta was 36 mm and therefore he was thought to be at low risk for rAAD. The medical history included arterial hypertension, diabetes mellitus, smoking (>40 pack years) and previous visceral surgery. The patient was deemed to be high risk for conventional open surgery because of the challenging location of his aortic aneurysm at the origin of the aberrant right subclavian artery (i.e. Kommerell's diverticulum).
The patient underwent supra-aortic de-branching and concomitant off-pump OPCAB surgery. TEVAR of the transverse arch, using one stent graft (Valiant®), was performed as a second-stage repair 1 week later. The postoperative course was uneventful; however, routine predischarge surveillance CT revealed an rAAD involving the non-coronary sinus and proximal ascending aorta, without signs of acute cardiac tamponade.
Urgent supracoronary ascending replacement with a 30-mm Hemashield prosthesis was performed. The de-branching prosthesis was cannulated in order to perform bilateral antegrade selective cerebral perfusion during mild hypothermic (34°C) arrest. Additional distal perfusion (2.5 l/min) of the lower body was performed via a balloon occlusion catheter that was placed within the arch stent graft, in order to lower the risk of ischaemic damage to the spinal cord and visceral organs. The dissection entry was located in the proximal ascending aorta directly adjacent to the bare metal springs of the stent graft. During antegrade cerebral and lower body (distal) perfusion, the ascending aorta was resected and an ‘elephant trunk procedure’ with a 30-mm Hemashield prosthetic graft was performed as described above. The aortic valve was competent and therefore the dissected layers of the non-coronary sinus were re-approximated with fibrin glue. Circulatory arrest time was 25 min, aortic cross-clamp time was 43 min and CPB time was 85 min.
The patient was extubated on the second postoperative day, but required another reoperation for sternal instability 10 days later. In addition, a right posterolateral cerebral infarction (3.7 × 2.7 cm) was diagnosed via cranial CT scan after the patient had developed left arm weakness. No further neurological deficits occurred and the patient was discharged home 6 weeks after the initial de-branching operation.
DISCUSSION
rAAD has been recognized as an uncommon but frequently lethal aortic disease in the era of transcutaneous thoracic aortic intervention [5, 9]. Post-TEVAR rAAD was initially reported as single case reports following the treatment of acute type B aortic dissection [10, 11]. In 2009, a multicentre study of the European Registry on Endovascular Aortic Repair Complications reported on an incidence of rAAD post-TEVAR for either acute or chronic type B dissection of 1.33% (95% CI 0.75–2.40) [12]. Although rAAD post-TEVAR is uncommon, its associated mortality is higher than that observed in patients presenting with conventional type A aortic dissection, being 50% if rAAD is diagnosed in-hospital and 70% if diagnosed during the TEVAR procedure [12].
Today, hybrid endovascular procedures are increasingly used in high-risk patients deemed unsuitable for conventional aortic arch surgery [5–7]. Most recently, the incidence of early rAAD after partial (Zone 1 and 2) and complete (Zone 0) supra-aortic re-routing followed by TEVAR of the aortic arch was reported to be 1.9%, with a 30-day hospital mortality rate of 33% [9]. However, the incidence of rAAD after stent graft deployment in the ascending aorta (Zone 0) with complete arch coverage was even higher when compared with more distal endografting of the aortic arch (Zone 1 or 2): 6.9 vs 1.4% [9]. Czerny et al. reported an overall rAAD incidence of 8% in a multicentre study of 66 patients with total arch de-branching and TEVAR (Zone 0), with an early (<7 days postoperatively) and delayed (>7 days postoperatively) incidence of 3 and 5%, respectively [5]. At our institution, 2 (22%) of 9 patients who underwent hybrid arch repair developed delayed rAAD. Even though emergency surgery was successful with no in-hospital mortality, the high occurrence of rAAD and the accompanying stroke rate raises concerns about the safety of complete supra-aortic de-branching and TEVAR of the aortic arch.
Williams et al. reviewed their institutional database with regard to supra-aortic de-branching and TEVAR of the aortic arch (Zones 0–2) and identified only 6 (1.9%) of 309 patients who developed early rAAD [9]. Their patient analysis revealed an increased rAAD incidence in patients with an aortic diameter of more than >40 mm and after stent graft deployment in the native ascending aorta (Zone 0) of 4.8 and 6.9%, respectively. A combination of increased ascending aortic diameter (>40 mm), a proximal landing zone in the native aorta (Zone 0), and the existence of dissection pathology increased the incidence of rAAD up to 25% [9]. All of these potential risk factors applied to the first patient of our series who developed rAAD: ascending aortic diameter of 42 mm, proximal landing zone in the native ascending aorta (Zone 0) and pre-existing type B aortic dissection. In addition, it was noted at the time of reoperation that the entry site was directly adjacent to the proximal bare springs of the stent graft.
Other investigators have noted an association between proximal bare springs and the occurrence of rAAD [12, 13]. However, a definite causation has not yet been established. Czerny et al. identified several factors that may contribute to the development of rAAD after surgical de-branching and complete TEVAR of the arch: ascending aortic injury after partial clamping for the proximal anastomosis, compliance mismatch (rigidity of the stent graft vs highly compliant ascending aorta) and blood flow alterations after transposition [5]. Aortic injury due to partial clamping of the ascending aorta was unlikely to have been the cause of rAAD in our second patient, since he underwent a repeat CT scan between his de-branching and TEVAR procedures. This interval CT did not reveal any evidence of rAAD, which was first diagnosed after the TEVAR procedure. Intraoperative examination again revealed an entry site that occurred directly adjacent to the proximal bare metal springs that may have caused the initial aortic wall injury. Kpodonu et al. suggested that stent graft oversizing to >20% with regard to aortic diameter may also be a potential risk factor for rAAD [14]. Of note, 4 patients in our series (including both patients with rAAD) required balloon expansion after stent graft deployment in the transverse arch.
Another issue regarding rAAD after TEVAR is the resulting neurological outcome after the reoperation. Both patients had been treated successfully with supra-aortic de-branching and TEVAR without any neurological complication postoperatively. However, both required emergent ascending aortic replacement with bilateral SCP—and ultimately suffered from stroke after reoperation. Clearly, any aortic operation bears a potential risk for occurrence of the new neurological events. However, CPR was required preoperatively in the first case and may have predisposed the patient to significant cerebral ischaemia. Avoiding rAAD—and thereby an aortic reoperation—is of outmost importance in these high-risk patients to lower the incidence of neurological complications and postoperative mortality.
The results of our study and others suggest that not every ‘high-risk’ patient deemed unsuitable for conventional aortic arch repair is a good candidate for off-pump supra-aortic de-branching and TEVAR of the aortic arch. When considering hybrid or conventional surgery, it is important to always individually estimate the patient's history, actual clinical status and anatomical variations. During a period of 29 months, we only classified 9 of 118 patients (7.6%) requiring aortic arch surgery as acceptable candidates for supra-aortic de-branching and TEVAR. However, the fact that both patients with rAAD (22%; n = 2) were successfully treated by emergent open surgery may even indicate that conventional surgery could have been successfully performed in the first place despite these significant comorbidities.
It is important for every surgeon to distinguish whether a patient is dying from aortic pathology (i.e. dissection or aneurysm) or with aortic pathology. Mack most recently described a method of patient selection for transcutaneous aortic valve replacement with regard to frailty and reported the usefulness of the ‘eyeball test’, grip strength and the 5-min walk test in order to assess whether a patient is best suited for conventional surgery, interventional treatment or conservative therapy [15]. Currently, there is no general consensus among the aortic experts in classifying ‘fitness for open surgery’, TEVAR or hybrid procedures, and therefore patient estimation of being ‘too high risk’ for open arch surgery depends on the surgeon's individual experience only!
Koullias and Wheatly suggested a new hybrid classification system for high-risk patients with regard to anatomy to allow for better decision making when deciding to replace the transverse arch surgically or to exclude the underlying aortic pathology by TEVAR: hybrid type 1 vs hybrid type 2 [16] (Fig. 2). With regard to their classification, the frozen elephant trunk technique could be used in patients requiring a hybrid type 1 repair, while off-pump supra-aortic de-branching with TEVAR of the arch would be indicated in a hybrid type 2 [16]. The Philadelphia group published another classification system (Type I–III) to address the varying pathologies of the transverse arch [7] (Fig. 2). They suggested the use of supra-aortic de-branching and TEVAR of the transverse arch in the ‘classic’ saccular aneurysm (Type I), while Type II and III have no suitable proximal landing zone (and distal landing zone in Type III) and therefore require reconstruction of Zone 0 by ascending aortic replacement [7]. In Type II or III, TEVAR may be performed antegradely during the same session (Type II, IIIa) or retrogradely via the femoral artery as a second-stage repair (Type IIIb).
![Suggested classification systems for hybrid aortic arch repair with regard to arch pathology by Koullias et al. [16] and Milewski et al. [7]. Supra-aortic de-branching followed by TEVAR of the arch; TEVAR procedure is primary (hybrid type 2/type I). Conventional hemiarch/arch replacement is performed; TEVAR procedure is secondary (hybrid type 1/type II and III).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/44/5/10.1093_ejcts_ezt180/1/m_ezt18002.gif?Expires=1750518184&Signature=huWtU8sHmSlFlOwcNGA9ohzRsHK7U9sMWkZThB2Hh42HGyvE58KkN5w5e-4V6Tcak2oUfzDyF9osIXhs0QEteDrvFbghVeSIhIeeDNg8TBzWeaBXicsbjnIaPBAwaqvv7ie7yOMm~ek7g2VDL5kSSmLdYo5vJWnVYbXb0MdwfAG37ISdXRE3OV98M8J4AwuB09XaZzJ2UpvsJDvEHgZIr0EBHqfT3jYl8gzBF-Y3summ7glKDdD-m6hKbFipiOelA9LhZuaLk41724oaw-JyKfmsa8yPCLQkTGLjWlC3YUj4gGrYh5R5OdxsaaJWytYbHEFsUb2qEYzUgSJISarD3Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Suggested classification systems for hybrid aortic arch repair with regard to arch pathology by Koullias et al. [16] and Milewski et al. [7]. Supra-aortic de-branching followed by TEVAR of the arch; TEVAR procedure is primary (hybrid type 2/type I). Conventional hemiarch/arch replacement is performed; TEVAR procedure is secondary (hybrid type 1/type II and III).
Perioperative diagnostics and imaging are of outmost importance to diagnose post-TEVAR rAAD. Intraoperative transoesophageal echocardiogram (TEE) allows for the detection of acute rAAD during TEVAR, and therefore should be applied in every case [9]. A high degree of clinical suspicion should be used in the postoperative period, particularly if sudden hypotension occurs. At our institution, postoperative routine chest CT scan is performed 1 week post-TEVAR in order to exclude any procedure-related complications (e.g. subacute rAAD, endoleaks etc.), and to verify the therapeutic success (e.g. aneurysm exclusion).
Although our incidence of rAAD is worrisomely high in this small patient cohort, it is possible that the true incidence of post-TEVAR rAAD is under-reported in the current literature. We strongly believe that routine predischarge CT scan examination should be performed in all patients treated by TEVAR regardless of symptoms or clinical suspection.
As a result of our high incidence of rAAD after hybrid aortic de-branching and stent-grafting in patients (with extensive aortic pathologies involving the arch and the descending aorta), we now electively perform ascending aortic replacement with a four-branched prefabricated graft in all patients with ascending aortic ectasia (aortic diameter >4.0 cm) or aneurysm (hybrid type 2/Type II and III as described above), in order to achieve an extended proximal landing zone (3–4 cm in length) and to avoid the potential risk of rAAD. Moreover, we also avoid the use of conventional endovascular stent graft prostheses with protruding, rigid proximal bare springs during all hybrid de-branching procedures, particularly in patients with a dilated ascending aorta or distorted vascular anatomy. Finally, we are very selective when identifying patients for hybrid therapy, with conventional aortic arch surgery being our procedure of choice in the vast majority of patients.
CONCLUSION
rAAD represents a new and possibly under-reported complication after hybrid endovascular treatment of the aortic arch. The use of stent grafts with protruding proximal bare stents and the implementation of oversizing and post-deployment ballooning should be avoided in this high-risk cohort, particularly if the ascending aorta is dilated. An expert consensus should attempt to more accurately identify high-risk patients unsuitable for conventional surgery who will benefit most from hybrid arch procedures.
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.
- aorta
- aortic arch
- stents
- hypothermia, natural
- descending aorta
- proximal aortic dissection
- cerebrovascular accident
- ischemic stroke
- ascending aorta
- hospital mortality
- metals
- repeat surgery
- surgical procedures, operative
- pathology
- transplantation
- endoluminal grafts
- aortoplasty
- military deployment
- thoracic endovascular aortic repair
- cerebral perfusion
- prostheses
- replacement of ascending aorta
- endovascular procedures
- prosthetic grafts




