-
PDF
- Split View
-
Views
-
Cite
Cite
Thomas Tovedal, Gunnar Myrdal, Ove Jonsson, Maria Bergquist, Vitas Zemgulis, Stefan Thelin, Fredrik Lennmyr, Experimental treatment of superior venous congestion during cardiopulmonary bypass, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages e239–e244, https://doi.org/10.1093/ejcts/ezt311
Close - Share Icon Share
Abstract
Superior venous outflow obstruction affects cerebral perfusion negatively by reducing cerebral perfusion pressure (CPP). We present a randomized study designed to compare two alternative strategies to preserve the CPP during superior vena cava (SVC) congestion and cardiopulmonary bypass (CPB).
Fourteen pigs on bi-caval CPB were subjected to 75% occlusion of the SVC flow. CPP was restored either by vasopressor treatment (VP, n = 7) or by partial relief (PR) of the congestion (n = 7). The cerebral effects of the interventions were studied for 60 min with intracranial pressure (ICP) monitoring, cerebral blood flow measurement, the near-infrared light spectroscopy tissue oxygen saturation index (StO2), arterial and venous blood gas analyses and serial measurements of the glial cell damage marker protein S100β.
Both strategies restored the CPP to baseline levels and no signs of severe ischaemia were observed. In the PR group, the venous and ICPs were normalized in response to the intervention, while in the VP group those parameters remained elevated throughout the experiment. The haemoglobin oxygen saturation in the sagittal sinus (SsagO2) was increased by both VP and PR, while significant improvement in the StO2 was observed only in the PR group. The S100β concentrations were similar in the two groups.
Experimental SVC obstruction during CPB may reduce the CPP, resulting in impaired cerebral perfusion. Both vasopressor treatment and improved venous drainage can, in the short term, individually restore the CPP during these circumstances.
INTRODUCTION
The incidence of cerebral injury associated with cardiopulmonary bypass (CPB) is reportedly 1.2–9.7% focal lesions [1–3]. Cognitive decline of up to 24% has been described after 6 months [4, 5]. A contributing factor may be superior venous congestion, which may occur with a single venous cannula in the right atrium or the superior vena cava (SVC) [6, 7], bi-caval [8–10] or dual-stage cannulation [11]. This promotes manifest ischaemia [12], but may also pass undetected with merely subtle changes in central venous pressure (CVP), total venous drainage and near-infrared light spectroscopy (NIRS) indexes [6, 13]. Although common, corrective measures are not always sufficient. In clinical practice, the phenomenon is difficult to avoid completely and occasionally, the benefit from repositioning of the SVC cannula needs to be balanced against the loss of precious time on CPB. In a previous paper, we demonstrated that during mild hypothermia, even with complete clamping, a substantial part of the subjects may escape clinically measurable signs of ischaemia [13], indicating that the phenomenon needs further exploration in order to control the damage when causal action is not possible. Others have shown that poor SVC drainage can result in elevated intracranial pressure (ICP) with a resulting reduction in cerebral perfusion pressure (CPP), and it has been suggested that impaired cerebral perfusion may be accessible by vasopressor treatment aiming to restore the CPP [6]. However, the efficacy of this approach has not been established.
We designed an experiment on pigs subjected to superior venous congestion during CPB, randomized either to treatment with partial relief (PR) of the congestion or to vasopressor treatment (VP) in order to restore the CPP. Cerebral perfusion was monitored with NIRS and blood gas analysis.
MATERIALS AND METHODS
Fourteen pigs of Norwegian Landrace breed (30.0–39.0, mean 36.0 kg) were subjected to CPB with bi-caval venous drainage during normocapnia and mild hypothermia (34°C). SVC obstruction was obtained by strangulating the SVC cannula to 25% of baseline flow using an adjustable gate clamp and an ultrasonic flow meter (M3, Spectrum Medical Ltd., Gloucester, UK). The animals were then allowed to stabilize for 10 min before being randomized to receiving either vasopressor treatment (VP, n = 7) or PR of the congestion (n = 7). Norepinephrine infusion (Noradrenalin, Abcur AB, Helsingborg, Sweden) was used as VP, and to relieve the congestion, the clamp was released. Both methods were adjusted so that the difference between mean arterial pressure (MAP) and sagittal sinus pressure (SagP) was restored. The duration of the intervention was 60 min, with sampling after 30 min (T2) and at 60 min (T3), after which the animals were euthanized by an intravenous potassium injection. Time points for sampling are specified in Table 1. The effects on cerebral perfusion were studied with blood gases, NIRS, cerebral blood flow (CBF) measuring by laser-Doppler, ICP monitoring and by analysis of the glial cell damage marker S100β in sagittal sinus blood.
| BL1 | After induction of anesthesia |
| BL2 | On stabile CPB |
| T1 | 10 min after superior venous congestion |
| T2 | 30 min after start of intervention |
| T3 | 60 min after start of intervention |
| BL1 | After induction of anesthesia |
| BL2 | On stabile CPB |
| T1 | 10 min after superior venous congestion |
| T2 | 30 min after start of intervention |
| T3 | 60 min after start of intervention |
Baseline 1 (BL1) after induction of anesthesia, Baseline 2 (BL2) on CPB, after which SVC obstruction was applied, and Time point 1 (T1) after SVC obstruction + 10 min. After T1, the animals were randomized, and intervention was applied. Time point 2 (T2) was defined as intervention + 30 min, and Time point 3 (T3) as intervention + 60 min.
| BL1 | After induction of anesthesia |
| BL2 | On stabile CPB |
| T1 | 10 min after superior venous congestion |
| T2 | 30 min after start of intervention |
| T3 | 60 min after start of intervention |
| BL1 | After induction of anesthesia |
| BL2 | On stabile CPB |
| T1 | 10 min after superior venous congestion |
| T2 | 30 min after start of intervention |
| T3 | 60 min after start of intervention |
Baseline 1 (BL1) after induction of anesthesia, Baseline 2 (BL2) on CPB, after which SVC obstruction was applied, and Time point 1 (T1) after SVC obstruction + 10 min. After T1, the animals were randomized, and intervention was applied. Time point 2 (T2) was defined as intervention + 30 min, and Time point 3 (T3) as intervention + 60 min.
For quantification of S100β, a commercially available human ELISA kit with documented porcine cross-reactivity was used (BioVendor, Brno, Czech Republic). The assay was optimized for optimal S100β recovery with regard to sample dilution, heparin and CaCl2 concentration (data not shown). Briefly, plasma (ethylenediaminetetraacetic acid) samples were heparinized using 25 U of heparin and diluted 1 : 3 in the kit dilution buffer. All standards were diluted according to the manufacturer's instructions. Optical density was read at 450 nm, with a correction at 630 nm using a Tecan Sunrise instrument (Tecan Nordic AB, Mölndal, Sweden) with the Magellan software. The limit of detection was 15 pg/ml, as determined by the manufacturer.
A 22-G venous catheter (BD Venflon®, Becton-Dickinson AB, Sweden) was inserted into the superior sagittal sinus through a burr craniotomy for pressure monitoring (SagP) and blood sampling. A laser-Doppler probe (Probe 407 connected to a PF 5010 LDPM Unit, Perimed AB, Järfälla, Sweden) was inserted through a dural incision in a PH 07-5 stabilizing holder onto the left parietal surface close to the midline of the brain for CBF measurements. The device presents unitless values, thus indicating relative changes. An 18-G epidural catheter (Portex®, Smiths Medical ASD, Inc., Keene, NH, USA) positioned 1 cm into a parietal sulcus was used for ICP registration. After skin closure, a NIRS sensor was placed parietally over the right hemisphere and connected to a Fore-Sight® Cerebral Oximeter (Casmed, Branford, CT, USA) for the registration of cerebral tissue oxygen saturation index (StO2cer). A second NIRS sensor was applied over the hepatic region for the registration of possible inferior tissue oxygen saturation (StO2abd) alterations during SVC obstruction and interventions. The SVC was cannulated with a DLP® 24-Fr venous catheter (Medtronic AB, Kista, Sweden), and the inferior vena cava (IVC) was cannulated with a DLP® 28-Fr venous catheter (Medtronic AB). Both cannulas were snared. Aortic cannulation was performed with an 18-Fr Fem-Flex II arterial cannula (Edwards Lifesciences Nordic AB, Malmö, Sweden). The venous anatomy differs in pigs compared with humans [14]. The azygos system connects the SVC and the IVC territories, and in contrast to humans, the left hemi-azygos vein drains into the coronary sinus in pigs. To reduce collateral flow from the SVC to the IVC territory during the experiment, both right and left azygos veins were ligated. The CPB flow was adjusted to 84–104 ml/kg/min to obtain adequate mixed venous oxygen saturation (SvO2) at a PaCO2 of 4.7 (4.3–5.0) kPa at time point BL2. All animals received human care in compliance with the European Convention on Animal Care. The study was approved by the Uppsala Ethical Committee on Laboratory Animal Research.
All data are expressed as means and standard error of means (SEMs). Directed within-group comparisons of interest were made using paired t-tests, and for comparisons between the groups at specified time points, unpaired t-tests were used. An alpha-level of 0.05 was considered statistically significant. Statistical processing was performed with GraphPad Prism 6.01 (GraphPad Software, Inc., La Jolla, CA, USA). One outlier (CBF, PR at T2) was removed using Grubbs' test.
RESULTS
General findings
All 14 experiments were carried out successfully. Prior to randomization, the PaO2, SvO2 and haematocrit were 56.2 ± 4.0 kPa, 53.1 ± 1.8% and 17.8 ± 1.6% in the VP and 50.9 ± 1.1 kPa, 58.5 ± 3.7% and 16.7 ± 0.5% in the PR group (Fig. 1 and Table 2).
Compilation of means and SEMs at various time points, with P-values from paired t-tests of differences between specified time points
| . | . | BL2 . | T1 . | T2 . | T3 . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | BL2–T1 . | T1–T2 . | ||
| CVP | VP | 1.0 | 1.5 | 22.4 | 4.2 | 27.0 | 5.0 | 26.4 | 5.2 | 0.0008 | 0.0363 |
| PR | 1.6 | 0.8 | 28.6 | 3.0 | 5.3 | 2.7 | 6.3 | 2.6 | <0.0001 | 0.0001 | |
| ICP | VP | 8.9 | 1.9 | 20.3 | 5.2 | 24.5 | 5.2 | 31.5 | 11.3 | 0.0269 | 0.0003 |
| PR | 9.1 | 1.7 | 29.4 | 3.6 | 12.6 | 2.0 | 13.0 | 1.8 | 0.0017 | 0.0003 | |
| SagP | VP | 7.0 | 0.6 | 22.1 | 3.9 | 27.7 | 4.9 | 27.6 | 5.1 | 0.0090 | 0.0170 |
| PR | 7.1 | 0.6 | 28.9 | 2.7 | 10.6 | 2.2 | 10.0 | 1.7 | <0.0001 | <0.0001 | |
| CPP | VP | 52.6 | 4.0 | 37.1 | 4.4 | 58.0 | 4.8 | 50.7 | 5.7 | 0.0127 | 0.0070 |
| PR | 51.6 | 3.1 | 29.1 | 5.4 | 48.6 | 5.2 | 51.1 | 4.3 | 0.0020 | 0.0036 | |
| StO2cer | VP | 59.0 | 1.8 | 53.7 | 2.8 | 55.9 | 1.8 | 52.1 | 3.8 | 0.0916 | 0.1868 |
| PR | 57.3 | 2.1 | 50.6 | 2.7 | 57.4 | 1.7 | 58.1 | 1.8 | 0.0003 | 0.0011 | |
| SsvcO2 | VP | 59.6 | 4.3 | 49.5 | 4.9 | 62.7 | 7.1 | 60.6 | 6.1 | 0.0156 | 0.0064 |
| PR | 68.9 | 5.4 | 55.8 | 7.2 | 66.8 | 4.6 | 69.0 | 4.0 | 0.0188 | 0.0669 | |
| SsagO2 | VP | 36.8 | 4.5 | 29.7 | 2.3 | 40.6 | 5.4 | 33.7 | 4.7 | 0.1313 | 0.0385 |
| PR | 44.1 | 5.9 | 28.8 | 1.5 | 34.0 | 2.3 | 34.9 | 3.1 | 0.0299 | 0.1163 | |
| SvO2 | VP | 60.0 | 2.1 | 53.1 | 1.8 | 56.9 | 2.4 | 57.1 | 4.0 | 0.0646 | 0.2468 |
| PR | 57.0 | 4.0 | 58.5 | 3.7 | 57.0 | 3.3 | 59.6 | 3.1 | 0.8300 | 0.7794 | |
| StO2abd | VP | 55.4 | 1.0 | 55.4 | 0.9 | 55.1 | 0.9 | 55.3 | 1.0 | >0.9999 | 0.7261 |
| PR | 53.6 | 0.7 | 52.7 | 0.9 | 53.4 | 1.0 | 53.9 | 0.6 | 0.2248 | 0.5265 | |
| CBF rel ch | VP | 4.2 | 30.9 | −4.6 | 22.9 | −5.0 | 19.1 | −13.5 | 21.2 | 0.8349 | 0.9787 |
| PR | 42.5 | 16.8 | −2.2 | 14.1 | 15.3 | 13.0 | 8.9 | 13.9 | 0.0522 | 0.1162 | |
| S100β | VP | 702.5 | 138.5 | 536.7 | 93.8 | 425.5 | 62.0 | 516.2 | 114.5 | 0.1895 | 0.0968 |
| PR | 648.2 | 150.0 | 428.9 | 46.9 | 530.0 | 110.3 | 544.6 | 121.9 | 0.0958 | 0.3278 | |
| . | . | BL2 . | T1 . | T2 . | T3 . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | BL2–T1 . | T1–T2 . | ||
| CVP | VP | 1.0 | 1.5 | 22.4 | 4.2 | 27.0 | 5.0 | 26.4 | 5.2 | 0.0008 | 0.0363 |
| PR | 1.6 | 0.8 | 28.6 | 3.0 | 5.3 | 2.7 | 6.3 | 2.6 | <0.0001 | 0.0001 | |
| ICP | VP | 8.9 | 1.9 | 20.3 | 5.2 | 24.5 | 5.2 | 31.5 | 11.3 | 0.0269 | 0.0003 |
| PR | 9.1 | 1.7 | 29.4 | 3.6 | 12.6 | 2.0 | 13.0 | 1.8 | 0.0017 | 0.0003 | |
| SagP | VP | 7.0 | 0.6 | 22.1 | 3.9 | 27.7 | 4.9 | 27.6 | 5.1 | 0.0090 | 0.0170 |
| PR | 7.1 | 0.6 | 28.9 | 2.7 | 10.6 | 2.2 | 10.0 | 1.7 | <0.0001 | <0.0001 | |
| CPP | VP | 52.6 | 4.0 | 37.1 | 4.4 | 58.0 | 4.8 | 50.7 | 5.7 | 0.0127 | 0.0070 |
| PR | 51.6 | 3.1 | 29.1 | 5.4 | 48.6 | 5.2 | 51.1 | 4.3 | 0.0020 | 0.0036 | |
| StO2cer | VP | 59.0 | 1.8 | 53.7 | 2.8 | 55.9 | 1.8 | 52.1 | 3.8 | 0.0916 | 0.1868 |
| PR | 57.3 | 2.1 | 50.6 | 2.7 | 57.4 | 1.7 | 58.1 | 1.8 | 0.0003 | 0.0011 | |
| SsvcO2 | VP | 59.6 | 4.3 | 49.5 | 4.9 | 62.7 | 7.1 | 60.6 | 6.1 | 0.0156 | 0.0064 |
| PR | 68.9 | 5.4 | 55.8 | 7.2 | 66.8 | 4.6 | 69.0 | 4.0 | 0.0188 | 0.0669 | |
| SsagO2 | VP | 36.8 | 4.5 | 29.7 | 2.3 | 40.6 | 5.4 | 33.7 | 4.7 | 0.1313 | 0.0385 |
| PR | 44.1 | 5.9 | 28.8 | 1.5 | 34.0 | 2.3 | 34.9 | 3.1 | 0.0299 | 0.1163 | |
| SvO2 | VP | 60.0 | 2.1 | 53.1 | 1.8 | 56.9 | 2.4 | 57.1 | 4.0 | 0.0646 | 0.2468 |
| PR | 57.0 | 4.0 | 58.5 | 3.7 | 57.0 | 3.3 | 59.6 | 3.1 | 0.8300 | 0.7794 | |
| StO2abd | VP | 55.4 | 1.0 | 55.4 | 0.9 | 55.1 | 0.9 | 55.3 | 1.0 | >0.9999 | 0.7261 |
| PR | 53.6 | 0.7 | 52.7 | 0.9 | 53.4 | 1.0 | 53.9 | 0.6 | 0.2248 | 0.5265 | |
| CBF rel ch | VP | 4.2 | 30.9 | −4.6 | 22.9 | −5.0 | 19.1 | −13.5 | 21.2 | 0.8349 | 0.9787 |
| PR | 42.5 | 16.8 | −2.2 | 14.1 | 15.3 | 13.0 | 8.9 | 13.9 | 0.0522 | 0.1162 | |
| S100β | VP | 702.5 | 138.5 | 536.7 | 93.8 | 425.5 | 62.0 | 516.2 | 114.5 | 0.1895 | 0.0968 |
| PR | 648.2 | 150.0 | 428.9 | 46.9 | 530.0 | 110.3 | 544.6 | 121.9 | 0.0958 | 0.3278 | |
Pressures (mmHg), saturations (%) and S100β (pg/ml).
CBF rel ch: stepwise cerebral blood flow changes; CPP: cerebral perfusion pressure; CVP: central venous pressure; ICP: intracranial pressure; PR: partial relief of congestion; S100β: glial protein S100β; SagP: sagittal sinus pressure; SsagO2: sagittal sinus oxygen saturation; SsvcO2: superior vena cava oxygen saturation; StO2abd: inferior tissue oxygen saturation index; StO2cer: cerebral tissue oxygen saturation index; SvO2: mixed venous oxygen saturation; VP: vasopressor treatment.
Compilation of means and SEMs at various time points, with P-values from paired t-tests of differences between specified time points
| . | . | BL2 . | T1 . | T2 . | T3 . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | BL2–T1 . | T1–T2 . | ||
| CVP | VP | 1.0 | 1.5 | 22.4 | 4.2 | 27.0 | 5.0 | 26.4 | 5.2 | 0.0008 | 0.0363 |
| PR | 1.6 | 0.8 | 28.6 | 3.0 | 5.3 | 2.7 | 6.3 | 2.6 | <0.0001 | 0.0001 | |
| ICP | VP | 8.9 | 1.9 | 20.3 | 5.2 | 24.5 | 5.2 | 31.5 | 11.3 | 0.0269 | 0.0003 |
| PR | 9.1 | 1.7 | 29.4 | 3.6 | 12.6 | 2.0 | 13.0 | 1.8 | 0.0017 | 0.0003 | |
| SagP | VP | 7.0 | 0.6 | 22.1 | 3.9 | 27.7 | 4.9 | 27.6 | 5.1 | 0.0090 | 0.0170 |
| PR | 7.1 | 0.6 | 28.9 | 2.7 | 10.6 | 2.2 | 10.0 | 1.7 | <0.0001 | <0.0001 | |
| CPP | VP | 52.6 | 4.0 | 37.1 | 4.4 | 58.0 | 4.8 | 50.7 | 5.7 | 0.0127 | 0.0070 |
| PR | 51.6 | 3.1 | 29.1 | 5.4 | 48.6 | 5.2 | 51.1 | 4.3 | 0.0020 | 0.0036 | |
| StO2cer | VP | 59.0 | 1.8 | 53.7 | 2.8 | 55.9 | 1.8 | 52.1 | 3.8 | 0.0916 | 0.1868 |
| PR | 57.3 | 2.1 | 50.6 | 2.7 | 57.4 | 1.7 | 58.1 | 1.8 | 0.0003 | 0.0011 | |
| SsvcO2 | VP | 59.6 | 4.3 | 49.5 | 4.9 | 62.7 | 7.1 | 60.6 | 6.1 | 0.0156 | 0.0064 |
| PR | 68.9 | 5.4 | 55.8 | 7.2 | 66.8 | 4.6 | 69.0 | 4.0 | 0.0188 | 0.0669 | |
| SsagO2 | VP | 36.8 | 4.5 | 29.7 | 2.3 | 40.6 | 5.4 | 33.7 | 4.7 | 0.1313 | 0.0385 |
| PR | 44.1 | 5.9 | 28.8 | 1.5 | 34.0 | 2.3 | 34.9 | 3.1 | 0.0299 | 0.1163 | |
| SvO2 | VP | 60.0 | 2.1 | 53.1 | 1.8 | 56.9 | 2.4 | 57.1 | 4.0 | 0.0646 | 0.2468 |
| PR | 57.0 | 4.0 | 58.5 | 3.7 | 57.0 | 3.3 | 59.6 | 3.1 | 0.8300 | 0.7794 | |
| StO2abd | VP | 55.4 | 1.0 | 55.4 | 0.9 | 55.1 | 0.9 | 55.3 | 1.0 | >0.9999 | 0.7261 |
| PR | 53.6 | 0.7 | 52.7 | 0.9 | 53.4 | 1.0 | 53.9 | 0.6 | 0.2248 | 0.5265 | |
| CBF rel ch | VP | 4.2 | 30.9 | −4.6 | 22.9 | −5.0 | 19.1 | −13.5 | 21.2 | 0.8349 | 0.9787 |
| PR | 42.5 | 16.8 | −2.2 | 14.1 | 15.3 | 13.0 | 8.9 | 13.9 | 0.0522 | 0.1162 | |
| S100β | VP | 702.5 | 138.5 | 536.7 | 93.8 | 425.5 | 62.0 | 516.2 | 114.5 | 0.1895 | 0.0968 |
| PR | 648.2 | 150.0 | 428.9 | 46.9 | 530.0 | 110.3 | 544.6 | 121.9 | 0.0958 | 0.3278 | |
| . | . | BL2 . | T1 . | T2 . | T3 . | P . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | Mean . | SEM . | BL2–T1 . | T1–T2 . | ||
| CVP | VP | 1.0 | 1.5 | 22.4 | 4.2 | 27.0 | 5.0 | 26.4 | 5.2 | 0.0008 | 0.0363 |
| PR | 1.6 | 0.8 | 28.6 | 3.0 | 5.3 | 2.7 | 6.3 | 2.6 | <0.0001 | 0.0001 | |
| ICP | VP | 8.9 | 1.9 | 20.3 | 5.2 | 24.5 | 5.2 | 31.5 | 11.3 | 0.0269 | 0.0003 |
| PR | 9.1 | 1.7 | 29.4 | 3.6 | 12.6 | 2.0 | 13.0 | 1.8 | 0.0017 | 0.0003 | |
| SagP | VP | 7.0 | 0.6 | 22.1 | 3.9 | 27.7 | 4.9 | 27.6 | 5.1 | 0.0090 | 0.0170 |
| PR | 7.1 | 0.6 | 28.9 | 2.7 | 10.6 | 2.2 | 10.0 | 1.7 | <0.0001 | <0.0001 | |
| CPP | VP | 52.6 | 4.0 | 37.1 | 4.4 | 58.0 | 4.8 | 50.7 | 5.7 | 0.0127 | 0.0070 |
| PR | 51.6 | 3.1 | 29.1 | 5.4 | 48.6 | 5.2 | 51.1 | 4.3 | 0.0020 | 0.0036 | |
| StO2cer | VP | 59.0 | 1.8 | 53.7 | 2.8 | 55.9 | 1.8 | 52.1 | 3.8 | 0.0916 | 0.1868 |
| PR | 57.3 | 2.1 | 50.6 | 2.7 | 57.4 | 1.7 | 58.1 | 1.8 | 0.0003 | 0.0011 | |
| SsvcO2 | VP | 59.6 | 4.3 | 49.5 | 4.9 | 62.7 | 7.1 | 60.6 | 6.1 | 0.0156 | 0.0064 |
| PR | 68.9 | 5.4 | 55.8 | 7.2 | 66.8 | 4.6 | 69.0 | 4.0 | 0.0188 | 0.0669 | |
| SsagO2 | VP | 36.8 | 4.5 | 29.7 | 2.3 | 40.6 | 5.4 | 33.7 | 4.7 | 0.1313 | 0.0385 |
| PR | 44.1 | 5.9 | 28.8 | 1.5 | 34.0 | 2.3 | 34.9 | 3.1 | 0.0299 | 0.1163 | |
| SvO2 | VP | 60.0 | 2.1 | 53.1 | 1.8 | 56.9 | 2.4 | 57.1 | 4.0 | 0.0646 | 0.2468 |
| PR | 57.0 | 4.0 | 58.5 | 3.7 | 57.0 | 3.3 | 59.6 | 3.1 | 0.8300 | 0.7794 | |
| StO2abd | VP | 55.4 | 1.0 | 55.4 | 0.9 | 55.1 | 0.9 | 55.3 | 1.0 | >0.9999 | 0.7261 |
| PR | 53.6 | 0.7 | 52.7 | 0.9 | 53.4 | 1.0 | 53.9 | 0.6 | 0.2248 | 0.5265 | |
| CBF rel ch | VP | 4.2 | 30.9 | −4.6 | 22.9 | −5.0 | 19.1 | −13.5 | 21.2 | 0.8349 | 0.9787 |
| PR | 42.5 | 16.8 | −2.2 | 14.1 | 15.3 | 13.0 | 8.9 | 13.9 | 0.0522 | 0.1162 | |
| S100β | VP | 702.5 | 138.5 | 536.7 | 93.8 | 425.5 | 62.0 | 516.2 | 114.5 | 0.1895 | 0.0968 |
| PR | 648.2 | 150.0 | 428.9 | 46.9 | 530.0 | 110.3 | 544.6 | 121.9 | 0.0958 | 0.3278 | |
Pressures (mmHg), saturations (%) and S100β (pg/ml).
CBF rel ch: stepwise cerebral blood flow changes; CPP: cerebral perfusion pressure; CVP: central venous pressure; ICP: intracranial pressure; PR: partial relief of congestion; S100β: glial protein S100β; SagP: sagittal sinus pressure; SsagO2: sagittal sinus oxygen saturation; SsvcO2: superior vena cava oxygen saturation; StO2abd: inferior tissue oxygen saturation index; StO2cer: cerebral tissue oxygen saturation index; SvO2: mixed venous oxygen saturation; VP: vasopressor treatment.
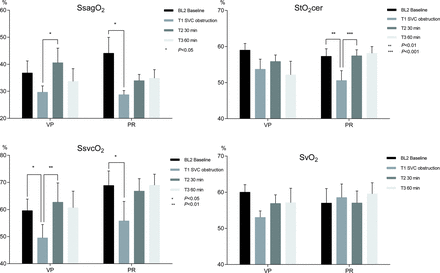
Sagittal sinus oxygen saturation, regional cerebral tissue oxygenation saturation, SVC oxygen saturation and mixed venous oxygen saturation in pigs during 34°C experimental CPB, with SVC obstruction and interventions in the form of VP respective PR of congestion. Means and SEMs. StO2cer, SsvcO2 and SsagO2 were significantly lowered by SVC obstruction (all pigs). Both intervention techniques increased all three variables (StO2cer: VP P = 0.1868, PR P = 0.0011; SsvcO2: VP P = 0.0064, PR P = 0.0669; SsagO2: VP P = 0.0385, PR P = 0.1163), whereas the SvO2 results were inconsistent. PR: partial relief of congestion; SsagO2: sagittal sinus oxygen saturation; SsvcO2: SVC oxygen saturation; StO2cer: cerebral tissue oxygen saturation index; SvO2: mixed venous oxygen saturation; VP: vasopressor treatment.
Superior venous obstruction causes a reversible decrease in cerebral perfusion pressure
The 75% obstruction of the SVC caused an almost 20-fold increase in CVP from an average of 1.3 to 25.5 mmHg (all pigs, P < 0.0001). The ICP and SagP values were closely correlated (data not shown). To avoid missing values related to ICP catheter obstruction at a few time points, the SagP was used for CPP calculations (CPP =MAP–SagP).
The CPP decreased by 19.0 mmHg on average (all pigs) in response to SVC obstruction (52.1 ± 2.5 vs 33.1 ± 3.5 mmHg, P < 0.0001) accompanied by a tendency towards a decrease in CBF by 28.2% points on average (P = 0.2233).
The CPP was effectively restored by both treatments, increasing by 21 mmHg in the VP (37.1 ± 4.3 vs 58.0 ± 4.8 mmHg; P = 0.0070) and by 19 mmHg on average in the PR group (29.1 ± 5.4 vs 48.6 ± 5.2 mmHg; P = 0.0036) (Fig. 2).
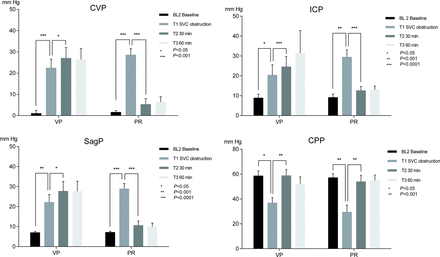
CVP, ICP, SagP and CPP in pigs during 34°C experimental CPB, with SVC obstruction and interventions in the form of VP respective PR of congestion. Means and SEMs. SVC obstruction caused a significant increase in CVP, ICP and SagP, and a corresponding decrease in the CPP. Both intervention methods restored CPP to baseline levels. Elevated pressures persisted in the VP group. CPP: cerebral perfusion pressure; CVP: central venous pressure; ICP: intracranial pressure; PR: partial relief of congestion; SagP: sagittal sinus pressure; VP: vasopressor treatment.
Superior venous congestion impairs regional perfusion
The SVC obstruction produced a prompt decrease in SsvcO2 by 18% on average (all pigs) (64.2 ± 3.5 vs 52.7 ± 4.3%; P = 0.0004), consistent with impaired perfusion in the superior venous territory. The regional venous saturations decreased significantly for both sagittal sinus oxygen saturation (SsagO2; 40.4 ± 3.7 vs 29.2 ± 1.3%; P = 0.0063) and the NIRS index StO2cer (58.1 ± 1.4 vs 52.1 ± 1.9%; P = 0.0007). The laser-Doppler CBF measurements did not reveal any significant differences in cortical CBF (Fig. 3).
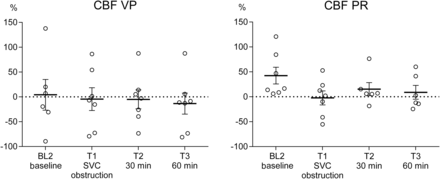
Stepwise cerebral blood flow (CBF) changes, measured by the cortical laser-Doppler technique in pigs during 34°C experimental CPB, with SVC obstruction and interventions in the form of VP respective PR of congestion. Means and SEMs. CBF decreased insignificantly upon SVC obstruction and was restored by PR alone (P = 0.1162). CBF: cerebral blood flow; PR: partial relief of congestion; VP: vasopressor treatment.
The mixed venous oxygen saturation was not significantly altered by the SVC obstruction, and similarly, there were no significant changes in the data from the abdominal NIRS probes in response to the experiment.
Both vasopressor treatment and partial obstruction relief improve short-term perfusion
The CPP was restored to approximate baseline levels in response to elevated MAP in the VP group and decreased CVP in the PR group.
In the VP group, the SsvcO2 increased significantly upon intervention (49.5 ± 4.9 vs 62.7 ± 7.1%; P = 0.0064), as did the SsagO2 (29.7 ± 2.3 vs 40.6 ± 5.4%; P = 0.0385). A similar tendency was observed for StO2cer (53.7 ± 2.8 vs 55.9 ± 1.8%; P = 0.1868).
The PR group pattern was similar to the VP; however, it was not statistically significant for SsvcO2 (55.8 ± 7.2 vs 66.8 ± 4.6%, P = 0.0669) or SsagO2 (28.8 ± 1.5 vs 34.0 ± 2.3%; P = 0.1163), while there was a statistically significant increase in StO2cer upon intervention (50.6 ± 2.7 vs 57.4 ± 1.7%; P = 0.0011).
The S100β concentrations in sagittal sinus venous blood were sampled and measured for each time point, without revealing any peaks in response to obstruction or intervention in either group. No significant difference in relative or absolute changes in S100β concentrations was seen between the groups, or between adjacent time points within the groups across the experiment (Fig. 4).
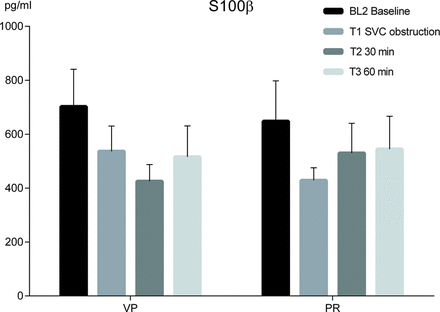
Release of glial cell S100β protein to sagittal sinus blood in pigs during 34oC experimental CPB, with SVC obstruction and interventions in the form of VP respective PR of congestion. Means and SEMs. Analyses were inconsistent, both regarding the SVC obstruction and the intervention strategies. However, the S100β concentration 30 min after intervention was 25% higher in PR than in VP (P = 0.4249), possibly due to facilitated cerebral venous outflow in PR. PR: partial relief of congestion; VP: vasopressor treatment.
DISCUSSION
This study demonstrates that 75% partial obstruction of the SVC flow elevates the CVP to a level where the ICP is clearly affected and the CPP is reduced. To our knowledge, this is the first controlled study to simultaneously describe the intracranial propagation of venous pressure with resulting CPP reduction and perfusion abnormalities upon SVC obstruction. Furthermore, we demonstrate the effects of improved drainage as well as vasopressor treatment with resulting improvement in CPP and perfusion by both strategies. However, it should be emphasized that the study covers only a short period of time, and that short-term improvements by means of restored CPP do not exclude subsequent effects of the intracranial hypertension.
The diagnosis of venous congestion may be difficult, since impaired SVC drainage may escape detection due to redirection of venous flow [13] and the SvO2 can be left largely unaffected despite congestion [10]. Standard monitoring of the CVP has been recommended based on accidental findings of intracranial hypertension due to venous obstruction during CPB [6]. However, the association between the degree of obstruction, the blood and ICP levels and the effects on perfusion remains to be outlined.
Venous congestion may cause impairment in cerebral perfusion [12], but clinical and experimental data also indicate that moderate obstruction may be relatively well tolerated at least in some cases [10, 13, 15]. Based on previous experience, we reduced the SVC flow by 75% in order to elevate the CVP to ∼25 mmHg [13]. The effects on the regional venous and NIRS saturations unanimously indicated a rapid impairment of the cerebral perfusion in response to obstruction. This is in agreement with the findings by Sakamoto et al. that 50% obstruction was well tolerated while total clamping caused cerebral desaturation. At the 50% obstruction level, the CVP increased to ∼ 20 mmHg, while no decline in cerebral perfusion was reported [10]. Similar observations of preserved cerebral oxygenation at a CVP of 20 mmHg were reported from clamping of the left SVC in patients with congenital heart disease, and a CVP of <30 mmHg was proposed to be safe in that context [15].
The absence of significantly decreased CBF in response to SVC obstruction is similar to the observations made by Sakamoto et al., where the CBF was unaffected by 50% obstruction and merely moderately reduced by total obstruction [10]. Thus, it is possible that there is a critical level of dangerously high CVP with respect to cerebral perfusion between 20 and 30 mmHg, and that the mechanism does not involve severe reductions in the CBF. Possibly, other drainage routes are recruited at elevated venous pressures [10, 13].
In contrast to the venous desaturation in the SVC territory, there were no effects on the inferior venous drainage or SvO2 during the SVC obstruction. The abdominal NIRS recordings were congruent with the SvO2, suggesting stable conditions in the inferior circulation. Possibly, NIRS may be a future tool for splanchnic monitoring during CPB, but further refinement and validation are warranted.
In the present protocol, the PR arm aimed at restoring the CPP by decreasing the CVP, representing the ideal scenario of successful repositioning of the SVC cannula, raising the operating bed, vacuum-assisted drainage or a combination thereof [16]. The VP arm aimed at shifting the arterial blood pressure upwards within the autoregulatory range of the brain to improve the CPP. However, the latter strategy cannot be assumed a priori efficacious, since common vasopressors have been described to reduce cerebral oxygenation and CBF dose-dependently in volunteers [17–19]. Nevertheless, the effects of the two treatments appeared largely comparable regarding the CPP and cerebral perfusion measures. Thus, it proved possible to restore the CPP despite venous congestion by making the arterial pressure rise supersede the venous and ICPs, and there were no signs of adverse effects of the vasopressors in terms of further impairment of the cerebral perfusion.
The autoregulatory pressure range during CPB is not entirely clear. Being modulated by factors such as haematocrit, carbon dioxide tension and temperature, it can also differ between populations [20, 21]. Haugen et al. [22] described that a CPP of 50 mmHg preserved the cerebral metabolism in pigs, while a CPP of 20–25 mmHg produced manifest ischaemia. It is not known whether venous congestion affects the cerebral autoregulation per se, but the present findings of moderate impairment by reduction of the CPP to 33 mmHg on average are compatible with the findings by Haugen et al. [22]. The characterization of the ischemic response at this particular CPP level is beyond the scope of the present study.
One limitation of our study is the lack of reliable markers of cerebral damage in the acute phase. The glial protein S100β used in the present study is a widely used marker of cerebral ischaemic injury [23]. After CPB, increased leakage of S100β has been associated with cognitive dysfunction [23, 24], but the prognostic value of S100β remains controversial due to extracerebral sources and the issue of appropriate time window for sampling [24]. The present data did not reveal any changes in S100β concentration indicating any structural short-term damage caused by either the intervention or by the obstruction per se. However, the possibility of Type 2 error should not be disregarded, and injurious effects of the SVC obstruction and/or interventions cannot be entirely excluded, particularly not long-term, by the present study.
In conclusion, the present study provides evidence that reduced CPP per se is a major determinant of cerebral perfusion during the acute phase of SVC congestion. Furthermore, short-term improvement can be obtained by either improved venous drainage or vasopressor treatment to restore the CPP.
Funding
This work was supported by the Erik, Karin and Gösta Selander's Foundation.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We wish to express our gratitude to Agneta Ronéus, Karin Fagerbrink, Maria Swälas and Birgitta Linder at the Department of Medical Sciences, Clinical Physiology, Uppsala University for invaluable assistance with the experiments.
REFERENCES
- ischemia
- cardiopulmonary bypass
- vasoconstrictor agents
- superior vena cava syndrome
- superior vena cava
- hemoglobin
- objective (goal)
- intracranial pressure
- neuroglia
- spectrum analysis
- suidae
- brain
- cerebral perfusion pressure
- sagittal sinus
- cerebral blood flow measurement
- oxygen saturation measurement
- experimental treatment
- venous blood gases
- blood outflow
- cerebral perfusion
- fluid flow




