-
PDF
- Split View
-
Views
-
Cite
Cite
Toru Kimura, Masayoshi Inoue, Yoshihisa Kadota, Hiroyuki Shiono, Yasushi Shintani, Tomoyuki Nakagiri, Soichiro Funaki, Noriyoshi Sawabata, Masato Minami, Meinoshin Okumura, The oncological feasibility and limitations of video-assisted thoracoscopic thymectomy for early-stage thymomas, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages e214–e218, https://doi.org/10.1093/ejcts/ezt305
Close - Share Icon Share
Abstract
Although video-assisted thoracoscopic thymectomy (VATS-Tx) for thymoma has been introduced, its oncological outcome remains unclear. Our institutional experience with early-stage thymoma was retrospectively reviewed to evaluate the oncological feasibility of thoracoscopic thymectomy.
A retrospective review consisting of 74 patients with Masaoka Stage I and II thymomas who had undergone thymectomy was performed. Forty-five patients underwent thoracoscopic thymectomy, while 29 underwent thymectomy through the open sternotomy approach. The clinical factors associated with the surgical outcome, including tumour recurrence, were investigated.
Neither operative death nor major postoperative complications were observed. The median intraoperative blood loss and operative time of thoracoscopic thymectomy were 50 ml and 180 min, respectively. Among the patients with thymomas >5 cm, the number of patients with operative time >4 h was 9 of 26 (34.6%) in the thoracoscopic thymectomy and 1 of 21 (4.8%) in the open sternotomy groups. Pleural recurrence was observed in 3 (6.7%) patients with thymoma >5 cm only in the thoracoscopic thymectomy group. Tumour capsule injury by manipulation during the operation was recorded in 2 of these 3 patients. In 2 of the 3 cases who had tumours with cystic portions on computed tomography, a tumour capsule injury occurred due to manipulation during thoracoscopic thymectomy.
VATS-Tx for early-stage thymomas is feasible, while the indications should be carefully considered in patients with large or cystic tumours. The conventional open sternotomy approach could be recommended in patients with thymomas >5 cm to avoid capsule injury.
INTRODUCTION
Thymoma is a malignant epithelial neoplasm arising from the thymus gland, and complete resection is the cornerstone of its treatment. Video-assisted thoracoscopic surgery now represents a useful approach for the management of non-invasive mediastinal tumours, including thymoma. We previously reported the use of video-assisted thoracoscopic thymectomy (VATS-Tx) [1–3], along with its technical usefulness. However, the oncological outcome of VATS-Tx for thymoma has rarely been reported because of its low-grade malignant behaviour. The oncological feasibility and the limitations of VATS-Tx for early-stage thymomas were evaluated.
MATERIALS AND METHODS
Consecutive cases of early-stage thymoma (Masaoka Stage I and II) who underwent thymectomy at Osaka University Hospital between July 2002 and December 2009 were retrospectively reviewed in order to evaluate the oncological feasibility of VATS-Tx. A total of 74 patients who underwent thymectomy for Stage I or II thymoma were extracted from 109 cases with all stages of thymomas (67.9%). We planned to use the VATS-Tx approach for Masaoka Stage I or II thymomas, except for the tumours that were expected to be difficult to manipulate with a thoracoscopic approach. In detail, tumours >6 cm in diameter or adjacent to the brachiocephalic vein were excluded from VATS-Tx based on the practical decision made by the attending surgeons. The decision on the surgical approach was made in a non-randomized way. An extended thymectomy including resection of the surrounding fat tissue was performed in patients who had associated myasthenia gravis (MG) by either a VATS-Tx or thymectomy through an open sternotomy (Open-Tx) approach [4]. Neither the preoperative diagnosis of MG nor the severity of its symptoms affected our decision to select the surgical approach. A thoracoscopic extended thymectomy was completed using bilateral access with an optional cervical incision [5]. In cases with non-myasthenic thymoma diagnosed as WHO Type A, AB or B1 with low-grade behaviour by computed tomography (CT) finding with sufficient surgical margin, we have occasionally indicated thoracoscopic hemithymectomy according to the attending physicians' decision, recently. In the VATS-Tx approach, the resected thymus with tumour was removed through one of the access windows with a plastic retrieval bag. Open-Tx was performed with a conventional median sternotomy. Neither postoperative adjuvant chemotherapy nor radiation therapy was given in completely resected Stage I–II thymoma as an institutional policy. The patients' characteristics, including the presence of associated MG and its Myasthenia Gravis Foundation of America (MGFA) clinical classification, CT findings of the thymoma with tumour diameter, Masaoka stage, surgical approach, intraoperative blood loss, operative time and oncological outcome, were collected from the patients' records. The mean postoperative follow-up period was 53.7 ± 24.5 (mean ± standard deviation) and 49.6 ± 25.3 months in the VATS-Tx and Open-Tx groups, respectively. The study was approved by our institutional review board, which waived the need for patient consent. The statistical calculations were performed using the Student t-test, Fisher's exact test and the χ2 test to compare the data for VATS-Tx with that for Open-Tx groups.
RESULTS
The patients' characteristics are summarized in Table 1. Among the patients who underwent Open-Tx, the bilateral or unilateral thoracic cavity was approached through the mediastinum in 26 patients because of mediastinal pleural invasion or adhesion of the tumour. Injury of the tumour capsule during intraoperative manipulation was recorded in 3 (6.7%) patients in VATS-Tx, but there were no events of capsule injury in Open-Tx. Regarding the medication for MG, anticholinesterase agents were used in 13 and 5 patients in the VATS-Tx and Open-Tx groups, respectively. Corticosteroid was given in 4 and 3 patients in the VATS-Tx and Open-Tx groups, respectively. One patient in the Open-Tx group had preoperatively received intravenous immunoglobulin treatment, and the operation was performed after the improvement of MG symptoms. Neither plasmapheresis nor intravenous immunoglobulin treatment was performed in all MG patients during the intraoperative or postoperative periods. Temporary exacerbation of myasthenia symptoms was postoperatively observed in 2 patients in VATS-Tx and in 3 in Open-Tx, but anticholinesterase and corticosteroid medication could improve the symptoms in these 5 patients.
| . | VATS-Tx (N = 45) . | Open-Tx (N = 29) . | P-value . |
|---|---|---|---|
| Gender | |||
| Male | 19 | 10 | 0.5056 |
| Female | 26 | 19 | |
| Age | 35–81 | 32–75 | 0.4154 |
| Median (IR) | 55 (47–60) | 59 (50–65) | |
| Average ± SD | 55 ± 12 | 57 ± 12 | |
| Masaoka stage | |||
| Stage I | 41 | 17 | 0.0025 |
| Stage II | 4 | 12 | |
| WHO classification | |||
| A | 5 | 1 | 0.9954 |
| AB | 16 | 11 | |
| B1 | 11 | 13 | |
| B2 | 11 | 2 | |
| B3 | 2 | 2 | |
| Tumour size (cm) | 0.8–10 | 2.1–14 | 0.0028 |
| Median (IR) | 5.0 (3.5–5.5) | 6.0 (5.0–7.5) | |
| Average ± SD | 4.8 ± 2.1 | 6.5 ± 2.5 | |
| Cystic/necrotic findings in CT | |||
| Yes | 3 | 6 | 0.1414 |
| No | 42 | 23 | |
| Myasthenia gravis | |||
| Yes | 14 | 9 | 0.9945 |
| No | 31 | 20 | |
| MGFA classification | |||
| Class I | 3 | 4 | 0.2794 |
| Class IIa | 5 | 4 | |
| Class IIb | 6 | 1 | |
| Operative time (min) | 50–545 | 105–280 | 0.1381 |
| Median (IR) | 180 (117–250) | 165 (130–185) | |
| Average ± SD | 197 ± 102 | 167 ± 42 | |
| Blood loss (ml) | 0–700 | 0–1000 | 0.0002 |
| Median (IR) | 50 (20–135) | 220 (150–300) | |
| Average ± SD | 105 ± 133 | 262 ± 205 | |
| Hospital stay (days) | 4–64 | 8–62 | 0.0714 |
| Median (IR) | 11 (9–14) | 15 (12–17) | |
| Average ± SD | 14 ± 9 | 19 ± 13 | |
| . | VATS-Tx (N = 45) . | Open-Tx (N = 29) . | P-value . |
|---|---|---|---|
| Gender | |||
| Male | 19 | 10 | 0.5056 |
| Female | 26 | 19 | |
| Age | 35–81 | 32–75 | 0.4154 |
| Median (IR) | 55 (47–60) | 59 (50–65) | |
| Average ± SD | 55 ± 12 | 57 ± 12 | |
| Masaoka stage | |||
| Stage I | 41 | 17 | 0.0025 |
| Stage II | 4 | 12 | |
| WHO classification | |||
| A | 5 | 1 | 0.9954 |
| AB | 16 | 11 | |
| B1 | 11 | 13 | |
| B2 | 11 | 2 | |
| B3 | 2 | 2 | |
| Tumour size (cm) | 0.8–10 | 2.1–14 | 0.0028 |
| Median (IR) | 5.0 (3.5–5.5) | 6.0 (5.0–7.5) | |
| Average ± SD | 4.8 ± 2.1 | 6.5 ± 2.5 | |
| Cystic/necrotic findings in CT | |||
| Yes | 3 | 6 | 0.1414 |
| No | 42 | 23 | |
| Myasthenia gravis | |||
| Yes | 14 | 9 | 0.9945 |
| No | 31 | 20 | |
| MGFA classification | |||
| Class I | 3 | 4 | 0.2794 |
| Class IIa | 5 | 4 | |
| Class IIb | 6 | 1 | |
| Operative time (min) | 50–545 | 105–280 | 0.1381 |
| Median (IR) | 180 (117–250) | 165 (130–185) | |
| Average ± SD | 197 ± 102 | 167 ± 42 | |
| Blood loss (ml) | 0–700 | 0–1000 | 0.0002 |
| Median (IR) | 50 (20–135) | 220 (150–300) | |
| Average ± SD | 105 ± 133 | 262 ± 205 | |
| Hospital stay (days) | 4–64 | 8–62 | 0.0714 |
| Median (IR) | 11 (9–14) | 15 (12–17) | |
| Average ± SD | 14 ± 9 | 19 ± 13 | |
Hospital stay is calculated from the operation.
VATS-Tx: video-assisted thoracoscopic thymectomy; Open-Tx: thymectomy through open sternotomy; IR: inter-quartile range; SD: standard deviation; WHO: World Health Organization; MGFA: Myasthenia Gravis Foundation of America.
| . | VATS-Tx (N = 45) . | Open-Tx (N = 29) . | P-value . |
|---|---|---|---|
| Gender | |||
| Male | 19 | 10 | 0.5056 |
| Female | 26 | 19 | |
| Age | 35–81 | 32–75 | 0.4154 |
| Median (IR) | 55 (47–60) | 59 (50–65) | |
| Average ± SD | 55 ± 12 | 57 ± 12 | |
| Masaoka stage | |||
| Stage I | 41 | 17 | 0.0025 |
| Stage II | 4 | 12 | |
| WHO classification | |||
| A | 5 | 1 | 0.9954 |
| AB | 16 | 11 | |
| B1 | 11 | 13 | |
| B2 | 11 | 2 | |
| B3 | 2 | 2 | |
| Tumour size (cm) | 0.8–10 | 2.1–14 | 0.0028 |
| Median (IR) | 5.0 (3.5–5.5) | 6.0 (5.0–7.5) | |
| Average ± SD | 4.8 ± 2.1 | 6.5 ± 2.5 | |
| Cystic/necrotic findings in CT | |||
| Yes | 3 | 6 | 0.1414 |
| No | 42 | 23 | |
| Myasthenia gravis | |||
| Yes | 14 | 9 | 0.9945 |
| No | 31 | 20 | |
| MGFA classification | |||
| Class I | 3 | 4 | 0.2794 |
| Class IIa | 5 | 4 | |
| Class IIb | 6 | 1 | |
| Operative time (min) | 50–545 | 105–280 | 0.1381 |
| Median (IR) | 180 (117–250) | 165 (130–185) | |
| Average ± SD | 197 ± 102 | 167 ± 42 | |
| Blood loss (ml) | 0–700 | 0–1000 | 0.0002 |
| Median (IR) | 50 (20–135) | 220 (150–300) | |
| Average ± SD | 105 ± 133 | 262 ± 205 | |
| Hospital stay (days) | 4–64 | 8–62 | 0.0714 |
| Median (IR) | 11 (9–14) | 15 (12–17) | |
| Average ± SD | 14 ± 9 | 19 ± 13 | |
| . | VATS-Tx (N = 45) . | Open-Tx (N = 29) . | P-value . |
|---|---|---|---|
| Gender | |||
| Male | 19 | 10 | 0.5056 |
| Female | 26 | 19 | |
| Age | 35–81 | 32–75 | 0.4154 |
| Median (IR) | 55 (47–60) | 59 (50–65) | |
| Average ± SD | 55 ± 12 | 57 ± 12 | |
| Masaoka stage | |||
| Stage I | 41 | 17 | 0.0025 |
| Stage II | 4 | 12 | |
| WHO classification | |||
| A | 5 | 1 | 0.9954 |
| AB | 16 | 11 | |
| B1 | 11 | 13 | |
| B2 | 11 | 2 | |
| B3 | 2 | 2 | |
| Tumour size (cm) | 0.8–10 | 2.1–14 | 0.0028 |
| Median (IR) | 5.0 (3.5–5.5) | 6.0 (5.0–7.5) | |
| Average ± SD | 4.8 ± 2.1 | 6.5 ± 2.5 | |
| Cystic/necrotic findings in CT | |||
| Yes | 3 | 6 | 0.1414 |
| No | 42 | 23 | |
| Myasthenia gravis | |||
| Yes | 14 | 9 | 0.9945 |
| No | 31 | 20 | |
| MGFA classification | |||
| Class I | 3 | 4 | 0.2794 |
| Class IIa | 5 | 4 | |
| Class IIb | 6 | 1 | |
| Operative time (min) | 50–545 | 105–280 | 0.1381 |
| Median (IR) | 180 (117–250) | 165 (130–185) | |
| Average ± SD | 197 ± 102 | 167 ± 42 | |
| Blood loss (ml) | 0–700 | 0–1000 | 0.0002 |
| Median (IR) | 50 (20–135) | 220 (150–300) | |
| Average ± SD | 105 ± 133 | 262 ± 205 | |
| Hospital stay (days) | 4–64 | 8–62 | 0.0714 |
| Median (IR) | 11 (9–14) | 15 (12–17) | |
| Average ± SD | 14 ± 9 | 19 ± 13 | |
Hospital stay is calculated from the operation.
VATS-Tx: video-assisted thoracoscopic thymectomy; Open-Tx: thymectomy through open sternotomy; IR: inter-quartile range; SD: standard deviation; WHO: World Health Organization; MGFA: Myasthenia Gravis Foundation of America.
No surgical mortality or major complications requiring additional treatment were seen in any patients of either group. As summarized in Table 1, the median intraoperative blood loss and operative time of VATS-Tx were 50 ml and 180 min, respectively. Because the surgical approach was selected with consideration of tumour size, it was significantly smaller in VATS-Tx (P = 0.0028) and the proportion of patients with Masaoka Stage I was significantly higher in VATS-Tx (P = 0.0025). The intraoperative blood loss was significantly lower in VATS-Tx, and there was no patient requiring transfusion during VATS-Tx. The median postoperative hospital stay was 11 days with VATS-Tx and 15 days with Open-Tx. Prolonged hospital stay was observed in some patients with MG due to medical management. The postoperative hospital stay among the patients without MG was significantly shorter in the VATS-Tx group (P = 0.0233, Table 2). All patients are alive as of the writing of this report, during the follow-up period.
| . | VATS-Tx . | Open-Tx . | P-value . |
|---|---|---|---|
| With MG | 9–64 | 13–62 | 0.3001 |
| Median (IR) | 14 (11–25) | 17 (14–33) | |
| Average ± SD | 20 ± 14 | 28 ± 20 | |
| Without MG | 4–33 | 8–30 | 0.0233 |
| Median (IR) | 11 (9–13) | 14 (12–17) | |
| Average ± SD | 11 ± 5 | 15 ± 5 |
| . | VATS-Tx . | Open-Tx . | P-value . |
|---|---|---|---|
| With MG | 9–64 | 13–62 | 0.3001 |
| Median (IR) | 14 (11–25) | 17 (14–33) | |
| Average ± SD | 20 ± 14 | 28 ± 20 | |
| Without MG | 4–33 | 8–30 | 0.0233 |
| Median (IR) | 11 (9–13) | 14 (12–17) | |
| Average ± SD | 11 ± 5 | 15 ± 5 |
MG: myasthenia gravis; VATS-Tx: video-assisted thoracoscopic thymectomy; Open-Tx: thymectomy through open sternotomy; IR: inter-quartile range; SD: standard deviation.
| . | VATS-Tx . | Open-Tx . | P-value . |
|---|---|---|---|
| With MG | 9–64 | 13–62 | 0.3001 |
| Median (IR) | 14 (11–25) | 17 (14–33) | |
| Average ± SD | 20 ± 14 | 28 ± 20 | |
| Without MG | 4–33 | 8–30 | 0.0233 |
| Median (IR) | 11 (9–13) | 14 (12–17) | |
| Average ± SD | 11 ± 5 | 15 ± 5 |
| . | VATS-Tx . | Open-Tx . | P-value . |
|---|---|---|---|
| With MG | 9–64 | 13–62 | 0.3001 |
| Median (IR) | 14 (11–25) | 17 (14–33) | |
| Average ± SD | 20 ± 14 | 28 ± 20 | |
| Without MG | 4–33 | 8–30 | 0.0233 |
| Median (IR) | 11 (9–13) | 14 (12–17) | |
| Average ± SD | 11 ± 5 | 15 ± 5 |
MG: myasthenia gravis; VATS-Tx: video-assisted thoracoscopic thymectomy; Open-Tx: thymectomy through open sternotomy; IR: inter-quartile range; SD: standard deviation.
With regard to the relationship between tumour size and operative time, a proportion of patients with large tumours required a prolonged operative time with VATS-Tx (Fig. 1). Among the patients with thymomas >5 cm, the number of patients with operative time >4 h was 9 of 26 (34.6%) in the VATS-Tx group and 1 of 21 (4.8%) in the Open-Tx group (P = 0.0152), though we found no significant relationship between tumour size and operative time as a whole.
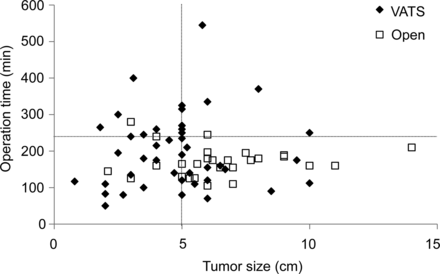
The relationship between the tumour size and length of the operation. Among the subset with thymomas >5 cm, the percentage of patients with operative time >4 h was significantly higher in the VATS-Tx than that in the Open-Tx group.
Three (6.7%) patients in the VATS-Tx group had tumours with a cystic or necrotic appearance on CT examination, and intraoperative injury of the tumour capsule occurred in 1 of these patients (Fig. 2). Hypotonic mediastinal and pleural lavage treatment was added after extirpation of the thymoma, and no local recurrence was observed for 4 years in this case. Although the Open-Tx group had 6 patients with cystic tumours (20.1%), no case of capsule injury was observed during the operation.
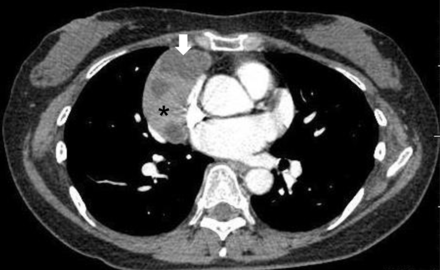
Contrast-enhanced chest CT findings of a Masaoka Stage I and WHO Type B2 thymoma in a 56-year old female patient. This is the CT scan of a representative case with a cystic or necrotic component in the mediastinal tumour. CT Hounsfield units of this area (arrow) and solid component (star) are 10 and 80, respectively. The capsule of this cystic portion was injured during VATS-Tx. This patient has had no relapse for 4 years after the operation.
Among the patients who underwent a hemithymectomy, no local recurrence has been observed in the remaining thymic tissue, while 1 patient with VATS-Tx had a second thymoma in the remaining thymic tissue. In the VATS-Tx group, 3 (6.7%) patients had recurrent disease with pleural dissemination (Table 3). The tumour size in these 3 patients was >5 cm, including 2 patients with Masaoka Stage I/WHO Type AB thymoma, which is relatively low grade (Table 3). The CT findings for these 3 recurrent cases are shown in Fig. 3. In the 2 patients with Stage I disease, the capsules of the tumours were injured during VATS-Tx. Although these cases did not have either a cystic nor a necrotic portion on CT examination, one showed a small area of low attenuation (Fig. 3).
| Age/gender . | Stage . | WHO type . | Size (cm) . | Operative time (min) . | Blood loss (ml) . | Time to recurrence (months) . |
|---|---|---|---|---|---|---|
| 56/Fa | I | AB | 6.5 | 160 | 130 | 24.4 |
| 35/Fa | I | AB | 5 | 260 | 120 | 60 |
| 36/Mb | II | B2 | 5.8 | 545 | 190 | 24.1 |
| Age/gender . | Stage . | WHO type . | Size (cm) . | Operative time (min) . | Blood loss (ml) . | Time to recurrence (months) . |
|---|---|---|---|---|---|---|
| 56/Fa | I | AB | 6.5 | 160 | 130 | 24.4 |
| 35/Fa | I | AB | 5 | 260 | 120 | 60 |
| 36/Mb | II | B2 | 5.8 | 545 | 190 | 24.1 |
Stage: Masaoka stage.
aCases with the capsule injury during operation.
bThe case with the longest operation time among all patients.
| Age/gender . | Stage . | WHO type . | Size (cm) . | Operative time (min) . | Blood loss (ml) . | Time to recurrence (months) . |
|---|---|---|---|---|---|---|
| 56/Fa | I | AB | 6.5 | 160 | 130 | 24.4 |
| 35/Fa | I | AB | 5 | 260 | 120 | 60 |
| 36/Mb | II | B2 | 5.8 | 545 | 190 | 24.1 |
| Age/gender . | Stage . | WHO type . | Size (cm) . | Operative time (min) . | Blood loss (ml) . | Time to recurrence (months) . |
|---|---|---|---|---|---|---|
| 56/Fa | I | AB | 6.5 | 160 | 130 | 24.4 |
| 35/Fa | I | AB | 5 | 260 | 120 | 60 |
| 36/Mb | II | B2 | 5.8 | 545 | 190 | 24.1 |
Stage: Masaoka stage.
aCases with the capsule injury during operation.
bThe case with the longest operation time among all patients.
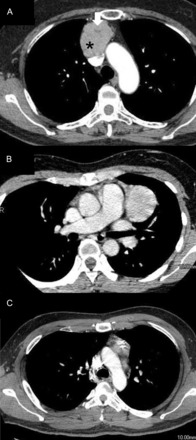
Contrast-enhanced chest CT findings of the pleural recurrent thymoma cases listed in Table 3 following VATS-Tx. (A) Masaoka Stage I and WHO Type AB thymoma in a 56-year old female. The CT image shows an anterior mediastinal mass with a small area of low attenuation (arrow). CT Hounsfield units of this low attenuation area (arrow) and other components (star) are 27 and 100, respectively. Capsule injury was found during surgery. (B) Masaoka Stage I and WHO Type AB thymoma in a 35-year old male. The CT image shows a homogenous, anterior mediastinal mass. The capsule was injured during surgery. (C) Masaoka Stage II and WHO Type B2 thymoma associated with MG in a 36-year old male. The CT image shows a mass in the anterior portion of the mediastinum.
The remaining patient with Stage II had the longest operative time (545 min) of all patients in the present study (Table 3). All recurrent patients were treated with pleural resection of the disseminated lesions through an open thoracotomy with video-assistance. With follow-up examinations performed at 6, 14 and 44 months after reoperation, these 3 patients are alive, with no evidence of relapse.
DISCUSSION
Median sternotomy has been the standard approach for the surgical resection of thymomas. In recent years, thoracoscopic surgery to excise mediastinal thymic lesions has been reported to be an effective procedure even for the resection of early-stage thymoma [6–9]. To the best of our knowledge, the present investigation is one of the largest series that has assessed the perioperative and oncological outcomes of VATS-Tx for early-stage thymoma.
Pennathur et al. [8] presented their results from a comparative study of Open-Tx (n = 22) vs VATS-Tx (n = 18) for Masaoka Stage I or II thymomas and noted a significant difference in the hospital stay, favouring the VATS-Tx group. Cheng et al. prospectively compared the outcomes of VATS-Tx (n = 12) and Open-Tx (n = 10) in patients with Stage II thymomas and concluded that the amount of intraoperative blood loss was lower when the VATS-Tx approach was used [6]. Bachmann et al. [10], Meyer et al. [11] and Liu et al. [12] also reported the efficacy and feasibility of thoracoscopic thymectomy for MG with or without thymoma. The shorter hospital stay in the VATS-Tx group in patients without MG might suggest a contribution to early postoperative recovery. Given the benefit of minimal invasiveness, we suppose that the resection of early-stage thymomas by the VATS-Tx currently meets the technical requirements for thymoma treatment.
However, the oncological outcome following VATS-Tx for thymoma remains unclear. The recurrence rates of patients following complete resection of the thymoma, stratified by Masaoka Stage I and II, were relatively low (0–5 and 4.1–21%, respectively), with a typical pattern of disease recurrence being pleural dissemination [13–17]. In addition, our previous study showed that the WHO histological classification system reflects the oncological nature of thymomas, and that Types A, AB and B1 tumours have a less malignant nature than Type B2 and B3 tumours in terms of prognosis and tumour recurrence [18, 19]. In the present study, the disease recurrence with pleural dissemination that was observed in the 2 patients with Masaoka Stage I/WHO Type AB thymoma might have been caused by intraoperative capsule injury of the tumours. Therefore, en bloc resection of the tumour with surrounding thymus and fat tissue is mandatory using either VATS-Tx or the Open-Tx approach, even for early-stage and low-grade thymomas. Additionally, one case developed a second thymoma in remaining thymic tissue after hemithymectomy with VATS-Tx. Thus, long-term follow-up for secondary thymoma or local recurrence is required in patients who do not undergo an extended thymectomy.
Some authors have raised concerns about minimally invasive approaches for thymectomy, particularly the possibility of capsular rupture and the subsequent risk of pleural spread [20, 21]. However, no previous study has focused on postoperative recurrence after VATS-Tx, along with the technical risk of capsule injury. In the present study, 3 patients had tumour capsule injuries, and 2 of these 3 patients had postoperative recurrence with pleural dissemination. Lucchi et al. [20] reported 2 cases of pleural recurrence after VATS-Tx, although the authors did not document the intraoperative findings of these patients. They speculated that the VATS approach could expose the patients to a higher risk of pleural recurrence, because the incision of the mediastinal pleura might lead to the seeding of tumour cells into the thoracic cavity [20]. However, a mediastinal pleural incision was made in 89.7% of the patients in the Open-Tx group in the present study, and no pleural recurrence was observed in these patients. This observation suggests that the risk of pleural relapse, particularly after complete resection of early-stage thymoma, might be caused by manipulating the tumour rather than by opening the pleural cavity.
With regard to the technical difficulty in handling the tumour, Girard et al. [22] stated that VATS is contraindicated for large tumours to avoid the intraoperative spread of tumour cells in the pleural space. However, as of yet, no consensus has been reached on the indications for VATS-Tx regarding tumour size [7, 8, 22]. In the present series, all of the patients with recurrence had thymomas 5 cm or larger in diameter, and over one-third of patients with thymomas >5 cm required a more-than 4-h operation when using the VATS-Tx approach. Intraoperative manipulation during VATS-Tx may increase the technical difficulty and the risk of capsule injury. Thus, we currently choose Open-Tx in patients with thymomas >5 cm in principle.
Furthermore, the CT findings of the tumours were also assessed in order to evaluate their vulnerability. CT images of thymomas occasionally showed low-density areas, suggesting cystic or necrotic changes [23]. In 1 of the 3 cases who had tumours with such CT findings, a tumour capsule injury occurred due to manipulation during VATS-Tx. The presence of the cystic portion was also confirmed macroscopically after resection. In addition, 1 of the 3 patients with postoperative recurrence had a tumour with a small area of low attenuation on CT. Thus, thymomas with a low-density area on CT images may have a risk of capsule injury during intraoperative manipulation and require particularly careful handling when VATS-Tx is indicated. We recommend conversion to Open-Tx when frequent manipulation of the tumour is required, particularly for tumours with cystic changes.
The present study has several limitations. First, a non-randomized retrospective study might include the potential selection bias of the treating surgeon. In addition, the study sample size was small, and only a few outcome events were observed. Although all patients, including the recurrent cases, in this series are still alive, further long-term follow-up is necessary to identify the final oncological outcomes, because thymoma is an indolent disease. In addition, further multicentre studies with higher volumes are necessary to obtain the definitive conclusion of this study.
In conclusion, although VATS-Tx is oncologically feasible for non-invasive thymomas as long as en bloc resection of the tumour is achieved, careful attention is needed for large or cystic thymomas, which have a potential risk of intraoperative capsule injury and subsequent pleural dissemination.
Conflict of interest: none declared.




