-
PDF
- Split View
-
Views
-
Cite
Cite
Michal Nozdrzykowski, Christian D. Etz, Maximilian Luehr, Jens Garbade, Martin Misfeld, Michael A. Borger, Friedrich-Wilhelm Mohr, Optimal treatment for patients with chronic Stanford type B aortic dissection: endovascularly, surgically or both?, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages e165–e174, https://doi.org/10.1093/ejcts/ezt291
Close - Share Icon Share
Abstract
Patients with chronic Stanford type B aortic dissections (TBAD) are traditionally treated medically, but some of the affected thoracic and thoracoabdominal aortic segments progress to large aneurysms with a significant risk of rupture. The purpose of this study is to retrospectively evaluate, with an ‘all-comers’ approach, the survival and the outcome of patients following thoracic endovascular aortic repair (TEVAR) or conventional open surgery for chronic TBAD as a first-line therapy or a secondary option after failed medical treatment.
Between January 2000 and May 2010, 80 consecutive patients (59 males, median age 63, inter-quartile range (IQR) 55–69) suffering from chronic TBAD were treated at our institution. Thirty-three were treated medically (Group A, median age: 65, IQR: 58.5–71.5), 32 received TEVAR (Group B, median age: 62, IQR: 54–67.5) and 15 patients underwent conventional open surgery (Group C, median age: 61, IQR: 54–66). The median follow-up was 42 months (range: 0.1–124.7) and 100% complete.
There were no significant differences with regard to age, gender and associated comorbidities between the treatment groups. The overall hospital mortality for chronic TBAD was 6.3% (n = 5); in-hospital mortalities for Groups A, B and C were 3.0, 6.2 and 13.4%, respectively. The incidence of major complications, such as paraplegia, malperfusion, renal failure and cardiac arrhythmia, did not significantly differ between the three groups. Postoperative stroke occurred more often after conventional open surgery (Group C: 13.3%; P = 0.07). Reintervention for TBAD pathology was required in Groups A, B and C in 12.1, 28.1 and 0%, respectively (P = 0.03). Secondary open surgery post-TEVAR was required in 7 cases (21.8%) with no postoperative paraplegia.
Open surgery for extensive thoracic and thoracoabdominal repair in chronic TBAD may be performed with acceptable early and mid-term outcomes. TEVAR for aortic complications in patients with chronic dissection may be successfully performed as a first-stage procedure in order to stabilize the patient and serve as a ‘bridge’ to secondary open surgery. However, close surveillance is mandatory for the timely detection of aneurysm enlargement, malperfusion or impending rupture after TEVAR.
INTRODUCTION
In contrast to acute type A aortic dissection, the optimal management of type B aortic dissection (TBAD) remains controversial. A recent expert consensus and a recommendation by the International Registry of Aortic Dissection (IRAD) investigators suggested a conservative approach to address acute, but uncomplicated—TBADs [1, 2]. However, the question is: does an uncomplicated TBAD actually exist?
In the acute phase, medical management on the intensive care unit (ICU) aims for immediate anti-impulse therapy to effectively control blood pressure, heart rate (often applied as a rule of thumb aiming for an invasive mean arterial blood pressure of 60 mmHg, and a target heart rate of 60 bpm) and pain, to decrease aortic wall stress. Conservative acute medical therapy also aims to reduce pending secondary adverse events, such as extension of the intimal tear and the false lumen (potentially resulting in an acute malperfusion syndrome converting acute, yet uncomplicated, into a complicated TBAD), retrograde type A dissection or acute rupture. However, in the presence of malperfusion, intractable pain or other severe complications, conventional open surgery or thoracic endovascular aortic repair (TEVAR) becomes necessary [1–3].
The purpose of this study is to evaluate the survival and the outcome of patients following TEVAR or conventional open surgery for complicated chronic TBAD as a first-line therapy or secondary procedure after failed medical therapy.
PATIENTS AND METHODS
In an ‘all-comers’ approach, we evaluated 80 consecutive patients between January 2000 and May 2010, treated for either primarily uncomplicated (n = 33) or complicated (n = 47) chronic TBAD at our institution. The median patient age was 63 years (IQR: 55–69) and 21 (26.2%) were females. Four patients (5%) were previously diagnosed with Marfan's syndrome (n = 4).
All patients were treated medically on admission; however, the optimal management strategy for patients suffering from chronic TBAD was discussed on an individual basis by an interdisciplinary ‘aortic’ team consisting of a cardiothoracic surgeon, a vascular surgeon, a diagnostic radiologist and an interventional cardiologist.
Thirty-three patients were initially treated conservatively (Group A) by medical (antihypertensive/anti-impulse) therapy and close haemodynamic monitoring at our ICU, while 47 received either TEVAR (Group B; n = 32) or conventional open surgery (Group C; n = 15). Demographics and comorbidities of all patients presenting with chronic TBAD are summarized in Table 1.
| . | Overall (n = 80) . | Medical (Group A; n = 33) . | TEVAR (Group B; n = 32) . | Open surgery (Group C; n = 15) . |
|---|---|---|---|---|
| Age (years; median; IQR) | 63 (55–69) | 65 (58.5–71.5) | 62 (54–67.5) | 61 (54.0–66.0) |
| Female | 21 (26.3%) | 7 (21.2%) | 9 (28.1%) | 5 (33.3%) |
| Hypertension | 79 (98.8%) | 32 (97.0%) | 32 (100%) | 15 (100%) |
| CHD | 17 (21.3%) | 9 (27.3%) | 6 (18.8%) | 2 (13.3%) |
| Marfan’s syndrome | 4 (5.0%) | 2 (6.1%) | 0 (0.0%) | 2 (13.3%) |
| COPD | 16 (20.0%) | 7 (21.2%) | 7 (21.9%) | 2 (13.3%) |
| IDDM | 16 (20.0%) | 7 (21.2%) | 9 (28.1%) | 0 (0.0%) |
| Obesity (BMI ≥ 30) | 33 (41.3%) | 9 (27.3%) | 18 (56.3%) | 6 (40.0%) |
| Renal insufficiency | 28 (35.0%) | 11 (33.3%) | 13 (40.6%) | 4 (26.7%) |
| Previous neurological dysfunction | ||||
| Paraplegia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Stroke | 8 (10.0%) | 4 (12.1%) | 2 (6.2%) | 2 (13.4%) |
| Complicated dissection | 37 (46.3%) | 3 (9.1%)* | 19 (59.4%) | 15 (100%) |
| Aortic size (mm; median; IQR) | 48.0 (40.0–58.0) | 45 (36.5–50.5) | 44.5 (39.0–54.0) | 59.0 (53.0–60)*** |
| Aneurysma | 53 (66.2%) | 18 (54.5%) | 21 (65.6%) | 14 (93.3%)** |
| Thrombosis of false lumen (number of patients) | ||||
| Partial | 31 (38.8%) | 16 (48.5.3%) | 9 (28.1%) | 6 (40.0%) |
| Complete | 12 (15.0%) | 5 (15.2%) | 6 (18.8%) | 1 (6.7%) |
| Previous cardio-aortic surgery | 22 (27.5%) | (36.4%) | 6 (18.8%) | 4 (26.7%) |
| . | Overall (n = 80) . | Medical (Group A; n = 33) . | TEVAR (Group B; n = 32) . | Open surgery (Group C; n = 15) . |
|---|---|---|---|---|
| Age (years; median; IQR) | 63 (55–69) | 65 (58.5–71.5) | 62 (54–67.5) | 61 (54.0–66.0) |
| Female | 21 (26.3%) | 7 (21.2%) | 9 (28.1%) | 5 (33.3%) |
| Hypertension | 79 (98.8%) | 32 (97.0%) | 32 (100%) | 15 (100%) |
| CHD | 17 (21.3%) | 9 (27.3%) | 6 (18.8%) | 2 (13.3%) |
| Marfan’s syndrome | 4 (5.0%) | 2 (6.1%) | 0 (0.0%) | 2 (13.3%) |
| COPD | 16 (20.0%) | 7 (21.2%) | 7 (21.9%) | 2 (13.3%) |
| IDDM | 16 (20.0%) | 7 (21.2%) | 9 (28.1%) | 0 (0.0%) |
| Obesity (BMI ≥ 30) | 33 (41.3%) | 9 (27.3%) | 18 (56.3%) | 6 (40.0%) |
| Renal insufficiency | 28 (35.0%) | 11 (33.3%) | 13 (40.6%) | 4 (26.7%) |
| Previous neurological dysfunction | ||||
| Paraplegia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Stroke | 8 (10.0%) | 4 (12.1%) | 2 (6.2%) | 2 (13.4%) |
| Complicated dissection | 37 (46.3%) | 3 (9.1%)* | 19 (59.4%) | 15 (100%) |
| Aortic size (mm; median; IQR) | 48.0 (40.0–58.0) | 45 (36.5–50.5) | 44.5 (39.0–54.0) | 59.0 (53.0–60)*** |
| Aneurysma | 53 (66.2%) | 18 (54.5%) | 21 (65.6%) | 14 (93.3%)** |
| Thrombosis of false lumen (number of patients) | ||||
| Partial | 31 (38.8%) | 16 (48.5.3%) | 9 (28.1%) | 6 (40.0%) |
| Complete | 12 (15.0%) | 5 (15.2%) | 6 (18.8%) | 1 (6.7%) |
| Previous cardio-aortic surgery | 22 (27.5%) | (36.4%) | 6 (18.8%) | 4 (26.7%) |
*P < 0.01; **P < 0.05 and ***P ≤ 0.02.
| . | Overall (n = 80) . | Medical (Group A; n = 33) . | TEVAR (Group B; n = 32) . | Open surgery (Group C; n = 15) . |
|---|---|---|---|---|
| Age (years; median; IQR) | 63 (55–69) | 65 (58.5–71.5) | 62 (54–67.5) | 61 (54.0–66.0) |
| Female | 21 (26.3%) | 7 (21.2%) | 9 (28.1%) | 5 (33.3%) |
| Hypertension | 79 (98.8%) | 32 (97.0%) | 32 (100%) | 15 (100%) |
| CHD | 17 (21.3%) | 9 (27.3%) | 6 (18.8%) | 2 (13.3%) |
| Marfan’s syndrome | 4 (5.0%) | 2 (6.1%) | 0 (0.0%) | 2 (13.3%) |
| COPD | 16 (20.0%) | 7 (21.2%) | 7 (21.9%) | 2 (13.3%) |
| IDDM | 16 (20.0%) | 7 (21.2%) | 9 (28.1%) | 0 (0.0%) |
| Obesity (BMI ≥ 30) | 33 (41.3%) | 9 (27.3%) | 18 (56.3%) | 6 (40.0%) |
| Renal insufficiency | 28 (35.0%) | 11 (33.3%) | 13 (40.6%) | 4 (26.7%) |
| Previous neurological dysfunction | ||||
| Paraplegia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Stroke | 8 (10.0%) | 4 (12.1%) | 2 (6.2%) | 2 (13.4%) |
| Complicated dissection | 37 (46.3%) | 3 (9.1%)* | 19 (59.4%) | 15 (100%) |
| Aortic size (mm; median; IQR) | 48.0 (40.0–58.0) | 45 (36.5–50.5) | 44.5 (39.0–54.0) | 59.0 (53.0–60)*** |
| Aneurysma | 53 (66.2%) | 18 (54.5%) | 21 (65.6%) | 14 (93.3%)** |
| Thrombosis of false lumen (number of patients) | ||||
| Partial | 31 (38.8%) | 16 (48.5.3%) | 9 (28.1%) | 6 (40.0%) |
| Complete | 12 (15.0%) | 5 (15.2%) | 6 (18.8%) | 1 (6.7%) |
| Previous cardio-aortic surgery | 22 (27.5%) | (36.4%) | 6 (18.8%) | 4 (26.7%) |
| . | Overall (n = 80) . | Medical (Group A; n = 33) . | TEVAR (Group B; n = 32) . | Open surgery (Group C; n = 15) . |
|---|---|---|---|---|
| Age (years; median; IQR) | 63 (55–69) | 65 (58.5–71.5) | 62 (54–67.5) | 61 (54.0–66.0) |
| Female | 21 (26.3%) | 7 (21.2%) | 9 (28.1%) | 5 (33.3%) |
| Hypertension | 79 (98.8%) | 32 (97.0%) | 32 (100%) | 15 (100%) |
| CHD | 17 (21.3%) | 9 (27.3%) | 6 (18.8%) | 2 (13.3%) |
| Marfan’s syndrome | 4 (5.0%) | 2 (6.1%) | 0 (0.0%) | 2 (13.3%) |
| COPD | 16 (20.0%) | 7 (21.2%) | 7 (21.9%) | 2 (13.3%) |
| IDDM | 16 (20.0%) | 7 (21.2%) | 9 (28.1%) | 0 (0.0%) |
| Obesity (BMI ≥ 30) | 33 (41.3%) | 9 (27.3%) | 18 (56.3%) | 6 (40.0%) |
| Renal insufficiency | 28 (35.0%) | 11 (33.3%) | 13 (40.6%) | 4 (26.7%) |
| Previous neurological dysfunction | ||||
| Paraplegia | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Stroke | 8 (10.0%) | 4 (12.1%) | 2 (6.2%) | 2 (13.4%) |
| Complicated dissection | 37 (46.3%) | 3 (9.1%)* | 19 (59.4%) | 15 (100%) |
| Aortic size (mm; median; IQR) | 48.0 (40.0–58.0) | 45 (36.5–50.5) | 44.5 (39.0–54.0) | 59.0 (53.0–60)*** |
| Aneurysma | 53 (66.2%) | 18 (54.5%) | 21 (65.6%) | 14 (93.3%)** |
| Thrombosis of false lumen (number of patients) | ||||
| Partial | 31 (38.8%) | 16 (48.5.3%) | 9 (28.1%) | 6 (40.0%) |
| Complete | 12 (15.0%) | 5 (15.2%) | 6 (18.8%) | 1 (6.7%) |
| Previous cardio-aortic surgery | 22 (27.5%) | (36.4%) | 6 (18.8%) | 4 (26.7%) |
*P < 0.01; **P < 0.05 and ***P ≤ 0.02.
Indications for TEVAR vs conventional open surgery
The decision for either TEVAR or conventional open surgery depended on the patient's clinical status on admission and on individually present complications with regard to the technical feasibility of the intended procedure. Patients who presented with more proximal aneurysms of the descending aorta—in which a sufficient proximal und distal landing zone allowed for endovascular stent-grafting—were more likely to be treated by TEVAR while those with extensive aneurysms (often involving the entire thoracic and/or thoracoabdominal aorta and its branches) were treated by conventional open surgery. All patients with connective tissue disease, such as Marfan's syndrome, were considered ineligible for TEVAR due to the increased risk of additional aortic wall injury, dissection or rupture triggered by the stent-graft device.
The indications for TEVAR in Group B (n = 32) were enlarged aortic diameter (median diameter = 44.5 mm, range: 39.0–54.0; n = 17), impending rupture (n = 6), end-organ malperfusion (n = 6) and recurrent pain (n = 3). The interval between the initial diagnosis of TBAD and TEVAR procedure was 3.2 months (median; IQR = 0.7–9.2). The indications for conventional open surgery (Group C) were progressive enlargement of the diameter of the dissected segment of the aorta (median diameter: 59.0 mm, range: 53.0–60.0 mm; n = 12), free rupture (n = 2) and impending rupture (n = 1). The median interval after initial diagnosis of TBAD and open surgery was 6.6 months (median; IQR = 1.6–39.5).
The time interval between TEVAR and conventional open surgery as a secondary procedure was 14.6 months (median; IQR = 5.8–46.8).
Subgroup analysis: primary urgent open repair vs emergency surgery post-TEVAR
The 15 patients who underwent primary conventional open surgery (Group C) and those with complications after TEVAR (n = 7; Group post-TEVAR) were analysed separately, as two subgroups according to the primary treatment strategy: (i) conventional open surgery vs (ii) TEVAR. The average patient age of these two subgroups was 61 ± 8 years (median; range 45–75 years) and 14 patients were male (63.6%). All of the patients had a history of hypertension, whereas a minority had a history of coronary artery disease (n = 4; 18.2%), smoking (n = 5; 22.7%) or chronic obstructive pulmonary disease (n = 5; 22.7%).
Five of the 22 patients (22.7%) had prior cardiac surgery before admission for chronic TBAD (4 vs 1; P = 1.0). Three patients had undergone aortic valve replacement (n = 1) or reconstruction (n = 2) with ascending aortic and partial (n = 2) or total (n = 1) arch replacement for acute type A aortic dissection. These patients had previously not undergone a second-stage procedure to address the remaining dissected descending aorta, and were therefore prone to develop a chronically dissected aneurysm of their downstream aorta. One patient had been operated on for aortic isthmus stenosis during childhood. Emergency surgery was required in 3 (20%; Group C) and 2 (28%; post-TEVAR group) patients, respectively.
Four patients suffered from neurological complications prior to open surgery: preoperative paraplegia (n = 2) was exclusively present in the post-TEVAR group: 1 patient had been suffering from delayed paraplegia due to a ruptured aneurysm while the other developed acute paraplegia after TEVAR (P = 0.09). In contrast, severe stroke with resulting hemiparesis was diagnosed prior to conventional open surgery in 2 patients of Group C (P = 1.0). The prevalence of preoperative renal failure was not significantly different between both subgroups (P = 0.63).
Demographics and comorbidities of both subgroups (Group C vs post-TEVAR group) are summarized in Table 2.
Preoperative patient demographics and comorbidities of the 22 patients treated by conventional surgery for complicated chronic TBAD as a first or secondary procedure
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value (open surgery vs TEVAR) . |
|---|---|---|---|
| Age (years; median; IQR) | 62 (54–69) | 61 (53–66) | 0.49 |
| Female | 5 (33.3%) | 3 (42.9%) | 1.00 |
| Hypertension | 15 (100%) | 7 (100%) | – |
| CHD | 2 (13.3%) | 2 (28.6%) | 0.56 |
| Marfan’s syndrome | 2 (13.3%) | 0 (0.0%) | 1.00 |
| COPD | 2 (13.3%) | 3 (42.9%) | 0.27 |
| IDDM | 0 (0.0%) | 1 (14.3%) | 0.31 |
| Obesity (BMI ≥ 30) | 6 (40.0%) | 7 (100%) | 0.02 |
| Renal insufficiency | 4 (26.7%) | 3 (42.9%) | 0.63 |
| Previous neurological dysfunction | |||
| Paraplegia | 0 (0.0%) | 2 (28.6%) | 0.09 |
| Stroke | 2 (13.3%) | 0 (0.0%) | 1.00 |
| Urgent/emergent operation | 3 (20.0%) | 2 (28.6%) | 1.00 |
| Aortic size (mm; median; IQR) | 59 (53–60) | 70 (58–83) | 0.09 |
| Thrombosis of false lumen (number of patients) | |||
| Partial | 6 (40%) | 2 (28.6%) | 0.60 |
| Complete | 1 (6.7%) | 2 (28.6%) | 0.22 |
| Previous cardio-aortic surgery | 4 (26.7%) | 1 (14.3%) | 1.00 |
| Interval after initial diagnosis and surgery (months) | 6.6 (1.6–39.5) | 35.3 (7.5–70.8) | 0.40 |
| Interval after initial diagnosis and TEVAR (months) | – | 1.7 (0.1–24.0) | |
| Interval after TEVAR and surgery (months) | – | 14.6 (5.8–46.8) | – |
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value (open surgery vs TEVAR) . |
|---|---|---|---|
| Age (years; median; IQR) | 62 (54–69) | 61 (53–66) | 0.49 |
| Female | 5 (33.3%) | 3 (42.9%) | 1.00 |
| Hypertension | 15 (100%) | 7 (100%) | – |
| CHD | 2 (13.3%) | 2 (28.6%) | 0.56 |
| Marfan’s syndrome | 2 (13.3%) | 0 (0.0%) | 1.00 |
| COPD | 2 (13.3%) | 3 (42.9%) | 0.27 |
| IDDM | 0 (0.0%) | 1 (14.3%) | 0.31 |
| Obesity (BMI ≥ 30) | 6 (40.0%) | 7 (100%) | 0.02 |
| Renal insufficiency | 4 (26.7%) | 3 (42.9%) | 0.63 |
| Previous neurological dysfunction | |||
| Paraplegia | 0 (0.0%) | 2 (28.6%) | 0.09 |
| Stroke | 2 (13.3%) | 0 (0.0%) | 1.00 |
| Urgent/emergent operation | 3 (20.0%) | 2 (28.6%) | 1.00 |
| Aortic size (mm; median; IQR) | 59 (53–60) | 70 (58–83) | 0.09 |
| Thrombosis of false lumen (number of patients) | |||
| Partial | 6 (40%) | 2 (28.6%) | 0.60 |
| Complete | 1 (6.7%) | 2 (28.6%) | 0.22 |
| Previous cardio-aortic surgery | 4 (26.7%) | 1 (14.3%) | 1.00 |
| Interval after initial diagnosis and surgery (months) | 6.6 (1.6–39.5) | 35.3 (7.5–70.8) | 0.40 |
| Interval after initial diagnosis and TEVAR (months) | – | 1.7 (0.1–24.0) | |
| Interval after TEVAR and surgery (months) | – | 14.6 (5.8–46.8) | – |
CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; IDDM: insulin dependent diabetes mellitus; TEVAR: thoracic endovascular aortic repair.
Preoperative patient demographics and comorbidities of the 22 patients treated by conventional surgery for complicated chronic TBAD as a first or secondary procedure
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value (open surgery vs TEVAR) . |
|---|---|---|---|
| Age (years; median; IQR) | 62 (54–69) | 61 (53–66) | 0.49 |
| Female | 5 (33.3%) | 3 (42.9%) | 1.00 |
| Hypertension | 15 (100%) | 7 (100%) | – |
| CHD | 2 (13.3%) | 2 (28.6%) | 0.56 |
| Marfan’s syndrome | 2 (13.3%) | 0 (0.0%) | 1.00 |
| COPD | 2 (13.3%) | 3 (42.9%) | 0.27 |
| IDDM | 0 (0.0%) | 1 (14.3%) | 0.31 |
| Obesity (BMI ≥ 30) | 6 (40.0%) | 7 (100%) | 0.02 |
| Renal insufficiency | 4 (26.7%) | 3 (42.9%) | 0.63 |
| Previous neurological dysfunction | |||
| Paraplegia | 0 (0.0%) | 2 (28.6%) | 0.09 |
| Stroke | 2 (13.3%) | 0 (0.0%) | 1.00 |
| Urgent/emergent operation | 3 (20.0%) | 2 (28.6%) | 1.00 |
| Aortic size (mm; median; IQR) | 59 (53–60) | 70 (58–83) | 0.09 |
| Thrombosis of false lumen (number of patients) | |||
| Partial | 6 (40%) | 2 (28.6%) | 0.60 |
| Complete | 1 (6.7%) | 2 (28.6%) | 0.22 |
| Previous cardio-aortic surgery | 4 (26.7%) | 1 (14.3%) | 1.00 |
| Interval after initial diagnosis and surgery (months) | 6.6 (1.6–39.5) | 35.3 (7.5–70.8) | 0.40 |
| Interval after initial diagnosis and TEVAR (months) | – | 1.7 (0.1–24.0) | |
| Interval after TEVAR and surgery (months) | – | 14.6 (5.8–46.8) | – |
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value (open surgery vs TEVAR) . |
|---|---|---|---|
| Age (years; median; IQR) | 62 (54–69) | 61 (53–66) | 0.49 |
| Female | 5 (33.3%) | 3 (42.9%) | 1.00 |
| Hypertension | 15 (100%) | 7 (100%) | – |
| CHD | 2 (13.3%) | 2 (28.6%) | 0.56 |
| Marfan’s syndrome | 2 (13.3%) | 0 (0.0%) | 1.00 |
| COPD | 2 (13.3%) | 3 (42.9%) | 0.27 |
| IDDM | 0 (0.0%) | 1 (14.3%) | 0.31 |
| Obesity (BMI ≥ 30) | 6 (40.0%) | 7 (100%) | 0.02 |
| Renal insufficiency | 4 (26.7%) | 3 (42.9%) | 0.63 |
| Previous neurological dysfunction | |||
| Paraplegia | 0 (0.0%) | 2 (28.6%) | 0.09 |
| Stroke | 2 (13.3%) | 0 (0.0%) | 1.00 |
| Urgent/emergent operation | 3 (20.0%) | 2 (28.6%) | 1.00 |
| Aortic size (mm; median; IQR) | 59 (53–60) | 70 (58–83) | 0.09 |
| Thrombosis of false lumen (number of patients) | |||
| Partial | 6 (40%) | 2 (28.6%) | 0.60 |
| Complete | 1 (6.7%) | 2 (28.6%) | 0.22 |
| Previous cardio-aortic surgery | 4 (26.7%) | 1 (14.3%) | 1.00 |
| Interval after initial diagnosis and surgery (months) | 6.6 (1.6–39.5) | 35.3 (7.5–70.8) | 0.40 |
| Interval after initial diagnosis and TEVAR (months) | – | 1.7 (0.1–24.0) | |
| Interval after TEVAR and surgery (months) | – | 14.6 (5.8–46.8) | – |
CHD: coronary heart disease; COPD: chronic obstructive pulmonary disease; IDDM: insulin dependent diabetes mellitus; TEVAR: thoracic endovascular aortic repair.
Surgical procedures
The details of our institutional surgical technique of addressing chronic TBAD have been described elsewhere [4]. In brief, surgical access was achieved via a left posterolateral thoracotomy in the majority of patients (n = 21). In 1 patient with retrograde type A dissection extending into the distal arch, a left posterolateral thoracotomy and a full sternotomy were performed. In this single case, the right axillary artery was cannulated to allow for selective cerebral perfusion (SCP) along with direct cannulation of the right atrium for venous drainage.
Distal hypothermic circulatory arrest was induced at profound hypothermia of 20°C by cross-clamping the lower descending aortic segment. However, intraoperative body core temperatures for distal circulatory arrest were gradually increased to moderate hypothermic conditions (Group C: 32°C, range 24–32; post-TEVAR group: 30°C, range: 20–33) in the past few years. This particularly correlated with the increased use of SCP and selective visceral perfusion.
In 12 patients, abdominal aortic replacement was performed using a quattrofurcated prosthesis and with additional selective visceral perfusion utilizing pruitt-like perfusion catheters maintaining a flow of 800–1000 ml/min at a perfusate temperature of 24–32°C.
Profound hypothermic circulatory arrest (HCA) without SCP—as the only neurological protection method—was utilized in 4 (Group C) and 3 (post-TEVAR group) patients, respectively. The head was packed externally in ice during HCA (n = 7).
In 1 patient previously treated by TEVAR of the distal arch and proximal descending aorta, a left posterolateral thoracotomy with cannulation of the right axillary artery and the femoral vein was utilized to allow for cold antegrade SCP (perfusate temperature of 22°C) for 20 min and lower body circulatory arrest at moderate hypothermia (30°C).
In all 7 patients of the post-TEVAR group, the stent-grafts were successfully removed and the progressively enlarged thoracic or thoracoabdominal aorta (median diameter = 70.0 mm, range: 58.0–83.0 mm) subsequently replaced by a Hemashield prosthesis (Marquet, Inc., Wayne, NJ, USA; median diameter 24 mm; range 20–30 mm).
Reimplantation of the coeliac trunk, the superior mesenteric artery and the renal arteries into the prosthetic graft was performed in 9 (58%), 7 (47%) and 6 (40%) cases of Group C and 3 (43%) cases in the post-TEVAR group.
Prior to the implementation of the collateral network concept in clinical practice, aortic segmental arteries were reimplanted into the Hemashield graft in 5 (Group C) and 3 (Group post-TEVAR), respectively [4]. Perioperative cerebrospinal fluid (CSF) drainage as an additional measure to minimize the risk of paraplegia was used up to 72 h postoperatively in 18 patients (Group C: n = 13; post-TEVAR group: n = 5). As a safety measure for spinal cord ischaemic damage, CSF drainage was performed to reach a target pressure in the subarachnoid space of 10 mmHg or below the ‘opening pressure’ (baseline measurement).
The intraoperative details are summarized in Table 3.
Intraoperative data of the 22 patients treated by conventional surgery for complicated chronic TBAD as a first or secondary procedure
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Mean temperature | 32°C (24–32) | 30°C (20–33) | 0.630 |
| HCA (number of patients) | 4 (26.7%) | 3 (42.9%) | 0.630 |
| HCA time (min) | 16.2 ± 3.8 | 18.3 ± 9.5 | 0.418 |
| Operation time (min) | 285 (210–365) | 295 (190–365) | 0.680 |
| Duration of CPB or LHB (min) | 144 (125–204) | 178 (96–228) | 0.783 |
| CSF drainage | 13 (86.7%) | 5 (71.4%) | 0.565 |
| Concomitant CABG | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Reimplanted visceral arteries (number of patients) | |||
| Coeliac | 9 (58.0%) | 3 (42.9%) | 0.652 |
| Superior mesenteric | 7 (46.7%) | 3 (42.9%) | 1.000 |
| Renal | 6 (40.0%) | 3 (42.9%) | 1.000 |
| Reimplanted segmental artery (number of patients) | 5 (33.3%) | 3 (42.9%) | 1.000 |
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Mean temperature | 32°C (24–32) | 30°C (20–33) | 0.630 |
| HCA (number of patients) | 4 (26.7%) | 3 (42.9%) | 0.630 |
| HCA time (min) | 16.2 ± 3.8 | 18.3 ± 9.5 | 0.418 |
| Operation time (min) | 285 (210–365) | 295 (190–365) | 0.680 |
| Duration of CPB or LHB (min) | 144 (125–204) | 178 (96–228) | 0.783 |
| CSF drainage | 13 (86.7%) | 5 (71.4%) | 0.565 |
| Concomitant CABG | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Reimplanted visceral arteries (number of patients) | |||
| Coeliac | 9 (58.0%) | 3 (42.9%) | 0.652 |
| Superior mesenteric | 7 (46.7%) | 3 (42.9%) | 1.000 |
| Renal | 6 (40.0%) | 3 (42.9%) | 1.000 |
| Reimplanted segmental artery (number of patients) | 5 (33.3%) | 3 (42.9%) | 1.000 |
HCA: hypothermic circulatory arrest; CPB: cardiopulmonary bypass; LHB: left heart bypass; CSF: cerebrospinal fluid; CABG: coronary artery bypass graft.
Intraoperative data of the 22 patients treated by conventional surgery for complicated chronic TBAD as a first or secondary procedure
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Mean temperature | 32°C (24–32) | 30°C (20–33) | 0.630 |
| HCA (number of patients) | 4 (26.7%) | 3 (42.9%) | 0.630 |
| HCA time (min) | 16.2 ± 3.8 | 18.3 ± 9.5 | 0.418 |
| Operation time (min) | 285 (210–365) | 295 (190–365) | 0.680 |
| Duration of CPB or LHB (min) | 144 (125–204) | 178 (96–228) | 0.783 |
| CSF drainage | 13 (86.7%) | 5 (71.4%) | 0.565 |
| Concomitant CABG | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Reimplanted visceral arteries (number of patients) | |||
| Coeliac | 9 (58.0%) | 3 (42.9%) | 0.652 |
| Superior mesenteric | 7 (46.7%) | 3 (42.9%) | 1.000 |
| Renal | 6 (40.0%) | 3 (42.9%) | 1.000 |
| Reimplanted segmental artery (number of patients) | 5 (33.3%) | 3 (42.9%) | 1.000 |
| . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Mean temperature | 32°C (24–32) | 30°C (20–33) | 0.630 |
| HCA (number of patients) | 4 (26.7%) | 3 (42.9%) | 0.630 |
| HCA time (min) | 16.2 ± 3.8 | 18.3 ± 9.5 | 0.418 |
| Operation time (min) | 285 (210–365) | 295 (190–365) | 0.680 |
| Duration of CPB or LHB (min) | 144 (125–204) | 178 (96–228) | 0.783 |
| CSF drainage | 13 (86.7%) | 5 (71.4%) | 0.565 |
| Concomitant CABG | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Reimplanted visceral arteries (number of patients) | |||
| Coeliac | 9 (58.0%) | 3 (42.9%) | 0.652 |
| Superior mesenteric | 7 (46.7%) | 3 (42.9%) | 1.000 |
| Renal | 6 (40.0%) | 3 (42.9%) | 1.000 |
| Reimplanted segmental artery (number of patients) | 5 (33.3%) | 3 (42.9%) | 1.000 |
HCA: hypothermic circulatory arrest; CPB: cardiopulmonary bypass; LHB: left heart bypass; CSF: cerebrospinal fluid; CABG: coronary artery bypass graft.
Study variables and definitions
The records of all patients were retrospectively reviewed. The follow-up was 100% complete, with a median follow-up time of 42 months (range 0.1–124.7). Aortic dissection was classified as type B according to the Stanford classification, that is, a dissection involving only the descending and/or throacoabdominal aorta with a dissection entry distally to the left subclavian artery. Diagnosis was based on conclusive medical history and imaging results (CT-angiography, magnetic resonance imaging). Dissections are considered chronic after 14 days following the first symptom onset or documentation of an intimal entry tear. The term complicated chronic TBAD was defined according to the recently published recommendations given by an expert consensus, that is, the presence of one or more of the following conditions: malperfusion syndrome (mesenteric ischaemia/infarction, or acute renal failure, limb ischaemia, spinal cord ischaemia), recurrent pain, refractory hypertension or covered or impending rupture [2].
Hospital mortality was defined according to current guidelines as death in hospital prior to discharge or within 30 days after surgery (regardless of location).
Stroke was defined as new-onset neurological deficit and/or evident brain injury visualized by CT-scan after TEVAR or surgery. Paraplegia was defined as permanent bilateral motor deficit of the lower extremities. However, we differentiated between early—immediately after the procedure—and delayed onset of paraplegia, which occurred after a period of intact motor function (e.g. after successful mobilization) during the further clinical course.
Renal failure was defined as an increase in serum creatinine >1.5 mg/dl, temporary (resolved by the time of discharge) or permanent need for haemodialysis.
Statistical methods
Frequencies are reported as percentages and continuous variables as median (inter-quartile range), respectively.
Treatment groups were compared by the Fisher's exact test for categorical variables and the Mann–Whitney U-test for continuous variables. Long-term survival was analysed with the log-rank test and depicted by a Kaplan–Meier chart. All tests were performed as two-sided at significance level 5%.
A search for the variables associated with time to death was carried out in two steps. First, a stepwise Cox regression with common risk and clinical parameters of the 80 patients was performed to identify the predictors of long-term mortality. Fifteen different variables were included: complicated dissection, previous cardiac surgery, male gender, age (>70 years), diabetes, obesity, hypertension, chronic obstructive pulmonary disease (COPD), renal failure, coronary artery disease, connective tissue disease, preoperative neurological complications, aortic aneurysm diameter, false lumen thrombosis, dissection entry site and emergency. All variables in the model (i.e. diameter, genetic defect and coronary artery disease (CAD)) were forced into a final model to get correct estimates for the odds ratios inclusive of confidence intervals.
Statistical analyses were performed using SPSS 14.0 and 19.0 for Windows (IBM SPSS Statistics, IBM Corp., 1989, 2011). Statistical support was supplied by the Department of Biostatistics of the University of Leipzig.
RESULTS
Hospital outcome—longevity in each group
The respective 30-day, 1- and 3-year mortality rates were 3, 3 and 9% for Group A (medical treatment), 6.2, 12.5, 12.5% for Group B (TEVAR) and 13.4, 26.7 and 26.7% for Group C (conventional open surgery) (Table 4). At the end of the study period, the survival rate for all 80 patients was 80% (n = 16).
Overall mortality of all 80 patients treated for chronic TBAD (Medical vs TEVAR vs Open surgery): both subgroups of patients treated by conventional surgery (Group C vs post-TEVAR group; n = 22) were additionally compared with regard to mortality
| Mortality . | Overall (n = 80) . | Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | P-value . |
|---|---|---|---|---|---|
| Hospital | 5 (6.3%) | 1 (3.0%) | 2 (6.2%) | 2 (13.4%) | 0.393 |
| 1 year | 9 (11.2%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.054 |
| 3 years | 11 (13.7%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.252 |
| Mortality | Post-TEVAR (n = 7) | Open surgery (n = 15) | P-value | ||
| Hospital | 1 (14.3%) | 2 (13.4%) | 1.000 | ||
| 1 year | 1 (14.3%) | 4 (26.7%) | 0.482 | ||
| 3 years | 1 (14.3%) | 4 (26.7%) | 0.482 |
| Mortality . | Overall (n = 80) . | Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | P-value . |
|---|---|---|---|---|---|
| Hospital | 5 (6.3%) | 1 (3.0%) | 2 (6.2%) | 2 (13.4%) | 0.393 |
| 1 year | 9 (11.2%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.054 |
| 3 years | 11 (13.7%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.252 |
| Mortality | Post-TEVAR (n = 7) | Open surgery (n = 15) | P-value | ||
| Hospital | 1 (14.3%) | 2 (13.4%) | 1.000 | ||
| 1 year | 1 (14.3%) | 4 (26.7%) | 0.482 | ||
| 3 years | 1 (14.3%) | 4 (26.7%) | 0.482 |
Overall mortality of all 80 patients treated for chronic TBAD (Medical vs TEVAR vs Open surgery): both subgroups of patients treated by conventional surgery (Group C vs post-TEVAR group; n = 22) were additionally compared with regard to mortality
| Mortality . | Overall (n = 80) . | Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | P-value . |
|---|---|---|---|---|---|
| Hospital | 5 (6.3%) | 1 (3.0%) | 2 (6.2%) | 2 (13.4%) | 0.393 |
| 1 year | 9 (11.2%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.054 |
| 3 years | 11 (13.7%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.252 |
| Mortality | Post-TEVAR (n = 7) | Open surgery (n = 15) | P-value | ||
| Hospital | 1 (14.3%) | 2 (13.4%) | 1.000 | ||
| 1 year | 1 (14.3%) | 4 (26.7%) | 0.482 | ||
| 3 years | 1 (14.3%) | 4 (26.7%) | 0.482 |
| Mortality . | Overall (n = 80) . | Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | P-value . |
|---|---|---|---|---|---|
| Hospital | 5 (6.3%) | 1 (3.0%) | 2 (6.2%) | 2 (13.4%) | 0.393 |
| 1 year | 9 (11.2%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.054 |
| 3 years | 11 (13.7%) | 1 (3.0%) | 4 (12.5%) | 4 (26.7%) | 0.252 |
| Mortality | Post-TEVAR (n = 7) | Open surgery (n = 15) | P-value | ||
| Hospital | 1 (14.3%) | 2 (13.4%) | 1.000 | ||
| 1 year | 1 (14.3%) | 4 (26.7%) | 0.482 | ||
| 3 years | 1 (14.3%) | 4 (26.7%) | 0.482 |
Multivariate analysis identified aortic aneurysm diameter (hazard ratio: 1.055; P < 0.001), emergency (hazard ratio: 6.847; P < 0.002), Marfan's syndrome (hazard ratio: 6.055; P = 0.034) and coronary artery disease (hazard ratio: 4.808; P = 0.009) to be independent predictors of mortality during the follow-up (Table 5).
Cox regression analysis of independent predictors of death during the follow-up
| Risk factor . | HR . | CI . | P-value . |
|---|---|---|---|
| Marfan’s syndrome | 6.055 | 1.143–32.063 | 0.034 |
| Aortic diameter | 1.055 | 1.026–1.086 | <0.001 |
| CAD | 4.808 | 1.489–15.523 | 0.009 |
| Emergency | 6.847 | 2.023–23.176 | <0.002 |
| Risk factor . | HR . | CI . | P-value . |
|---|---|---|---|
| Marfan’s syndrome | 6.055 | 1.143–32.063 | 0.034 |
| Aortic diameter | 1.055 | 1.026–1.086 | <0.001 |
| CAD | 4.808 | 1.489–15.523 | 0.009 |
| Emergency | 6.847 | 2.023–23.176 | <0.002 |
HR: hazard ratio; CI: confidence interval; CAD: coronary artery disease.
Cox regression analysis of independent predictors of death during the follow-up
| Risk factor . | HR . | CI . | P-value . |
|---|---|---|---|
| Marfan’s syndrome | 6.055 | 1.143–32.063 | 0.034 |
| Aortic diameter | 1.055 | 1.026–1.086 | <0.001 |
| CAD | 4.808 | 1.489–15.523 | 0.009 |
| Emergency | 6.847 | 2.023–23.176 | <0.002 |
| Risk factor . | HR . | CI . | P-value . |
|---|---|---|---|
| Marfan’s syndrome | 6.055 | 1.143–32.063 | 0.034 |
| Aortic diameter | 1.055 | 1.026–1.086 | <0.001 |
| CAD | 4.808 | 1.489–15.523 | 0.009 |
| Emergency | 6.847 | 2.023–23.176 | <0.002 |
HR: hazard ratio; CI: confidence interval; CAD: coronary artery disease.
The incidence of emergency procedures correlated with the incidence of complicated chronic TBAD, and therefore, was significantly higher in Groups B and C (P < 0.01). Emergency procedures were performed due to aortic rupture (n = 6), organ malperfusion/ischaemic complications (n = 2) or complications of TEVAR (n = 7).
Postoperative complications
All endovascular and open surgical procedures were technically successful. However, 4 patients died within 30 days, resulting in an in-hospital mortality of 8.5% for both groups (B vs C) (Table 4). Postoperative overall follow-up for Groups B and C was available for all patients and 100% complete: after a median time period of 39.4 months, 39 patients were still alive. Overall survival was 76.5% (26/34) at 1 year and 65.2% (15/23) at 3 years after interventional or surgical treatment. Figure 1A shows the survival curves estimated by the Kaplan–Meier method stratified by the management strategy of Groups B and C.
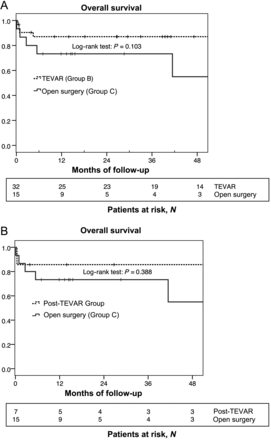
Kaplan–Meier survival curve stratified by the two in-hospital treatment strategies for complicated chronic TBAD. There is no significant difference in survival rates (log-rank test, P = 0.103) after 36 months (A). Kaplan–Meier survival curve stratified by the two groups who underwent open surgery as a first or secondary procedure. There is no significant difference in survival rates (log-rank test, P = 0.388) after 36 months (B).
The rate of major complications such as paraplegia, stroke, renal failure, malperfusion syndrome and cardiac arrhythmia did not significantly differ between the three groups (A vs B vs C). Postoperative complications for each group are listed in Table 6.
Postoperative complications and reintervention procedures according to initial management strategy (medical vs TEVAR vs open surgery)
| Treatment strategy . | Overall (n = 80) . | Uncomplicated TBAD . | Complicated TBAD . | P-value . | |
|---|---|---|---|---|---|
| Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | |||
| Paraplegia | 5 (5.0%) | 0 (0.0%) | 3 (9.3%) | 2 (13.3%) | 0.13 |
| Early | – | 2 (6.2%) | 1 (6.7%) | – | |
| Delayed | – | 1 (3.1%) | 1 (6.7%) | – | |
| Stroke | 3 (3.7%) | 0 (0.0%) | 1 (3.1%) | 2 (13.3%) | 0.07 |
| Renal failure | 21 (26.3%) | 5 (15.2%) | 11 (34.4%) | 5 (33.3%) | 0.16 |
| Malperfusion | 7 (8.8%) | 2 (6.2%) | 3 (9.3%) | 2 (13.3%) | 0.70 |
| Cardiac arrhythmia | 7 (8.8%) | 3 (9.1%) | 2 (6.2%) | 2 (13.3%) | 0.72 |
| Reintervention | 13 (16.3%) | 4 (12.1%) | 9 (28.1%) | 0 (0.0%) | 0.03 |
| Stenting (iliac/renal/visceral arteries) | 3 (9.1%) | 2 (6.2%) | – | – | |
| Fenestration | 1 (3.0%) | – | – | – | |
| Open surgery | – | 7 (21.8%) | – | – | |
| Treatment strategy . | Overall (n = 80) . | Uncomplicated TBAD . | Complicated TBAD . | P-value . | |
|---|---|---|---|---|---|
| Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | |||
| Paraplegia | 5 (5.0%) | 0 (0.0%) | 3 (9.3%) | 2 (13.3%) | 0.13 |
| Early | – | 2 (6.2%) | 1 (6.7%) | – | |
| Delayed | – | 1 (3.1%) | 1 (6.7%) | – | |
| Stroke | 3 (3.7%) | 0 (0.0%) | 1 (3.1%) | 2 (13.3%) | 0.07 |
| Renal failure | 21 (26.3%) | 5 (15.2%) | 11 (34.4%) | 5 (33.3%) | 0.16 |
| Malperfusion | 7 (8.8%) | 2 (6.2%) | 3 (9.3%) | 2 (13.3%) | 0.70 |
| Cardiac arrhythmia | 7 (8.8%) | 3 (9.1%) | 2 (6.2%) | 2 (13.3%) | 0.72 |
| Reintervention | 13 (16.3%) | 4 (12.1%) | 9 (28.1%) | 0 (0.0%) | 0.03 |
| Stenting (iliac/renal/visceral arteries) | 3 (9.1%) | 2 (6.2%) | – | – | |
| Fenestration | 1 (3.0%) | – | – | – | |
| Open surgery | – | 7 (21.8%) | – | – | |
Postoperative complications and reintervention procedures according to initial management strategy (medical vs TEVAR vs open surgery)
| Treatment strategy . | Overall (n = 80) . | Uncomplicated TBAD . | Complicated TBAD . | P-value . | |
|---|---|---|---|---|---|
| Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | |||
| Paraplegia | 5 (5.0%) | 0 (0.0%) | 3 (9.3%) | 2 (13.3%) | 0.13 |
| Early | – | 2 (6.2%) | 1 (6.7%) | – | |
| Delayed | – | 1 (3.1%) | 1 (6.7%) | – | |
| Stroke | 3 (3.7%) | 0 (0.0%) | 1 (3.1%) | 2 (13.3%) | 0.07 |
| Renal failure | 21 (26.3%) | 5 (15.2%) | 11 (34.4%) | 5 (33.3%) | 0.16 |
| Malperfusion | 7 (8.8%) | 2 (6.2%) | 3 (9.3%) | 2 (13.3%) | 0.70 |
| Cardiac arrhythmia | 7 (8.8%) | 3 (9.1%) | 2 (6.2%) | 2 (13.3%) | 0.72 |
| Reintervention | 13 (16.3%) | 4 (12.1%) | 9 (28.1%) | 0 (0.0%) | 0.03 |
| Stenting (iliac/renal/visceral arteries) | 3 (9.1%) | 2 (6.2%) | – | – | |
| Fenestration | 1 (3.0%) | – | – | – | |
| Open surgery | – | 7 (21.8%) | – | – | |
| Treatment strategy . | Overall (n = 80) . | Uncomplicated TBAD . | Complicated TBAD . | P-value . | |
|---|---|---|---|---|---|
| Medical (n = 33) . | TEVAR (n = 32) . | Open surgery (n = 15) . | |||
| Paraplegia | 5 (5.0%) | 0 (0.0%) | 3 (9.3%) | 2 (13.3%) | 0.13 |
| Early | – | 2 (6.2%) | 1 (6.7%) | – | |
| Delayed | – | 1 (3.1%) | 1 (6.7%) | – | |
| Stroke | 3 (3.7%) | 0 (0.0%) | 1 (3.1%) | 2 (13.3%) | 0.07 |
| Renal failure | 21 (26.3%) | 5 (15.2%) | 11 (34.4%) | 5 (33.3%) | 0.16 |
| Malperfusion | 7 (8.8%) | 2 (6.2%) | 3 (9.3%) | 2 (13.3%) | 0.70 |
| Cardiac arrhythmia | 7 (8.8%) | 3 (9.1%) | 2 (6.2%) | 2 (13.3%) | 0.72 |
| Reintervention | 13 (16.3%) | 4 (12.1%) | 9 (28.1%) | 0 (0.0%) | 0.03 |
| Stenting (iliac/renal/visceral arteries) | 3 (9.1%) | 2 (6.2%) | – | – | |
| Fenestration | 1 (3.0%) | – | – | – | |
| Open surgery | – | 7 (21.8%) | – | – | |
The overall reintervention rate was 16.3% (n = 13); reinterventions were only required after medical therapy (Group A; in 4 patients:12.1%) and after TEVAR (Group B) in 9 (28.1%) cases, and involved stenting of aortic side branches to restore distal-organ perfusion in 5 (Group A: n = 3; Group B: n = 2), fenestration of the dissection membrane to allow for lower body perfusion in 1 (Group A), and open surgery with stent-graft removal in 7 (Group B) patients. No reintervention for TBAD pathology was required in patients after conventional open surgery (Group C; P = 0.03); however, reoperation for postoperative haemorrhage was required in 5 cases (33.3%) (Table 7).
Postoperative complications following conventional open surgery with regard to both subgroups (Group C vs post-TEVAR group)
| Post operative complications . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Neurological complications | |||
| Early paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Delayed paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Stroke | 2 (13.3%) | 2 (28.6%) | 0.565 |
| Renal insufficiency | |||
| Creatinin >1.5 mg/dl | 3 (20.0%) | 2 (28.6%) | 1.000 |
| Temporary HD | 1 (6.7%) | 2 (28.6%) | 0.220 |
| Permanent HD | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Respiratory complications—Tracheostomy | 5 (33.3%) | 2 (28.6%) | 1.000 |
| Bleeding requiring reoperation | 5 (33.3%) | 1 (28.6%) | 0.616 |
| Cardiac complications | |||
| Cardiac arrest | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Pacemaker | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Sepsis | 2 (13.3%) | 1 (14.3%) | 1.000 |
| MODS | 0 (0.0%) | 1 (14.3%) | 0.318 |
| Malperfusion | 2 (13.3%) | 1 (14.3%) | 1.000 |
| Blood transfusion | 14 (6–23) | 12 (8–24) | 0.837 |
| Transfusion of FFP | 6 (3–15) | 9 (4–16) | 0.680 |
| ICU stay (days) | 7 (3–29) | 15 (4–20) | 0.837 |
| Hospital stay (days) | 24 (21–34) | 5 (12–35) | 0.490 |
| Post operative complications . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Neurological complications | |||
| Early paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Delayed paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Stroke | 2 (13.3%) | 2 (28.6%) | 0.565 |
| Renal insufficiency | |||
| Creatinin >1.5 mg/dl | 3 (20.0%) | 2 (28.6%) | 1.000 |
| Temporary HD | 1 (6.7%) | 2 (28.6%) | 0.220 |
| Permanent HD | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Respiratory complications—Tracheostomy | 5 (33.3%) | 2 (28.6%) | 1.000 |
| Bleeding requiring reoperation | 5 (33.3%) | 1 (28.6%) | 0.616 |
| Cardiac complications | |||
| Cardiac arrest | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Pacemaker | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Sepsis | 2 (13.3%) | 1 (14.3%) | 1.000 |
| MODS | 0 (0.0%) | 1 (14.3%) | 0.318 |
| Malperfusion | 2 (13.3%) | 1 (14.3%) | 1.000 |
| Blood transfusion | 14 (6–23) | 12 (8–24) | 0.837 |
| Transfusion of FFP | 6 (3–15) | 9 (4–16) | 0.680 |
| ICU stay (days) | 7 (3–29) | 15 (4–20) | 0.837 |
| Hospital stay (days) | 24 (21–34) | 5 (12–35) | 0.490 |
MODS: multi-organ dysfunction syndrome; FFP: fresh frozen plasma; ICU: intensive care unit; HD: haemodialysis.
Postoperative complications following conventional open surgery with regard to both subgroups (Group C vs post-TEVAR group)
| Post operative complications . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Neurological complications | |||
| Early paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Delayed paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Stroke | 2 (13.3%) | 2 (28.6%) | 0.565 |
| Renal insufficiency | |||
| Creatinin >1.5 mg/dl | 3 (20.0%) | 2 (28.6%) | 1.000 |
| Temporary HD | 1 (6.7%) | 2 (28.6%) | 0.220 |
| Permanent HD | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Respiratory complications—Tracheostomy | 5 (33.3%) | 2 (28.6%) | 1.000 |
| Bleeding requiring reoperation | 5 (33.3%) | 1 (28.6%) | 0.616 |
| Cardiac complications | |||
| Cardiac arrest | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Pacemaker | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Sepsis | 2 (13.3%) | 1 (14.3%) | 1.000 |
| MODS | 0 (0.0%) | 1 (14.3%) | 0.318 |
| Malperfusion | 2 (13.3%) | 1 (14.3%) | 1.000 |
| Blood transfusion | 14 (6–23) | 12 (8–24) | 0.837 |
| Transfusion of FFP | 6 (3–15) | 9 (4–16) | 0.680 |
| ICU stay (days) | 7 (3–29) | 15 (4–20) | 0.837 |
| Hospital stay (days) | 24 (21–34) | 5 (12–35) | 0.490 |
| Post operative complications . | Open surgery (Group C; n = 15) . | Post-TEVAR group (n = 7) . | P-value . |
|---|---|---|---|
| Neurological complications | |||
| Early paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Delayed paraplegia | 1 (6.7%) | 0 (0.0%) | 1.000 |
| Stroke | 2 (13.3%) | 2 (28.6%) | 0.565 |
| Renal insufficiency | |||
| Creatinin >1.5 mg/dl | 3 (20.0%) | 2 (28.6%) | 1.000 |
| Temporary HD | 1 (6.7%) | 2 (28.6%) | 0.220 |
| Permanent HD | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Respiratory complications—Tracheostomy | 5 (33.3%) | 2 (28.6%) | 1.000 |
| Bleeding requiring reoperation | 5 (33.3%) | 1 (28.6%) | 0.616 |
| Cardiac complications | |||
| Cardiac arrest | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Pacemaker | 1 (6.7%) | 1 (14.3%) | 1.000 |
| Sepsis | 2 (13.3%) | 1 (14.3%) | 1.000 |
| MODS | 0 (0.0%) | 1 (14.3%) | 0.318 |
| Malperfusion | 2 (13.3%) | 1 (14.3%) | 1.000 |
| Blood transfusion | 14 (6–23) | 12 (8–24) | 0.837 |
| Transfusion of FFP | 6 (3–15) | 9 (4–16) | 0.680 |
| ICU stay (days) | 7 (3–29) | 15 (4–20) | 0.837 |
| Hospital stay (days) | 24 (21–34) | 5 (12–35) | 0.490 |
MODS: multi-organ dysfunction syndrome; FFP: fresh frozen plasma; ICU: intensive care unit; HD: haemodialysis.
In almost one quarter of patients (7 of 32) primarily treated by TEVAR, endovascular therapy resulted in a severe complication during the follow-up. Initially, TEVAR was performed in these 7 patients due to the development of aortic aneurysms (n = 4) and imminent limb ischaemia, mesenteric ischaemia or covered aortic rupture in 1 patient each. Complications post-TEVAR leading to secondary conventional open surgery were, in detail: type I endoleak (n = 2), covered rupture with new onset of paraplegia (n = 1), infection of the endoprosthesis (n = 1), stent-graft migration (n = 1), aorto-bronchial fistula (n = 1) and progressive enlargement of the aortic aneurysm (most likely due to endoleak type II or III; n = 1). Emergency surgery was immediately warranted due to the onset of life-threatening complications in 5 of these patients.
Twenty-one (26.3%) patients developed renal failure during their early postoperative clinical course: 5 (15.2%) patients with uncomplicated TBAD (Group A) vs 11 (34.4%) and 5 (33.3%) with complicated chronic TBAD in Groups B and C, respectively (P = 0.16).
Postopertive malperfusion syndrome was diagnosed in 7 patients (8.8%) with an occurrence of 6.2% in Group A (n = 2), 9.3% in Group B (n = 3) and 13.3% in Group C (n = 2). Successful reintervention was performed as described above in 5 cases.
Essential cardiac arrhythmias occurred in 7 (8.8%) and were successfully treated by pharmaceutical or electrical cardioversion. There were no cardiac ischaemic events, but 2 patients required implantation of a permanent pacemaker for atrioventricular block III.
Severe neurological complications only occurred after TEVAR and open surgery in Groups B and C, respectively. Postoperative stroke, as confirmed by computed tomography (CT), occurred more often in Group C (13.3%, n = 2) than in Group B (n = 1), however, without statistical significance (P = 0.07).
The incidence of procedure-related paraplegia was higher in the TEVAR and open surgery groups (Group B: 9.3% vs Group C: 13.3%). Two patients in Group C developed postoperative paraplegia: 1 patient developed a permanent, immediate deficit and 1 developed delayed paraplegia 3 days after surgery. Improvement of neurological symptoms was achieved by medical therapy with dexamethasone, control of CSF pressure and increase of mean arterial pressure (MAP), but permanent motor function deficits remained. In comparison, 2 patients after TEVAR developed early paraplegia immediately after stent-graft deployment, while 1 suffered from delayed paraplegia 32.4 months after endovascular repair due to enlarged aneurysm with acute rupture and resulting spinal cord ischaemia—most likely due to persistent false lumen perfusion (endoleak type II). However, the incidence of early (Group B: 6.2% vs Group C: 6.7%) or delayed (Group B: 3.1% vs Group C: 6.7%) paraplegia did not significantly differ between both groups (P = 0.13) (Table 6).
Overall median ICU stay was 11.5 days; median time to hospital discharge was 23 days.
Outcome after conventional open surgery: first-line treatment vs secondary (staged) procedure
In-hospital mortality among patients who had undergone primary emergency TEVAR (n = 7) prior to definite surgical repair was 14.3% (n = 1). No additional patient died during the critical first year and 3-year survival was still 85.7%. Overall, patients after first-line treatment by conventional surgery (Group C) had no significantly different in-hospital mortality when compared with their peers who had undergone TEVAR pretreatment (P = 1.0) (Table 4).
After the first year, slightly more patients in the post-TEVAR group were alive (6/7, no additional deaths after hospital discharge) as opposed to the patients directly treated by conventional surgery (Group C: 11/15; P = 0.482). Figure 1B shows the estimated Kaplan–Meier survival curves for Groups C and post-TEVAR.
Cause of death was multiorgan failure and sepsis in 2 patients; 1 patient died after hospital discharge of unknown causes. Two patients died 5 and 42 months postoperatively during the follow-up.
Five patients (71.4%) developed renal failure post-TEVAR: 2 of them (40%) required continuous veno-venous haemodialysis during the early postoperative course, while another (n = 1, 20%) required subsequent life-long haemodialysis. In comparison, only 1 (20%) of 5 patients who developed acute renal failure of Group C required continuous veno-venous haemodialysis early postoperatively but, again, 1 (20%) required life-long haemodialysis thereafter. The incidence of permanent renal failure was therefore also not significantly different between both groups (P = 1.000).
Further postoperative complications such as stroke, respiratory complications requiring temporary tracheostomy, cardiac arrhythmias, need for pacemaker implantation, sepsis or malperfusion syndrome were not significantly different between both groups (Table 7).
There were more reoperations due to postoperative bleeding in Group C (P = 0.616), but no significant differences were found in the amount of postoperative transfusions of red blood cells (P = 0.837) or fresh frozen plasma (P = 0.680) required between both groups.
Interestingly, no patient in the post-TEVAR group developed a clinically relevant ischaemic spinal cord injury after conventional open surgery.
The median ICU stay was 7 (IQR: 3–29) and 15 (IQR: 4–20) days for Groups C and post-TEVAR, respectively.
DISCUSSION
Medical therapy remains the gold standard for uncomplicated acute TBAD with favourable hospital survival rates between 85 and 95% after the initial hospital admission [1, 2]. However, it is questionable whether an ‘uncomplicated’ TBAD exists as conservative treatment (by means of medical therapy and monitoring only) that may result in an unfavourable long-term survival (5-year mortality of up to 50%) caused by severe complications, such as progressive aortic dilatation and rupture [5]. Bavaria et al. referred to this phenomenon in chronic aortic dissection patients [6] as the rule-of-ten: patients are treated successfully for the next 10 years and then they will return.
In 1980, Wheat [7] reported progressive aortic enlargement in 14–20% of TBAD patients managed medically over a 10-year follow-up. Most recently, the IRAD reported progressive aortic dilatation in 59% of medically treated TBAD patients with a mean expansion rate of 1.7 ± 7 mm/year [8]. Caucasian origin and a small initial aortic diameter were associated with increased aortic expansion during the follow-up, while decreased aortic expansion was observed among women, patients with intramural haematoma and those on calcium-channel blockers [8].
With regard to the natural history, an uncomplicated stable TBAD may suddenly deteriorate into a severe, acutely life-threatening clinical scenario requiring immediate surgical intervention [6].
Bozinovski and Coselli [9] reported a perioperative mortality rate of 22.4% and a paraplegia rate of 6.6% in surgically treated patients suffering from primary, complicated TBAD. Recently, IRAD investigators reported an overall mortality of 29.3% and an even higher in-hospital mortality of 39.2% in TBAD patients; mortality even increased to 50% if aortic rupture was present prior to surgery [10]. Open surgery was considered the best treatment option for most patients presenting with progressive (or acute) aneurysmal dilatation complicating their chronic TBAD, particularly since TEVAR requires an appropriate proximal and distal landing zone for successful stent-graft deployment. Therefore, 15 of 80 TBAD patients with progressive aneurysm of their thoracic and thoracoabdominal aorta were treated by conventional open repair, while the ‘aortic team’ opted for further surveillance or an endovascular intervention in the remaining 65 patients—if aneurysm size was not extensively enlarged (with an appropriate proximal and distal landing zone), or if acute malperfusion syndrome or imminent rupture did occur despite optimal medical treatment.
Spontaneous complete thrombosis of the false lumen occurs very rarely (<4% of patients) [11], most likely due to persistent false lumen perfusion, and thereby predisposing patients to aortic enlargement, rupture or retrograde dissection with an increased mortality rate [5, 12]. TEVAR has been reported to allow for closure of the proximal entry tear—which is essential to achieve false lumen thrombosis, aortic remodelling, and false lumen reduction and improved outcome—and may be achieved in up to 79% of endovascularly treated TBAD patients [2]. However, controversies with regard to the high success rate of TEVAR with regard to false lumen thrombosis remain. Guangqi et al. [13] reported a series of 121 consecutive patients undergoing TEVAR for acute and chronic TBAD with an incidence of postoperative endoleaks in 22% and a 30-day mortality of 8.2%. In our TBAD cohort, a persisting false lumen (including partial thrombosis) was observed in up to 85% (n = 68) of cases with 59% (n = 47) of them requiring endovascular or open surgery.
Initially, TEVAR was used as a last-resort procedure for high-risk patients with unclear overall life expectancy or to achieve rapid aortic stabilization in the acutely unstable patient. However, procedural success—in combination with the potential advantage of less invasive repair—has led to a progressive use of this technique, with TEVAR now being proposed by some surgeons as an alternative for the most thoracic aortic pathologies affecting the descending or thoracoabdominal aorta [6].
TEVAR offers potential benefits in comparison to open surgery since no extensive thoracotomy is required and the procedure may be performed with local anaesthesia, while conventional open repair requires intubation, cardiopulmonary bypass, aortic cross-clamping and single-lung ventilation. CSF drainage for spinal cord protection, however, is recommended for both procedures [14, 15].
However, TEVAR—as most catheter-based techniques—is not risk free: the 30-day mortality rate following endovascular repair of TBAD may be as high as 20% [16] and severe complications, such as early or delayed retrograde type A dissection (5–22%) [12, 17], stroke (4.6%) and paraplegia (1.9–4.4%) [18, 19] may occur and require open surgical repair. Additionally, TEVAR-specific complications (e.g. endoleaks, limb ischaemia and various access-related complications) have also been reported, leading to substantial reintervention and reoperation rates [6, 20].
The incidence of acute or delayed complications requiring reintervention (i.e. surgery or TEVAR) after stent-graft repair is significant and affects roughly 1 of 5 patients—ranging from 17 to 22% in published series [16, 21].
Patients with chronic TBAD represent a very heterogeneous patient cohort, which requires optimized medical therapy and frequent surveillance to allow for optimal timing of interventional or surgical treatment. Most patients were treated medically after admission, but complications—either immediately or delayed—will occur as a matter of the follow-up time and will frequently require urgent or emergent treatment.
Medically treated patients for uncomplicated TBAD (Group A) were stabilized, monitored and thoroughly examined by the ‘aortic team’ before discharge. All patients were included in our institutional surveillance programme, including routine clinical examinations and CT scans with a survival at 1 and 3 years of 97 and 91%, respectively. During their clinical course, minor reinterventions, including stenting of aortic side branches (n = 3) and fenestration (n = 1), were required in 12% of patients. The critical interval after the acute event, however, is likely not yet reached by the majority of these patients as the mean follow-up time is yet too short.
As mentioned above, 15 patients were treated by conventional surgery (Group C) due to the acuteness of new complications (e.g. rupture) and/or the presence of an extensive thoracic or thoracoabdominal aneurysm on admission. In-hospital mortality was 13% (n = 2), followed by a postoperative mortality of 15% during the first year, however, at 3 years overall survival of patients treated for extensive aortic aneurysm of the thoracic and thoracoabdominal aorta was 73% (n = 11). These results for conventional open surgery are acceptable with regard to reported in-hospital mortality between 9.6 and 22.4% [9, 22] and 5-year survival of 78% [22] in other recently published series of thoracoabdominal aortic replacements.
TEVAR was successfully performed in 32 patients with in-hospital and 3-year mortalities of 6.2 and 12.5%, respectively. Although no patient required immediate conversion to open surgery during the TEVAR procedure, immediate stroke and early paraplegia occurred in 3.1% (n = 1) and 6.2% (n = 2) postoperatively. A single patient who had undergone TEVAR without complications suddenly developed aortic rupture of a progressively enlarged aneurysm—most likely due to non-diagnosed type II endoleak—resulting in acute paraplegia during the follow-up. Reinterventions for complications after aortic stent-grafting were necessary in almost one-third of TEVAR cases: 9 patients (28.1%) required a secondary procedure. In almost one quarter (n = 7) of post-TEVAR patients, urgent or emergent open surgery was required. Initially, 3 patients had undergone emergent TEVAR due to impending rupture, however, the concept of TEVAR as a possible ‘bridge-to-open repair’ was not considered at that time.
Paraplegia still remains one of the most dreaded complications after surgical and after endovascular repair of TBAD; however, it is thought to occur less frequently in a chronic setting. Accordingly, the incidence after open repair for chronic complicated TBAD was—compared with open repair for regular descending/thoracoabdominal aneurysm disease—relatively low, with only 2 cases in our surgical cohort of 15 patients. One patient developed permanent (early) paraplegia postoperatively, while the other experienced delayed spinal cord ischaemia leading to paraplegia on day 3 after open surgery despite extensive segmental artery reimplantation.
In comparison, 3 of 32 patients who underwent TEVAR developed early permanent paraplegia postoperatively (n = 2) or delayed spinal cord injury with subsequent paraplegia during their later clinical course (n = 1). A recent multicentre study by the European Registry of Endovascular Aortic Repair Complications reported a significant association of spinal cord ischaemia after acute endovascular stent-graft coverage of at least two inflow territories of spinal cord blood supply (positive predictive value: 0.75; 95% confidence interval 0.38–0.75; P < 0.0001) [18].
However, no new-onset paraplegia occurred post-TEVAR (n = 5; excluding those with previous paraplegia!) after ‘reoperative’ open repair with resection of the progressively enlarged thoracic and thoracoabdominal aorta during the follow-up. This finding is not surprising considering the notion that a ‘staged-repair’ has been proposed to significantly reduce the risk of postoperative paraplegia by the proponents of the collateral network concept [23]. Open surgery after previous TEVAR for TBAD mimics the ‘staged repair’ by step-wise occlusion of segmental arteries potentially ‘priming’ the arterial paraspinous collateral network to provide improved spinal cord perfusion after segmental artery sacrifice during conventional repair.
Spinal cord protection during open and endovascular aortic repair is of utmost importance to avoid perioperative spinal cord ischaemia ultimately leading to permanent paraplegia. Therefore, our current strategy for neuroprotection—based on the recently introduced collateral network concept—during open aortic surgery comprises CSF drainage, deep-to-moderate hypothermia (26–32°C) in combination with distal (lower body perfusion) to minimize the duration of circulatory arrest, and maintenance of a supra-normal MAPs postoperatively for at least 72 h [24]. Most recently, we also began utilizing near-infrared spectroscopy (NIRS) to monitor the paraspinous collateral network to detect related spinal cord ischaemia by measuring regional tissue oxygenation during open aortic surgery and also sporadically in high-risk TEVAR. Perioperative monitoring was upgraded using NIRS optodes, bilaterally placed on the patient's paravertebral muscles at the thoracic and the lumbar levels, to monitor the paravertebral muscle oxygenation (paravertebral collateral network) as an indirect parameter for sufficient arterial collateral network supply to the spinal cord [25]. This approach can easily be used in any setting of aortic surgery, particularly during thoracoabdominal repair, to allow for the early intra- and postoperative detection of insufficient distal inflow to the paraspinous collateral network potentially indicating spinal cord malperfusion: a possible drop of oxygen saturation below the (preoperative) baseline indicates decreased perfusion of the paravertebral collateral network that may be addressed by increasing the systemic MAP.
The incidence of stroke was similar between the two surgical groups (Group C: n = 2 vs post-TEVAR, n = 2; P = 0.565). Although 2 patients were diagnosed with stroke and died during their clinical course, the other 2 recovered from surgery and the neurological symptoms markedly improved during the follow-up. However, no patient treated with TEVAR suffered from stroke postoperatively. Reflecting the fact that TEVAR was exclusively performed percuteanously via the femoral artery, however, the incidence of stroke after TEVAR may increase if stent-grafting of the distal or transverse arch is performed.
Five patients required haemodialysis for either transient (n = 3) or permanent (n = 2) renal failure (Group C vs post-TEVAR; P = 1.0). Both patients who required permanent haemodialysis postoperatively had a compromised renal function prior to surgery and underwent extensive thoracoabdominal replacement with re-implantation of both renal arteries. Moreover, massive transfusion was required in both due to postoperative haemorrhage.
It would be reasonable to expect that TBAD patients who were initially treated endovascularly—and had developed a TEVAR-associated complication during their clinical course—would have a worse outcome after open repair than patients treated directly by conventional surgery, however, this was not true in this series. Comparison of Groups C and post-TEVAR showed an equal in-hospital mortality with lower 1 and 3-year mortality rates and a trend for improved longevity (Table 4; Fig. 1), however, without any statistical significance. Also, no paraplegia occurred despite extensive aortic resection for stent-graft removal and prolonged operation times (possibly due to ischaemic preconditioning of the spinal cord).
Patients with chronic TBAD who develop a complication suddenly become high-risk cases and should always be treated as aortic emergencies. Interventional catheter-based treatment by TEVAR, aortic side branch stenting or fenestration offer potential bail-out procedures for these acute emergencies. However, conclusive long-term follow-up after TEVAR, particularly in young patients, is not yet available. In comparison, conventional open surgery for complicated TBAD has been reported to result in an increased 30-day mortality of up to 30%, whereas endovascular treatment is thought to significantly reduce hospital mortality down to 9.8% [2]. Importantly, TBAD patients suffering from extensive aneurysms, malperfusion or acute rupture are surgically challenging, and the use of TEVAR might be limited with regard to aneurysm size and location, occlusion by the dissection's false lumen or thrombus formation within the chronic aneurysm. Moreover, progressive thickening of the dissection's intimal flap—prohibitating re-approximation of the native aortic wall—may occur due to fibrosis during the chronic phase [1, 2].
TEVAR application in chronic TBAD remains controversial since stent-grafting bears the risk of only eliminating antegrade false lumen flow by coverage of the primary entry, while retrograde false lumen flow persists through potential dissection re-entry entries more distally. This may lead to an endoleak type I, ultimately leading to aneurysm enlargement and acute aortic rupture [1, 2, 6].
However, TEVAR may be successfully used—particularly in acute TBAD—with complications to abrogate impending aortic rupture and relieve organ malperfusion in order to stabilize the patient ‘as a bridge to open surgery’. At the cost of only insignificantly increased early mortality, this staged-approach might not only allow the eventual complete cure of the patient's aortic pathology, but also might eliminate the most devastating neurological complication: permanent paraplegia.
Conclusions
Medical treatment remains the gold standard for the initial treatment of uncomplicated (chronic) TBAD. Open surgery for extensive thoracic and thoracoabdominal repair in complicated chronic TBAD may be performed with acceptable early and mid-term outcomes. TEVAR for aortic complications in patients with chronic dissection may be successfully performed to stabilize the patient and serve as a ‘bridge to open surgery’. However, close surveillance is mandatory for the timely detection of aneurysm enlargement, malperfusion or impending rupture after TEVAR.
Study limitations
The current study is retrospective in nature. The limited number of patients enrolled is insufficiently powered to identify the subtle differences in outcomes between patient groups. However, we strongly believe that the report on this very specific cohort—patients with complicated chronic TBAD, and in particular, after conventional open surgery as a first-line treatment or secondary (staged) completion procedure—is important to identify an optimal treatment option for this heterogenous cohort in the future.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr C. Hagl(Munich, Germany): In their paper, the Leipzig group ambitiously tried to answer the question concerning the best treatment for chronic type B aortic dissection. During a period of 10 years, they identified 80 patients who were admitted due to chronic type B aortic dissection of whom 40 were treated conservatively, 17 received a stent implantation, and 22 had open surgery. Thirty one per cent had a complicated type B dissection.
Specific treatment options were discussed in a multidisciplinary team. Not unexpectedly, the proportion of complicated type Bs was significantly higher in the TEVAR and open surgery groups. Thirty per cent who were initially treated by TEVAR needed secondary surgery. The overall mortality in all groups was 20%.
In their manuscript the authors compared the outcome of different treatment groups and subgroups. The inhomogeneous numbers of patients as well as the heterogeneity in each group makes a proper conclusion difficult.
My first question deals with your decision-making process. What were the indications for TEVAR compared to open surgery in the complicated and the uncomplicated type Bs? I feel that there is some mix-up of both definitions. And secondly, you showed that a significant number of the TEVAR patients needed open surgery. Will that have an influence on your treatment protocol in the future?
Dr Nozdrzykowski: In terms of the first question, we had 24 patients with TEVAR; 17 patients underwent only TEVAR and seven were operated on following serious complications after stent graft implantation. The majority of indications for initial TEVAR for these 24 patients were diametric expansion from the aorta and rupture. In these seven patients, in the secondary surgery group, there were three patients with expansion of the diameter of the aorta by endoleak type I, two patients with rupture, and one patient with a covered rupture.
Could you repeat the second question?
Dr Hagl: Did the results of your TEVAR procedures have an effect on your protocol for the future?
Dr Nozdrzykowski: Yes. We have seen that the patients who required later surgery were not suitable for TEVAR because of, first, these two patients with rupture, with haemothorax on the left side, and actually we think these patients should be treated with open surgery as a first option.
Dr Hagl: Let me try to be more precise. If you have a patient and you can treat him with TEVAR or open surgery, what would you choose?
Dr Nozdrzykowski: It's a difficult question. Actually, we think if the patient is suitable for TEVAR, when the landing zone is long enough, when the femoral vessels are suitable for cannulation, this patient should be treated with a stent graft. We think actually that in the acute phase, these patients, for example, with a covered rupture, should be treated initially with TEVAR. And when the patient achieves a stable state, secondary operation, open surgery, should be performed.
Dr L. Botta(Milan, Italy): Recently we had in our department two 80 year old patients with acute type B dissection with an associated acute intramural haematoma of the ascending aorta in the aortic arch. I didn't see this association in your experience. Have you encountered an association of acute type B dissection and acute intramural haematoma of arch and ascending aorta, and what is your suggested surgical strategy?
Dr Nozdrzykowski: In our cohort, we have no patients with intramural haematoma of the descending aorta. In the acute phase, we think these patients should be treated with TEVAR or stent graft when they have symptoms. When the patients are without symptoms, possibly a CT scan should be performed at 3 months or 6 months and then 1 year later.
Dr Botta: So you would suggest an endovascular procedure in acute type B with an intramural haematoma of the arch, you would put an endoprosthesis in the arch with an intramural haematoma? Or would you go for a complete open replacement of the thoracic aorta?
Dr Nozdrzykowski: In this case we would opt for open surgery of course. But I thought you wanted to know about intramural haematoma in the descending aorta.
Dr J. Garbade(Leipzig, Germany): Maybe I can expand his answer with just a brief comment on this topic. The intention of the paper was to discuss chronic type B and now we're speaking about acute type B or complicated type B with a haematoma of the arch. When we look to the guidelines in this case, we can also speak about retro type A dissection, and so we have to operate. And that's our principle strategy in Leipzig.
Author notes
Presented at the 26th Annual Meeting of the European Association of Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.
Both authors contributed equally to the manuscript.




