-
PDF
- Split View
-
Views
-
Cite
Cite
Yumi Tanaka, Tatsuya Yoshimasu, Shoji Oura, Yoshimitsu Hirai, Mitsumasa Kawago, Masako Ikeda, Yoshitaka Okamura, Preoperative serum pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen level predicts postoperative distant metastasis in patients with non-small-cell lung cancer, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages 539–543, https://doi.org/10.1093/ejcts/ezt076
Close - Share Icon Share
Abstract
To examine the relationship between preoperative serum pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen (I-CTP) levels and postoperative distant metastasis in patients with non-small-cell lung cancer (NSCLC).
We retrospectively reviewed 143 patients in whom preoperative serum I-CTP level was measured from January 2006 to March 2011, including 91 males and 52 females with an average age of 70.1 ± 8.2 years. Histological subtypes included adenocarcinoma (n = 95), squamous cell carcinoma (n = 34) and other (n = 14). Preoperative serum carcinoembryonic antigen (CEA) and cytokeratin-19 fragment (CYFRA) levels were also measured. Patients with abnormal renal function or preoperative bone fractures were excluded.
The mean preoperative serum I-CTP level was 4.1 ± 1.6 ng/ml, and the preoperative serum I-CTP level was elevated (>4.5 ng/ml) in 29 patients. Distant metastasis was detected in 21 patients during the 39 ± 18 (range 1–79) months of follow-up. The rate of distant metastasis was significantly higher in patients with elevated preoperative serum I-CTP levels than those with normal preoperative I-CTP levels (≤4.5 ng/ml) (P < 0.0001). The 5-year recurrence-free survival rate was lower in patients with elevated preoperative serum I-CTP levels than those with normal preoperative I-CTP levels (41.8 vs 92.9%; P < 0.0001).
An elevated preoperative serum I-CTP level predicts postoperative distant metastasis in patients with NSCLC.
INTRODUCTION
Lung cancer is the most common malignancy worldwide and is the leading cause of cancer death, particularly in men [1]. Non-small-cell lung cancer (NSCLC) accounts for 85% of lung cancers. Despite recent advances in therapy, the overall 5-year survival rate of patients with NSCLC is ∼15% [2]. Even among patients diagnosed during the early stage, more than one-third will experience local recurrence after curative resection and will ultimately die of their disease [3]. Distant metastasis is also common [4]. As it is difficult to predict which patients are at increased risk of local recurrence or distant metastasis after curative resection based on the pathological stage alone, it is essential to identify additional prognostic factors to guide management.
Type I collagen comprises ≥90% of the organic substance in bone. Serum pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen (I-CTP) is a metabolite that is released during the degradation of type I collagen, and is widely used as a sensitive and specific marker of bone resorption [5]. Lung cancer frequently metastasizes to bone [4], and a high preoperative serum I-CTP level might, therefore, indicate latent skeletal metastasis. In this study, we retrospectively examined the relationship between preoperative serum I-CTP levels and postoperative distant metastasis in patients with NSCLC.
PATIENTS AND METHODS
We retrospectively reviewed 143 patients in whom the preoperative serum I-CTP level was measured at our institute from January 2006 to March 2011, including 91 males and 52 females with a mean age of 70.1 ± 8.2 (range 41–87) years. Preoperative serum carcinoembryonic antigen (CEA) and cytokeratin-19 fragment (CYFRA) levels were measured at the same time as I-CTP level. Serum I-CTP levels were measured using radioimmunoassay, and serum CEA and CYFRA levels were measured using enzyme-linked immunosorbent assay. Serum tumour marker levels were defined as elevated when the I-CTP level was >4.5 ng/ml, CEA level was >5.0 ng/ml and CYFRA level was >3.5 ng/ml [6–8]. Patients with abnormal renal function or preoperative bone fractures were excluded. Histological subtypes included adenocarcinoma (n = 95), squamous cell carcinoma (n = 34), adenosquamous carcinoma (n = 6), large cell carcinoma (n = 2) and other (n = 6). Postoperative stage included IA (n = 83), IB (n = 24), IIA (n = 6), IIB (n = 12), IIIA (n = 15) and IIIB (n = 3) (Table 1).
| Age (years) | 70.1 ± 8.2 |
| Sex | |
| Male | 91 |
| Female | 52 |
| Histology | |
| Adenocarcinoma | 95 |
| Squamous cell carcinoma | 34 |
| Adenosquamous carcinoma | 6 |
| Large cell carcinoma | 2 |
| Other | 6 |
| Pathological stage | |
| IA | 83 |
| IB | 24 |
| IIA | 6 |
| IIB | 12 |
| IIIA | 15 |
| IIIB | 3 |
| Surgical procedure | |
| Wedge resection | 30 |
| Lobectomy | 111 |
| Pneumonectomy | 2 |
| Preoperative serum tumour marker level | |
| I-CTP (ng/ml) | 4.1 ± 1.6 |
| CEA (ng/ml) | 12.7 ± 64.8 |
| CYFRA (ng/ml) | 2.5 ± 4.2 |
| Age (years) | 70.1 ± 8.2 |
| Sex | |
| Male | 91 |
| Female | 52 |
| Histology | |
| Adenocarcinoma | 95 |
| Squamous cell carcinoma | 34 |
| Adenosquamous carcinoma | 6 |
| Large cell carcinoma | 2 |
| Other | 6 |
| Pathological stage | |
| IA | 83 |
| IB | 24 |
| IIA | 6 |
| IIB | 12 |
| IIIA | 15 |
| IIIB | 3 |
| Surgical procedure | |
| Wedge resection | 30 |
| Lobectomy | 111 |
| Pneumonectomy | 2 |
| Preoperative serum tumour marker level | |
| I-CTP (ng/ml) | 4.1 ± 1.6 |
| CEA (ng/ml) | 12.7 ± 64.8 |
| CYFRA (ng/ml) | 2.5 ± 4.2 |
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
| Age (years) | 70.1 ± 8.2 |
| Sex | |
| Male | 91 |
| Female | 52 |
| Histology | |
| Adenocarcinoma | 95 |
| Squamous cell carcinoma | 34 |
| Adenosquamous carcinoma | 6 |
| Large cell carcinoma | 2 |
| Other | 6 |
| Pathological stage | |
| IA | 83 |
| IB | 24 |
| IIA | 6 |
| IIB | 12 |
| IIIA | 15 |
| IIIB | 3 |
| Surgical procedure | |
| Wedge resection | 30 |
| Lobectomy | 111 |
| Pneumonectomy | 2 |
| Preoperative serum tumour marker level | |
| I-CTP (ng/ml) | 4.1 ± 1.6 |
| CEA (ng/ml) | 12.7 ± 64.8 |
| CYFRA (ng/ml) | 2.5 ± 4.2 |
| Age (years) | 70.1 ± 8.2 |
| Sex | |
| Male | 91 |
| Female | 52 |
| Histology | |
| Adenocarcinoma | 95 |
| Squamous cell carcinoma | 34 |
| Adenosquamous carcinoma | 6 |
| Large cell carcinoma | 2 |
| Other | 6 |
| Pathological stage | |
| IA | 83 |
| IB | 24 |
| IIA | 6 |
| IIB | 12 |
| IIIA | 15 |
| IIIB | 3 |
| Surgical procedure | |
| Wedge resection | 30 |
| Lobectomy | 111 |
| Pneumonectomy | 2 |
| Preoperative serum tumour marker level | |
| I-CTP (ng/ml) | 4.1 ± 1.6 |
| CEA (ng/ml) | 12.7 ± 64.8 |
| CYFRA (ng/ml) | 2.5 ± 4.2 |
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
Surgical procedures included wedge resection (n = 30), lobectomy (n = 111) and pneumonectomy (n = 2). Wedge resection was usually selected in patients with early-stage disease and poor pulmonary function.
Patients with T1b and T2 tumours without lymph-node metastasis received adjuvant chemotherapy with tegafur-uracil (UFT) [9], and patients with lymph-node metastasis received platinum-doublet chemotherapy with third-generation anticancer agents [10].
Follow-up
After surgery, tumour markers were measured at 1, 3 and 6 months, and every 6 months thereafter. Chest computed tomography (CT) was performed every 3 months during the first year, every 6 months during the second year and annually thereafter. Positron emission tomography was performed every year.
Statistical analysis
All values are presented as mean ± SD. Values were compared between groups using the t-test and χ2 test. Survival time was calculated from the time of surgery to the time of death from any cause, the time of recurrence or distant metastasis or the time of last follow-up. Five-year recurrence-free and overall survival curves were constructed using the Kaplan–Meier method, and the log-rank test was used to evaluate the statistical significance of differences between groups.
The Cox proportional hazards model for multivariate analysis was used to identify significant prognostic factors for postoperative distant metastasis. Multivariate analysis included age, gender, histological subtype, postoperative stage and preoperative serum I-CTP, CEA and CYFRA levels. A two-sided P-value of <0.05 was considered statistically significant.
RESULTS
The preoperative serum I-CTP, CEA and CYFRA levels were 4.1 ± 1.6 ng/ml, 12.7 ± 64.8 ng/ml and 2.5 ± 4.2 ng/ml, respectively. The preoperative serum I-CTP level was elevated in 29 patients, the preoperative serum CEA level was elevated in 37 patients and the preoperative serum CYFRA level was elevated in 20 patients. Distant metastasis was identified in 21 patients during the 39 ± 18 (range 1–79) months of follow-up. The sites of metastasis were the lung (n = 17), brain (n = 2), bone (n = 3) and adrenal gland (n = 1). Two patients had metastases at multiple sites. Local recurrence was identified in 10 patients during the follow-up, and distant metastasis was identified in 7 patients subsequent to recurrence. They were included the group without distant metastasis. Among patients with distant metastasis, the preoperative serum I-CTP level was elevated in 14 patients. The rate of postoperative distant metastasis was significantly higher in patients with an elevated preoperative serum I-CTP level than those with a normal preoperative I-CTP level (48.3 vs 6.1%; P < 0.0001). There were no significant differences in the rates of postoperative distant metastasis between patients with elevated and normal preoperative CEA levels (P = 0.0635) or between patients with elevated and normal preoperative CYFRA levels (P = 0.0801) (Table 2). The preoperative serum I-CTP level was significantly higher in patients with postoperative distant metastasis than those without (5.7 ± 2.3 ng/ml vs 3.8 ± 1.3 ng/ml; P < 0.0001). The preoperative serum CEA level was also significantly higher in patients with postoperative distant metastasis than those without (60.3 ± 163.4 ng/ml vs. 4.5 ± 7.4 ng/ml; P = 0.0002). There was no significant difference in the preoperative serum CYFRA level between patients with and without postoperative distant metastasis (Table 3).
Relationships between rate of distant metastasis and preoperative serum I-CTP, CEA and CYFRA levels
| . | Above cutoff, n (%) . | Normal, n (%) . | P-value . | ||
|---|---|---|---|---|---|
| Total . | Recurrence . | Total . | Recurrence . | ||
| I-CTP | 29 | 14 (42.9) | 114 | 7 (6.1) | <0.0001 |
| CEA | 37 | 9 (24.3) | 106 | 12 (11.3) | 0.0635 |
| CYFRA | 20 | 6 (30.0) | 123 | 15 (12.2) | 0.0801 |
| . | Above cutoff, n (%) . | Normal, n (%) . | P-value . | ||
|---|---|---|---|---|---|
| Total . | Recurrence . | Total . | Recurrence . | ||
| I-CTP | 29 | 14 (42.9) | 114 | 7 (6.1) | <0.0001 |
| CEA | 37 | 9 (24.3) | 106 | 12 (11.3) | 0.0635 |
| CYFRA | 20 | 6 (30.0) | 123 | 15 (12.2) | 0.0801 |
I-CTP: type I collagen; CEA: carcinoembryonic antigen; CYFRA: cytokeratin-19 fragment.
Relationships between rate of distant metastasis and preoperative serum I-CTP, CEA and CYFRA levels
| . | Above cutoff, n (%) . | Normal, n (%) . | P-value . | ||
|---|---|---|---|---|---|
| Total . | Recurrence . | Total . | Recurrence . | ||
| I-CTP | 29 | 14 (42.9) | 114 | 7 (6.1) | <0.0001 |
| CEA | 37 | 9 (24.3) | 106 | 12 (11.3) | 0.0635 |
| CYFRA | 20 | 6 (30.0) | 123 | 15 (12.2) | 0.0801 |
| . | Above cutoff, n (%) . | Normal, n (%) . | P-value . | ||
|---|---|---|---|---|---|
| Total . | Recurrence . | Total . | Recurrence . | ||
| I-CTP | 29 | 14 (42.9) | 114 | 7 (6.1) | <0.0001 |
| CEA | 37 | 9 (24.3) | 106 | 12 (11.3) | 0.0635 |
| CYFRA | 20 | 6 (30.0) | 123 | 15 (12.2) | 0.0801 |
I-CTP: type I collagen; CEA: carcinoembryonic antigen; CYFRA: cytokeratin-19 fragment.
Serum I-CTP, CEA and CYFRA levels in patients with and without distant metastasis
| . | Distant metastasis (+) . | Distant metastasis (−) . | P-value . |
|---|---|---|---|
| I-CTP (ng/ml) | 5.7 ± 2.3 | 3.8 ± 1.3 | <0.0001 |
| CEA (ng/ml) | 60.3 ± 163.4 | 4.5 ± 7.4 | 0.0002 |
| CYFRA (ng/ml) | 2.9 ± 2.9 | 2.5 ± 4.5 | 0.6745 |
| . | Distant metastasis (+) . | Distant metastasis (−) . | P-value . |
|---|---|---|---|
| I-CTP (ng/ml) | 5.7 ± 2.3 | 3.8 ± 1.3 | <0.0001 |
| CEA (ng/ml) | 60.3 ± 163.4 | 4.5 ± 7.4 | 0.0002 |
| CYFRA (ng/ml) | 2.9 ± 2.9 | 2.5 ± 4.5 | 0.6745 |
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
Serum I-CTP, CEA and CYFRA levels in patients with and without distant metastasis
| . | Distant metastasis (+) . | Distant metastasis (−) . | P-value . |
|---|---|---|---|
| I-CTP (ng/ml) | 5.7 ± 2.3 | 3.8 ± 1.3 | <0.0001 |
| CEA (ng/ml) | 60.3 ± 163.4 | 4.5 ± 7.4 | 0.0002 |
| CYFRA (ng/ml) | 2.9 ± 2.9 | 2.5 ± 4.5 | 0.6745 |
| . | Distant metastasis (+) . | Distant metastasis (−) . | P-value . |
|---|---|---|---|
| I-CTP (ng/ml) | 5.7 ± 2.3 | 3.8 ± 1.3 | <0.0001 |
| CEA (ng/ml) | 60.3 ± 163.4 | 4.5 ± 7.4 | 0.0002 |
| CYFRA (ng/ml) | 2.9 ± 2.9 | 2.5 ± 4.5 | 0.6745 |
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
Table 4 shows 5-year disease-free survival and overall survival rates according to the patient subgroup (univariate analysis). The significant factors for disease-free survival were N-factor, pathological stage, surgical technique, the preoperative serum I-CTP level, CEA level and CYFRA level. The most of them were pathological stage and the I-CTP level. The significant factors for overall survival were histology, T-factor, N-factor, pathological stage and the preoperative serum CYFRA level, furthermore, the most of them was N-factor.
Results of univariate analysis about 5-year disease-free survival and overall survival rates according to subgroup
| Characteristics . | Disease-free survival . | P-value . | Overall survival . | P-value . |
|---|---|---|---|---|
| Age (years) | ||||
| <69 | 0.81 | 0.92 | 0.79 | 0.96 |
| ≥70 | 0.84 | 0.73 | ||
| Gender | ||||
| Male | 0.83 | 0.51 | 0.72 | 0.34 |
| Female | 0.81 | 0.81 | ||
| Histology | ||||
| Adeno | 0.78 | 0.19 | 0.81 | 0.02 |
| Non-adeno | 0.91 | 0.65 | ||
| T-factor | ||||
| T1 | 0.88 | 0.05 | 0.9 | 0.001 |
| Over T1 | 0.73 | 0.58 | ||
| N-factor | ||||
| Negative | 0.86 | 0.007 | 0.84 | <0.001 |
| Positive | 0.63 | 0.44 | ||
| Pathological stage | ||||
| IA or IB | 0.89 | <0.001 | 0.83 | 0.002 |
| Over I | 0.61 | 0.53 | ||
| Technique | ||||
| Partial | 1 | 0.02 | 0.85 | 0.79 |
| Lobectomy | 0.78 | 0.73 | ||
| I-CTP (ng/ml) | ||||
| Elevated (>4.5) | 0.42 | <0.001 | 0.47 | 0.29 |
| Normal (≤4.5) | 0.93 | 0.8 | ||
| CEA (ng/ml) | ||||
| Elevated (>5.0) | 0.72 | 0.02 | 0.78 | 0.44 |
| Normal (≤5.0) | 0.86 | 0.75 | ||
| CYFRA (ng/ml) | ||||
| Elevated (>3.5) | 0.64 | 0.01 | 0.47 | 0.001 |
| Normal (≤3.5) | 0.85 | 0.81 | ||
| Characteristics . | Disease-free survival . | P-value . | Overall survival . | P-value . |
|---|---|---|---|---|
| Age (years) | ||||
| <69 | 0.81 | 0.92 | 0.79 | 0.96 |
| ≥70 | 0.84 | 0.73 | ||
| Gender | ||||
| Male | 0.83 | 0.51 | 0.72 | 0.34 |
| Female | 0.81 | 0.81 | ||
| Histology | ||||
| Adeno | 0.78 | 0.19 | 0.81 | 0.02 |
| Non-adeno | 0.91 | 0.65 | ||
| T-factor | ||||
| T1 | 0.88 | 0.05 | 0.9 | 0.001 |
| Over T1 | 0.73 | 0.58 | ||
| N-factor | ||||
| Negative | 0.86 | 0.007 | 0.84 | <0.001 |
| Positive | 0.63 | 0.44 | ||
| Pathological stage | ||||
| IA or IB | 0.89 | <0.001 | 0.83 | 0.002 |
| Over I | 0.61 | 0.53 | ||
| Technique | ||||
| Partial | 1 | 0.02 | 0.85 | 0.79 |
| Lobectomy | 0.78 | 0.73 | ||
| I-CTP (ng/ml) | ||||
| Elevated (>4.5) | 0.42 | <0.001 | 0.47 | 0.29 |
| Normal (≤4.5) | 0.93 | 0.8 | ||
| CEA (ng/ml) | ||||
| Elevated (>5.0) | 0.72 | 0.02 | 0.78 | 0.44 |
| Normal (≤5.0) | 0.86 | 0.75 | ||
| CYFRA (ng/ml) | ||||
| Elevated (>3.5) | 0.64 | 0.01 | 0.47 | 0.001 |
| Normal (≤3.5) | 0.85 | 0.81 | ||
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
Results of univariate analysis about 5-year disease-free survival and overall survival rates according to subgroup
| Characteristics . | Disease-free survival . | P-value . | Overall survival . | P-value . |
|---|---|---|---|---|
| Age (years) | ||||
| <69 | 0.81 | 0.92 | 0.79 | 0.96 |
| ≥70 | 0.84 | 0.73 | ||
| Gender | ||||
| Male | 0.83 | 0.51 | 0.72 | 0.34 |
| Female | 0.81 | 0.81 | ||
| Histology | ||||
| Adeno | 0.78 | 0.19 | 0.81 | 0.02 |
| Non-adeno | 0.91 | 0.65 | ||
| T-factor | ||||
| T1 | 0.88 | 0.05 | 0.9 | 0.001 |
| Over T1 | 0.73 | 0.58 | ||
| N-factor | ||||
| Negative | 0.86 | 0.007 | 0.84 | <0.001 |
| Positive | 0.63 | 0.44 | ||
| Pathological stage | ||||
| IA or IB | 0.89 | <0.001 | 0.83 | 0.002 |
| Over I | 0.61 | 0.53 | ||
| Technique | ||||
| Partial | 1 | 0.02 | 0.85 | 0.79 |
| Lobectomy | 0.78 | 0.73 | ||
| I-CTP (ng/ml) | ||||
| Elevated (>4.5) | 0.42 | <0.001 | 0.47 | 0.29 |
| Normal (≤4.5) | 0.93 | 0.8 | ||
| CEA (ng/ml) | ||||
| Elevated (>5.0) | 0.72 | 0.02 | 0.78 | 0.44 |
| Normal (≤5.0) | 0.86 | 0.75 | ||
| CYFRA (ng/ml) | ||||
| Elevated (>3.5) | 0.64 | 0.01 | 0.47 | 0.001 |
| Normal (≤3.5) | 0.85 | 0.81 | ||
| Characteristics . | Disease-free survival . | P-value . | Overall survival . | P-value . |
|---|---|---|---|---|
| Age (years) | ||||
| <69 | 0.81 | 0.92 | 0.79 | 0.96 |
| ≥70 | 0.84 | 0.73 | ||
| Gender | ||||
| Male | 0.83 | 0.51 | 0.72 | 0.34 |
| Female | 0.81 | 0.81 | ||
| Histology | ||||
| Adeno | 0.78 | 0.19 | 0.81 | 0.02 |
| Non-adeno | 0.91 | 0.65 | ||
| T-factor | ||||
| T1 | 0.88 | 0.05 | 0.9 | 0.001 |
| Over T1 | 0.73 | 0.58 | ||
| N-factor | ||||
| Negative | 0.86 | 0.007 | 0.84 | <0.001 |
| Positive | 0.63 | 0.44 | ||
| Pathological stage | ||||
| IA or IB | 0.89 | <0.001 | 0.83 | 0.002 |
| Over I | 0.61 | 0.53 | ||
| Technique | ||||
| Partial | 1 | 0.02 | 0.85 | 0.79 |
| Lobectomy | 0.78 | 0.73 | ||
| I-CTP (ng/ml) | ||||
| Elevated (>4.5) | 0.42 | <0.001 | 0.47 | 0.29 |
| Normal (≤4.5) | 0.93 | 0.8 | ||
| CEA (ng/ml) | ||||
| Elevated (>5.0) | 0.72 | 0.02 | 0.78 | 0.44 |
| Normal (≤5.0) | 0.86 | 0.75 | ||
| CYFRA (ng/ml) | ||||
| Elevated (>3.5) | 0.64 | 0.01 | 0.47 | 0.001 |
| Normal (≤3.5) | 0.85 | 0.81 | ||
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
The 5-year disease-free survival rate was lower in patients with elevated preoperative serum I-CTP levels than those with normal I-CTP levels (41.8 vs 92.9%; P < 0.0001) (Fig. 1a). There were also significant differences in 5-year recurrence-free survival rates between patients with elevated and normal preoperative serum CEA levels (71.7 vs 85.6%; P = 0.0221) and CYFRA levels (63.8 vs 84.7%; P = 0.0120). There was no significant difference in overall 5-year survival rate between patients with elevated and normal preoperative I-CTP levels (46.9 vs 79.9%; P = 0.2931) (Fig. 1b).
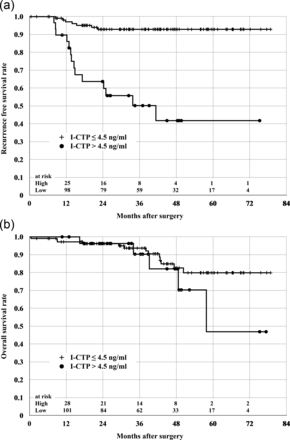
(a) 5-year distant metastasis-free survival rates. The 5-year disease-free survival rate was lower in patients with an elevated preoperative serum I-CTP level than those with a normal preoperative serum I-CTP level (41.8 vs 92.9%; P < 0.0001). (b) 5-year overall survival rates. There was no significant difference in overall 5-year survival rate between patients with an elevated preoperative serum I-CTP level and those with a normal preoperative serum I-CTP level (46.9 vs 79.9%; P = 0.2931).
Cox multivariate analysis for distant metastasis (Table 5) demonstrated the preoperative serum I-CTP level to be the strongest predictor (hazard ratio 10.030; P < 0.0001). On the other hand, Table 6 shows that pathological N-factor was the only significant predictor of overall survival of multivariate analysis (hazard ratio 3.066; P = 0.04). Both pathological stage and surgical technique were deleted in this multivariate analysis because they were covariates of N-factor.
| . | . | Distant metastasis . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| pN | >N1 | 1.795 | 0.2720 |
| I-CTP | >4.5 ng/ml | 10.030 | <0.0001 |
| CEA | >5.0 ng/ml | 2.993 | 0.0154 |
| CYFRA | >3.5 ng/ml | 2.369 | 0.1209 |
| . | . | Distant metastasis . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| pN | >N1 | 1.795 | 0.2720 |
| I-CTP | >4.5 ng/ml | 10.030 | <0.0001 |
| CEA | >5.0 ng/ml | 2.993 | 0.0154 |
| CYFRA | >3.5 ng/ml | 2.369 | 0.1209 |
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
| . | . | Distant metastasis . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| pN | >N1 | 1.795 | 0.2720 |
| I-CTP | >4.5 ng/ml | 10.030 | <0.0001 |
| CEA | >5.0 ng/ml | 2.993 | 0.0154 |
| CYFRA | >3.5 ng/ml | 2.369 | 0.1209 |
| . | . | Distant metastasis . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| pN | >N1 | 1.795 | 0.2720 |
| I-CTP | >4.5 ng/ml | 10.030 | <0.0001 |
| CEA | >5.0 ng/ml | 2.993 | 0.0154 |
| CYFRA | >3.5 ng/ml | 2.369 | 0.1209 |
CEA: carcinoembryonic antigen; I-CPT: type I collagen; CYFRA: cytokeratin-19 fragment.
| . | . | Overall survival . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| Histology | Adenocarcinoma | 2.385 | 0.0953 |
| pT | >T2a | 2.898 | 0.0905 |
| pN | >N1 | 3.066 | 0.0412 |
| CYFRA | >3.5 ng/ml | 1.372 | 0.5809 |
| . | . | Overall survival . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| Histology | Adenocarcinoma | 2.385 | 0.0953 |
| pT | >T2a | 2.898 | 0.0905 |
| pN | >N1 | 3.066 | 0.0412 |
| CYFRA | >3.5 ng/ml | 1.372 | 0.5809 |
CYFRA: cytokeratin-19 fragment.
| . | . | Overall survival . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| Histology | Adenocarcinoma | 2.385 | 0.0953 |
| pT | >T2a | 2.898 | 0.0905 |
| pN | >N1 | 3.066 | 0.0412 |
| CYFRA | >3.5 ng/ml | 1.372 | 0.5809 |
| . | . | Overall survival . | |
|---|---|---|---|
| Hazard ratio . | P-value . | ||
| Histology | Adenocarcinoma | 2.385 | 0.0953 |
| pT | >T2a | 2.898 | 0.0905 |
| pN | >N1 | 3.066 | 0.0412 |
| CYFRA | >3.5 ng/ml | 1.372 | 0.5809 |
CYFRA: cytokeratin-19 fragment.
DISCUSSION
Surgery remains the most effective treatment for early stage NSCLC. However, the 5-year survival rate in patients who underwent complete resection is only 45–75% [11]. Recently, several prognostic factors for NSCLC have been identified [8, 12]. Many of these factors are markers for the aggressiveness of the cancer, such as the Ki-67 level. CEA and CYFRA are produced by the cancer cells, and serum CEA and CYFRA levels are tumour markers for NSCLC [13]. Many reports have indicated that these markers can predict prognosis in patients with lung cancer [8, 14].
Type I collagen comprises ≥90% of the organic substance in bone. I-CTP is a metabolite that is released during the degradation of Type I collagen. It is thought that an increased serum I-CTP level may indicate bone resorption or accelerated bone metabolism due to bone metastasis [5]. Some other markers that are similar to I-CTP have been identified, such as type I collagen cross-linked N-telopeptide. The serum I-CTP level is more useful than these other markers for evaluating a disease because it has a smaller circadian variation and is less affected by menopause [15]. The serum I-CTP level has previously been reported to be a useful marker of bone metastasis in patients with lung cancer [5].
We measured pre- and postoperative serum I-CTP levels in patients with operable NSCLC because we speculated that an elevated serum I-CTP level might indicate bone metastasis. We retrospectively evaluated whether the preoperative serum I-CTP level is a prognostic indicator in patients with operable NSCLC.
The serum I-CTP level is increased in patients with bone metastasis, and also in patients with bone injuries such as fractures or renal dysfunction. We, therefore, excluded patients with bone diseases and abnormal renal function from this study.
The cutoff level of serum I-CTP for the diagnosis of bone metastasis is 4.5 ng/ml [6–8]. This is probably not the most suitable cutoff level for the diagnosis of distant metastasis. Receiver operating characteristic (ROC) analysis revealed that cutoff level that yielded the highest accuracy to predict distant metastasis in our patients was 6.7 ng/ml (Fig. 2). This cutoff level provided an accuracy of 88.1%, a sensitivity of 33.3% and a specificity of 97.5%. However, an accuracy that provided in the former cutoff level was 84.3%. There were no significant differences about these accuracies. Moreover, double standard cutoff levels are confusing. Therefore, we applied the cutoff level of serum I-CTP for the diagnosis of bone metastasis in our study.
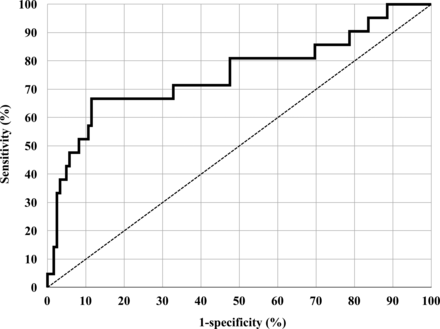
Receiver operating characteristic (ROC) curves of I-CTP. The solid black line indicates the ROC curve of I-CTP.
In our study, the preoperative serum I-CTP level was elevated in 29 patients. Preoperative bone metastasis was not identified in any of the patients, but we speculate that some had latent skeletal metastasis. Postoperative distant metastasis was detected in 21 patients during the 39 ± 18 (range 1–79) months of follow-up. Of these, 14 patients had an elevated preoperative serum I-CTP level. The rate of distant metastasis was significantly higher in patients with elevated preoperative serum I-CTP levels than those with normal preoperative I-CTP levels, and the preoperative serum I-CTP level was significantly higher in patients with metastasis than those without. Multivariate analysis identified an elevated preoperative serum I-CTP level as the strongest independent predictive factor only for postoperative distant metastasis. Together, these findings suggest that a high preoperative serum I-CTP level indicates microscopic bone metastasis, causing activation of bone metabolism. Some studies have reported that the serum I-CTP level is a prognostic factor in patients' lung and other cancers [16, 17]. However, it is unclear why preoperative serum I-CTP level predicts postoperative distant metastasis. It has been reported that the presence of tumour cells in bone marrow is associated with poor prognosis in other cancers [18, 19], and we speculate that the serum I-CTP level might reflect the presence of cancer cells in the bone marrow, even if such cells do not result in visible bone metastasis. We are planning further investigation to determine if cancer cells can be detected in the bone marrow of patients with high preoperative serum I-CTP levels.
Pathological T and N stage were not found to be independent predictive factors for distant metastasis in this study. This is probably because all patients in the study had operable cancer, and most were treated during the early stage. We, therefore, do not know whether the preoperative serum I-CTP level is a significant predictor of postoperative distant metastasis in patients with operable advanced NSCLC.
Postoperative bone metastasis was observed only in three patients in this study, contrary to our expectations. This does not mean that the preoperative serum I-CTP level does not appear to be a predictor of postoperative bone metastasis, but that it simply appears to be a predictor of postoperative distant metastasis as a whole. It has been reported that cancer cells frequently occur in the bone marrow of patients with solid cancers before surgical resection [20]. We speculate that this is because bone metastasis requires both the presence of cancer cells in bone marrow and osteoclast activation, and only cancer cells that stimulate osteoclast activation will cause bone destruction [21].
In conclusion, the preoperative serum I-CTP level is a predictor of distant metastasis after curative resection of NSCLC. We speculate that patients with high preoperative serum I-CTP levels have microscopic bone marrow metastases, although the mechanism of the increase in the serum I-CTP level is unclear.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr L. Spaggiari(Milan, Italy): The authors have had the good idea of looking for an innovative serum marker which could be related to the agressiveness of the tumour and to the prognosis of the patient with non-small cell lung cancer.
The aim of this study was in fact to examine the relationship between the preoperative and postoperative I-CTP levels, which is a metabolite released during the degradation of type I collagen, and postoperative distant metastasis in patients with non-small-cell lung cancer. Serum I-CTP level has previously been reported to be a useful marker of bone metastasis in patients with non-small-cell lung cancer, and based on that, the author speculated on the idea that an elevated serum I-CTP level may indicate bone metastasis. Unfortunately, in your results, preoperative serum I-CTP level was significantly higher in patients with postoperative distant metastasis, but only three patients out of 21 had bone metastasis. So you demonstrated a good relation between a high level of I-CTP and distant metastasis as well as a lower five-year recurrence-free survival rate, but you did not show any correlation between I-CTP and bone metastasis. The first question is: what is your point in continuing to support this speculation that I-CTP is correlated to bone metastasis? Why do you speculate this? The second question is: did you check the level of CTP in a group of patients with manifested bone metastasis to understand when the marker starts increasing? When it is already an evident metastasis or earlier?
Dr Tanaka: Concerning the first question, we speculate that serum I-CTP level might reflect the presence of cancer cells in the bone marrow, even if such cells do not result in visible bone metastasis. It has been reported that cancer cells frequently occur in the bone marrow of patients with solid cancers before resection. We speculate that this is because bone metastasis requires both the presence of cancer cells in bone marrow and osteoclast activation, and only cancer cells that stimulate osteoclast activation will cause bone destruction.
Dr Spaggiari: Have you analysed the level of CTP in a group of patients with manifested bone metastasis to study the behaviour of this marker?
Dr Tanaka: We have never re-examined the level of I-CTP in a group of patients who were not eligible for surgery.
Author notes
Presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.




