-
PDF
- Split View
-
Views
-
Cite
Cite
Niels J. Verberkmoes, Sander L. Wolters, Johannes C. Post, Mohamed A. Soliman-Hamad, Joost F. ter Woorst, Eric Berreklouw, Distal anastomotic patency of the Cardica C-PORT® xA system versus the hand-sewn technique: a prospective randomized controlled study in patients undergoing coronary artery bypass grafting, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 3, September 2013, Pages 512–519, https://doi.org/10.1093/ejcts/ezt079
Close - Share Icon Share
Abstract
The C-Port® Distal Anastomosis Systems (Cardica, Inc., Redwood City, CA, USA) demonstrated favourable results in feasibility trials. However, distal vein anastomoses created with the C-Port® or C-Port xA® system have never been compared with hand-sewn distal vein anastomoses. The objective of this study was to compare distal end-to-side anastomoses facilitated with the C-Port xA® System with the traditional hand-sewn method.
This single-centre prospective randomized controlled study comprised 71 patients (device group n = 35, control group n = 36) who underwent primary elective coronary artery bypass grafting between June 2008 and April 2011. The primary study end-point was 12-month distal anastomotic patency, which was assessed with prospective ECG-gated 256-multislice computed tomographic coronary angiography using a step-and-shoot scanning protocol. For the primary end-point, a per-protocol analysis was used.
In the device group, four (11%) anastomoses were converted to hand-sewn anastomoses, and additional stitches to achieve haemostasis were necessary in 22 (76%) patients. There was no hospital mortality in either group. During the 12-month follow-up, a single death occurred in the Device group and was unrelated to the device. Twenty-nine patients in the device group and 32 in the control group completed 12-month CT coronary angiography. The overall patency of 160 studied distal vein graft anastomoses was 93%. Comparison of the end-to-side target anastomosis showed 12-month patencies of 86 and 88% in the device group and the control group, respectively.
According to these preliminary results and despite the limited number of patients, the use of the C-Port xA® System is safe enough to perform distal end-to-side vein graft anastomosis, with respect to 12-month end-to-side distal venous anastomotic patency. Although there are some technical challenges with this device, the incidence of complications is comparable to the traditional hand-sewn technique.
INTRODUCTION
The introduction of minimally invasive direct coronary artery bypass grafting started in the mid-90s with single-vessel coronary artery bypass surgery [1, 2]. Nowadays, totally endoscopic coronary artery bypass grafting has become feasible. Most reports concern minimally invasive bypass grafting of only the left anterior descending artery (LAD) [3, 4]. In more recent years, totally endoscopic multivessel coronary artery bypass grafting (CABG) has also become feasible. Despite the satisfactory results [5], the procedure has not gained wide acceptance, because of the difficulty in performing anastomotic suturing. To resolve this challenge, different distal anastomotic devices have been designed [6–9]. However, most of these devices are no longer commercially available. In 2005, the first-generation C-Port® Distal Anastomosis Systems (Cardica, Inc., Redwood City, CA, USA) became available for use [10]. In November 2007, the second-generation C-Port® xA Distal Anastomosis Systems received an FDA 510k Premarket Notification [11] and was introduced for commercial distribution. The C-Port® Distal Anastomosis Systems demonstrated favourable results and promising short-term patency rates in non-randomized, observational, feasibility trials [10, 12]. However, distal vein anastomoses created with the C-port® or C-Port® xA system have never been compared with hand-sewn distal vein anastomoses in a prospective randomized controlled study. The objective of this study was to compare the 12-month end-to-side distal vein anastomotic patency facilitated with The C-Port® xA Distal Anastomosis System with the traditional hand-sewn method.
MATERIALS AND METHODS
Study design
This study was designed as a prospective, randomized, controlled, phase IV study and conducted at the Catharina Hospital, Eindhoven, between June 2008 and April 2011. The study was conducted in accordance with current good clinical practices and the ethical principles of the declaration of Helsinki. The study was conducted after institutional ethics committee approval and registered at the National Central Committee on Research Involving Human Subjects. According to the recommendations of the International Committee of Medical Journal Editors, the study was registered at http://www.trialregister.nl (NTR1409).
All patients who were referred to our centre for elective CABG and who met the inclusion and exclusion criteria were screened. Enrolment and randomization were performed after the patients had signed an informed consent. Inclusion and exclusion criteria are shown in Table 1.
| Inclusion criteria |
|
Additional intraoperative inclusion criteria
|
| Exclusion criteria |
|
| Inclusion criteria |
|
Additional intraoperative inclusion criteria
|
| Exclusion criteria |
|
CABG, coronary artery bypass grafting; IABP: intra-aortic balloon pump; PTCA: percutaneous transluminal coronary angioplasty.
| Inclusion criteria |
|
Additional intraoperative inclusion criteria
|
| Exclusion criteria |
|
| Inclusion criteria |
|
Additional intraoperative inclusion criteria
|
| Exclusion criteria |
|
CABG, coronary artery bypass grafting; IABP: intra-aortic balloon pump; PTCA: percutaneous transluminal coronary angioplasty.
Patients accepted in the study were randomized using the random drawing (envelope) method. All participants were blinded for group assignment. In the C-Port xA® group (device group), end-to-side venous distal anastomoses were performed with the C-Port xA® Distal Anastomosis System. In the control group, end-to-side venous distal anastomoses were performed with continues 7–0 polypropylene sutures. In both groups, side-to-side distal anastomoses and proximal anastomoses were performed with the traditional hand-sewn technique. Detailed operation techniques are outlined below.
Additional intraoperative inclusion criteria, according to the manufacturer's Instruction For Use, consisted of specific properties for the coronary artery site and the bypass conduit and are shown in Table 1. Severity of calcifications, wall thickness of the coronary artery and internal diameter were estimated by the surgeon. In the device group, if there was any doubt about the internal diameter, we inserted a 1.25-mm probe in the arteriotomy hole that was made to insert the C-Port Xa® anvil.
The C-port xA® distal anastomosis system
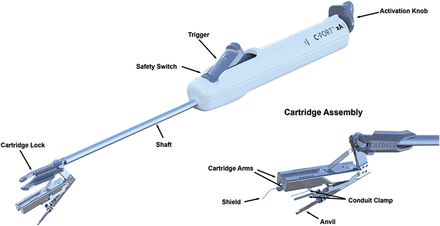
The Cardica C-Port® xA Distal Anastomosis Systems and other system components are intended to create a sutureless vascular anastomosis between blood vessels (Fig. 1). Specifically, the Cardica™ C-Port xA is indicated for creating end-to-side distal anastomosis between saphenous vein graft, radial artery or internal mammary artery grafts and the coronary arteries during CABG procedures. To create a complete end-to-side anastomosis, two arrays of six stainless steel micro-clips plus an extra heel clip are delivered by the device (Fig. 2). During deployment, the clips pierce the walls of both the coronary artery and the graft. The clips are then deformed by interacting with the anvil thereby firmly attaching the coronary artery to the graft. A knife located in the centre of the anvil creates an arteriotomy from the inside out of the coronary artery. The main components of the device are the cartridge with two conduit clamps, the anvil with the arteriotomy knife and the device holder containing a CO2 cartridge, an activation knob and a trigger (Fig. 1). The Cardica CPort® xA device is the second-generation C-Port Distal Anastomosis System. The C-Port® xA has been improved by changing the design of the toe clips to improve haemostasis at the toe. These particular clips have side arms that cover the toe. The use of the device consists of three main steps: precise graft, target vessel preparation and secure deployment (Fig. 3). For loading to the C-Port® xA device, the distal end of the saphenous vein has to be free of side branches for at least 15 mm and the conduit has to be incised at the heel. The conduit is inserted between the two cartridge arms. The heel of the conduit has to be everted over the heel clip. The selected target vessel needs to be prepared by dissecting the epicardial tissue, and an incision has to be made immediately proximal to the anastomosis site. The anvil of the C-Port® xA is inserted into the incision in the coronary artery. Finally, the anastomosis is completed by unlocking the safety switch and then compressing the trigger. The knife located inside the anvil creates the arteriotomy after the clips penetrated the graft and target vessel. By releasing the trigger, the cartridge unclamps and can be removed from the anastomosis site. As the last step, the surgeon places a stitch to close the anvil insertion site at the heel of the anastomosis.
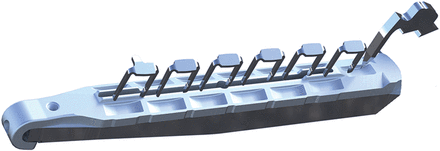
One array of six stainless steel micro-clips plus an extra heel clip.
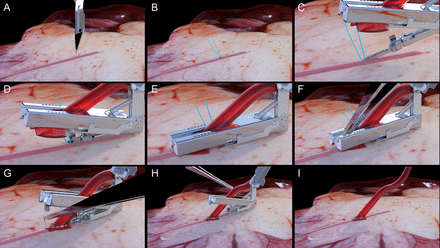
(A–C) Graft and target vessel preparation; (D–F) deployment of the C-Port device; (G–I) removal of the C-Port device.
Operative techniques
In all patients, a standard midline sternotomy was performed and the left internal mammary artery (LIMA) was used as bypass conduit for treatment of lesions in the left anterior descending artery (LAD). For the other coronary arteries, saphenous vein grafts, harvested by the open technique, were used. The use of sequential grafts or single grafts was dependent on the number of coronary lesions. In the device group, one device per patient was used. Heparin was given to achieve an activated clotted time of ∼440 s. Normothermic extracorporeal circulation was performed using non-pulsatile flow. Warm blood cardioplegia was used to induce and maintain cardioplegic arrest. In OPCAB surgery, no ischaemic preconditioning was performed and no coronary occluder clips or snares were used. A bloodless field was routinely achieved using Clearview intracoronary shunts (Medtronic, Minneapolis, MN, USA) in combination with the Accumist® (Medtronic, Minneapolis, MN, USA). Stabilization of the operated coronary artery was achieved using the Octopus II stabilizer (Medtronic, Minneapolis, MN, USA). In all patients, intraoperative transit time flow measurement with the Medistim's VeriQ flowmeter (Medistim, Oslo, Norway) was used to determine inadequate anastomosis. A revision of the anastomosis was mandated if graft flow was <20 ml/min or a Pulsatility Index >5. In both on-pump and off-pump CABG, heparin was neutralized by protamine chloride, with a dose of 1 mg protamine per 1 mg heparin. Postoperative antiplatelet treatment consisted of a low-dose acetylsalicylic acid (80 mg orally) on a daily basis, starting on the first postoperative day.
Study end-points
The primary end-points were acute and chronic anastomotic patency of the end-to-side anastomosis, as determined by intraoperative flow measurements and as determined by computed tomography angiography (CTA) at 12-month follow-up. Secondary end-points included incidence of device-related adverse events and convenience of the system.
Follow-up
Patients were asked to return at 6 months postoperatively for a clinical follow-up. A second follow-up was scheduled after 12 months to determine 12-month patency using a 256-slice Brilliance iCT scanner (Philips healthcare, Best, Netherlands). Prospective ECG triggering and the step-and-shoot (SAS) technique were used as scan algorithm. Scan parameters included the use of 120 kV with 200 mAs and a 112 × 0.625 collimation with a 512 matrix.
Patients with a heart rate higher than 70 beats per minute (bpm) were treated with 100 mg metoprolol tartrate (TEVA Pharmachemie, Haarlem, Netherlands) before scanning. If necessary, intravenous administration of 5–15 mg metoprolol tartrate, was given immediately before scanning. Patients were excluded from scanning if their heart rate remained over 70 bpm. A bolus of 85 ml Iomeron® contrast agent (Bracco Imaging Europe, Amsterdam, the Netherlands) was injected intravenously using an antecubital intravenous line with an automated infusion system (Medrad, Warrendale, PA, USA) at a flow rate of 5 ml/s. The threshold was set at 110 Houndsfield Units (HU). Patients with a creatinine clearance lower than 45 ml/min underwent prehydration. Patients were excluded if creatinine clearance was lower than 20 ml/min. Post-processing was performed by two radiologists and one cardiologist, who were blinded for group assignment, on an Extended Brilliance Workspace (Philips healthcare, Best, Netherlands). All data were volume rendered in different angles. A patent distal anastomosis was defined as <50% narrowing of the lumen.
Statistical design and analysis
For the primary end-points, a per-protocol analysis was used. Continuous data are presented as mean (standard deviation; range) and comparison was performed using the independent-sample t-test. For comparison of data that were not normally distributed, the Mann–Whitney U-test was used. Categorical data are presented as proportions and comparison was done using the Pearson's χ2 test or the Fisher's exact test when appropriate. All statistical tests were two sided and tests with a P-value of 0.05 or lower were considered significant. All statistical analyses were done using the Statistical Package for Social Sciences software, version 19.0 (SPSS, Chicago, IL, USA).
RESULTS
A total of 78 patients were enrolled in this study. In both the device group and the control group, 39 patients were included. Four patients (10%) in the device group and 3 (8%) in the control group did not met intraoperative inclusion criteria and were withdrawn from the study. Baseline characteristics of both patients groups are summarized in Table 2. Both groups were comparable with respect to age, sex, BMI, comorbidities, number of diseased vessels, left main coronary artery disease and left ventricular function.
| Variables . | C-Port® device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Mean age ± SD (years) | 67.6 ± 5.6 | 66.5 (5.7) | 0.40 |
| Male gender | 31 (89) | 32 (89) | 0.97 |
| BMI ± SD | 27.5 ± 3.7 | 27.1 ± 3.3 | 0.65 |
| Diabetes | 9 (26) | 11 (31) | 0.80 |
| Hypertension | 20 (57) | 20 (56) | 0.89 |
| COPD | 3 (9) | 5 (14) | 0.71 |
| PVD | 6 (17) | 5 (14) | 0.75 |
| Prior CVA | 4 (11) | 4 (11) | 1.00 |
| Previous myocardial infarction | 9 (26) | 15 (42) | 0.21 |
| Number of diseased vessels ± SD | 2.63 ± 0.49 | 2.61 ± 0.54 | 0.89 |
| LMCAD | 1 (3) | 6 (17) | 0.11 |
| LV function (%) | |||
| >50 | 32 (91) | 33 (92) | 0.97 |
| 35–50 | 3 (9) | 3 (8) | |
| Variables . | C-Port® device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Mean age ± SD (years) | 67.6 ± 5.6 | 66.5 (5.7) | 0.40 |
| Male gender | 31 (89) | 32 (89) | 0.97 |
| BMI ± SD | 27.5 ± 3.7 | 27.1 ± 3.3 | 0.65 |
| Diabetes | 9 (26) | 11 (31) | 0.80 |
| Hypertension | 20 (57) | 20 (56) | 0.89 |
| COPD | 3 (9) | 5 (14) | 0.71 |
| PVD | 6 (17) | 5 (14) | 0.75 |
| Prior CVA | 4 (11) | 4 (11) | 1.00 |
| Previous myocardial infarction | 9 (26) | 15 (42) | 0.21 |
| Number of diseased vessels ± SD | 2.63 ± 0.49 | 2.61 ± 0.54 | 0.89 |
| LMCAD | 1 (3) | 6 (17) | 0.11 |
| LV function (%) | |||
| >50 | 32 (91) | 33 (92) | 0.97 |
| 35–50 | 3 (9) | 3 (8) | |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; CVA: cerebrovascular accident; LMCD: left main coronary artery disease.
| Variables . | C-Port® device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Mean age ± SD (years) | 67.6 ± 5.6 | 66.5 (5.7) | 0.40 |
| Male gender | 31 (89) | 32 (89) | 0.97 |
| BMI ± SD | 27.5 ± 3.7 | 27.1 ± 3.3 | 0.65 |
| Diabetes | 9 (26) | 11 (31) | 0.80 |
| Hypertension | 20 (57) | 20 (56) | 0.89 |
| COPD | 3 (9) | 5 (14) | 0.71 |
| PVD | 6 (17) | 5 (14) | 0.75 |
| Prior CVA | 4 (11) | 4 (11) | 1.00 |
| Previous myocardial infarction | 9 (26) | 15 (42) | 0.21 |
| Number of diseased vessels ± SD | 2.63 ± 0.49 | 2.61 ± 0.54 | 0.89 |
| LMCAD | 1 (3) | 6 (17) | 0.11 |
| LV function (%) | |||
| >50 | 32 (91) | 33 (92) | 0.97 |
| 35–50 | 3 (9) | 3 (8) | |
| Variables . | C-Port® device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Mean age ± SD (years) | 67.6 ± 5.6 | 66.5 (5.7) | 0.40 |
| Male gender | 31 (89) | 32 (89) | 0.97 |
| BMI ± SD | 27.5 ± 3.7 | 27.1 ± 3.3 | 0.65 |
| Diabetes | 9 (26) | 11 (31) | 0.80 |
| Hypertension | 20 (57) | 20 (56) | 0.89 |
| COPD | 3 (9) | 5 (14) | 0.71 |
| PVD | 6 (17) | 5 (14) | 0.75 |
| Prior CVA | 4 (11) | 4 (11) | 1.00 |
| Previous myocardial infarction | 9 (26) | 15 (42) | 0.21 |
| Number of diseased vessels ± SD | 2.63 ± 0.49 | 2.61 ± 0.54 | 0.89 |
| LMCAD | 1 (3) | 6 (17) | 0.11 |
| LV function (%) | |||
| >50 | 32 (91) | 33 (92) | 0.97 |
| 35–50 | 3 (9) | 3 (8) | |
BMI: body mass index; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; CVA: cerebrovascular accident; LMCD: left main coronary artery disease.
Intraoperative characteristics are presented in Table 3. The majority of the end-to-side study anastomoses were performed on the right descending posterior coronary artery; namely in 63 and 81% (P = 0.42) of patients in the device group and control group, respectively. The majority of the study anastomoses were part of venous sequential grafts, 74 and 83% (P = 0.35) of patients in the device group and control group, respectively. Intraoperative graft flow was comparable between the device group and the control group (42.2 ± 19.9 ml/min vs 47.9 ± 16.9 ml/min, P = 0.41). In the device group, no revisions were needed after flow measurement at the end of the procedure. In four (11%) intent-to-treat C-Port xA® anastomoses, a conversion to hand-sewn anastomosis was required because of other complications. In one of these device cases, the CO2 cartridge could not be activated and the device did not deploy. In another device case, severe calcification, which was underestimated when choosing the arteriotomy site, may have resulted in an inadequate arteriotomy. In the third conversion, haemostasis of the C-Port xA® anastomosis could not be obtained and, in the last conversion, the venous conduit was thicker than 0.75 mm and therefore not suitable. These 4 patients were excluded from 6- and 12-month follow-up. Besides the necessary stitch to close the anvil insertion at the heel of the anastomosis, it was required in 22 (76%) patients to place one or more additional stitches at the toe (n = 13; 59%), the heel (n = 2; 9%), the heel and the toe (n = 8; 36%) or at the side (n = 1; 5%).
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Off-pump CABG | 5 (14) | 3 (8) | 0.43 |
| On-pump CABG | 30 (86) | 33 (92) | 0.43 |
| Cross-clamp time ± SD (min) | 46 ± 16 | 44 ± 18 | 0.74 |
| Perfusion time ± SD (min) | 81 ± 28 | 71 ± 24 | 0.13 |
| Number of distal anastomoses ± SD | 3.5 ± 1.0 | 3.8 ± 1.0 | 0.11 |
| LIMA to LAD | 32 (91) | 34 (94) | 0.62 |
| Target anastomose | |||
| PDA | 22 (63) | 29 (81) | 0.42 |
| PLRCA | 2 (6) | 1 (3) | |
| RCA | 4 (11) | 4 (11) | |
| OM1 | 4 (11) | 2 (6) | |
| OM2 | 2 (6) | 0 (0) | |
| D1 | 1 (3) | 0 (0) | |
| Diameter target anastomose ± SD (mm) | 1.48 ± 0.22 | 1.49 ± 0.32 | 0.91 |
| Jump graft | 26 (74) | 30 (83) | 0.35 |
| Single graft | 9 (26) | 6 (17) | 0.35 |
| Study graft − flow (ml/min) ± SD | 42.2 ± 19.9 | 47.9 ± 16.9 | 0.41 |
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Off-pump CABG | 5 (14) | 3 (8) | 0.43 |
| On-pump CABG | 30 (86) | 33 (92) | 0.43 |
| Cross-clamp time ± SD (min) | 46 ± 16 | 44 ± 18 | 0.74 |
| Perfusion time ± SD (min) | 81 ± 28 | 71 ± 24 | 0.13 |
| Number of distal anastomoses ± SD | 3.5 ± 1.0 | 3.8 ± 1.0 | 0.11 |
| LIMA to LAD | 32 (91) | 34 (94) | 0.62 |
| Target anastomose | |||
| PDA | 22 (63) | 29 (81) | 0.42 |
| PLRCA | 2 (6) | 1 (3) | |
| RCA | 4 (11) | 4 (11) | |
| OM1 | 4 (11) | 2 (6) | |
| OM2 | 2 (6) | 0 (0) | |
| D1 | 1 (3) | 0 (0) | |
| Diameter target anastomose ± SD (mm) | 1.48 ± 0.22 | 1.49 ± 0.32 | 0.91 |
| Jump graft | 26 (74) | 30 (83) | 0.35 |
| Single graft | 9 (26) | 6 (17) | 0.35 |
| Study graft − flow (ml/min) ± SD | 42.2 ± 19.9 | 47.9 ± 16.9 | 0.41 |
CABG: coronary artery bypass grafting; LAD: left anterior descending artery; LIMA: left internal mammary artery; PDA: posterior descending artery; PLRCA: right posterolateral artery; RCA: right coronary artery; OM: obtuse marginal; D: diagonal branch.
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Off-pump CABG | 5 (14) | 3 (8) | 0.43 |
| On-pump CABG | 30 (86) | 33 (92) | 0.43 |
| Cross-clamp time ± SD (min) | 46 ± 16 | 44 ± 18 | 0.74 |
| Perfusion time ± SD (min) | 81 ± 28 | 71 ± 24 | 0.13 |
| Number of distal anastomoses ± SD | 3.5 ± 1.0 | 3.8 ± 1.0 | 0.11 |
| LIMA to LAD | 32 (91) | 34 (94) | 0.62 |
| Target anastomose | |||
| PDA | 22 (63) | 29 (81) | 0.42 |
| PLRCA | 2 (6) | 1 (3) | |
| RCA | 4 (11) | 4 (11) | |
| OM1 | 4 (11) | 2 (6) | |
| OM2 | 2 (6) | 0 (0) | |
| D1 | 1 (3) | 0 (0) | |
| Diameter target anastomose ± SD (mm) | 1.48 ± 0.22 | 1.49 ± 0.32 | 0.91 |
| Jump graft | 26 (74) | 30 (83) | 0.35 |
| Single graft | 9 (26) | 6 (17) | 0.35 |
| Study graft − flow (ml/min) ± SD | 42.2 ± 19.9 | 47.9 ± 16.9 | 0.41 |
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Off-pump CABG | 5 (14) | 3 (8) | 0.43 |
| On-pump CABG | 30 (86) | 33 (92) | 0.43 |
| Cross-clamp time ± SD (min) | 46 ± 16 | 44 ± 18 | 0.74 |
| Perfusion time ± SD (min) | 81 ± 28 | 71 ± 24 | 0.13 |
| Number of distal anastomoses ± SD | 3.5 ± 1.0 | 3.8 ± 1.0 | 0.11 |
| LIMA to LAD | 32 (91) | 34 (94) | 0.62 |
| Target anastomose | |||
| PDA | 22 (63) | 29 (81) | 0.42 |
| PLRCA | 2 (6) | 1 (3) | |
| RCA | 4 (11) | 4 (11) | |
| OM1 | 4 (11) | 2 (6) | |
| OM2 | 2 (6) | 0 (0) | |
| D1 | 1 (3) | 0 (0) | |
| Diameter target anastomose ± SD (mm) | 1.48 ± 0.22 | 1.49 ± 0.32 | 0.91 |
| Jump graft | 26 (74) | 30 (83) | 0.35 |
| Single graft | 9 (26) | 6 (17) | 0.35 |
| Study graft − flow (ml/min) ± SD | 42.2 ± 19.9 | 47.9 ± 16.9 | 0.41 |
CABG: coronary artery bypass grafting; LAD: left anterior descending artery; LIMA: left internal mammary artery; PDA: posterior descending artery; PLRCA: right posterolateral artery; RCA: right coronary artery; OM: obtuse marginal; D: diagonal branch.
In-hospital postoperative data are presented in Table 4 and showed no significant differences in outcome between the two groups. In each group, one resternotomy was performed because of excessive postoperative bleeding. In both patients, bleeding was not related to the distal anastomoses. In the control group, one resternotomy was performed because of ischaemia. This particular patient had a calcified aorta, and a piece of calcium occluded the proximal anastomosis. In the device group, enzymatic infarction occurred in 2 patients (6%). One of these 2 patients is the same in whom conversion to hand-sewn anastomosis was necessary due to the technical failure of the device itself as described above. In the other patient, no intraoperative complications were reported and the cause of this enzymatic infarction is unclear. In the control group, two (6%) enzymatic infarctions occurred with unclear cause.
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Prolonged ventilation | 1 (3) | 1 (3) | 1.00 |
| Prolonged inotropics or IABP | 2 (6) | 2 (6) | 1.00 |
| In-hospital mortality | 0 (0) | 0 (0) | N.A. |
| Resternotomy | |||
| Bleeding | 1 (3) | 1 (3) | 1.00 |
| Ischaemia | 0 (0) | 1 (3) | 1.00 |
| Cardiac complications | |||
| Infarction | 2 (6) | 2 (6) | 1.00 |
| Atrial fibrillation | 7 (20) | 6 (17) | 0.76 |
| Pulmonary complications | 4 (11) | 3 (8) | 0.71 |
| Renal complications | 2 (6) | 0 (0 | 0.24 |
| Stroke | 0 (0) | 0 (0) | NA |
| Gastrointestinal complications | 0 (0) | 0 (0) | NA |
| Deep wound infection | 1 (3) | 0 (0) | 0.49 |
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Prolonged ventilation | 1 (3) | 1 (3) | 1.00 |
| Prolonged inotropics or IABP | 2 (6) | 2 (6) | 1.00 |
| In-hospital mortality | 0 (0) | 0 (0) | N.A. |
| Resternotomy | |||
| Bleeding | 1 (3) | 1 (3) | 1.00 |
| Ischaemia | 0 (0) | 1 (3) | 1.00 |
| Cardiac complications | |||
| Infarction | 2 (6) | 2 (6) | 1.00 |
| Atrial fibrillation | 7 (20) | 6 (17) | 0.76 |
| Pulmonary complications | 4 (11) | 3 (8) | 0.71 |
| Renal complications | 2 (6) | 0 (0 | 0.24 |
| Stroke | 0 (0) | 0 (0) | NA |
| Gastrointestinal complications | 0 (0) | 0 (0) | NA |
| Deep wound infection | 1 (3) | 0 (0) | 0.49 |
IABP: intra-aortic balloon pump; NA: not applicable.
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Prolonged ventilation | 1 (3) | 1 (3) | 1.00 |
| Prolonged inotropics or IABP | 2 (6) | 2 (6) | 1.00 |
| In-hospital mortality | 0 (0) | 0 (0) | N.A. |
| Resternotomy | |||
| Bleeding | 1 (3) | 1 (3) | 1.00 |
| Ischaemia | 0 (0) | 1 (3) | 1.00 |
| Cardiac complications | |||
| Infarction | 2 (6) | 2 (6) | 1.00 |
| Atrial fibrillation | 7 (20) | 6 (17) | 0.76 |
| Pulmonary complications | 4 (11) | 3 (8) | 0.71 |
| Renal complications | 2 (6) | 0 (0 | 0.24 |
| Stroke | 0 (0) | 0 (0) | NA |
| Gastrointestinal complications | 0 (0) | 0 (0) | NA |
| Deep wound infection | 1 (3) | 0 (0) | 0.49 |
| Variables . | C-Port® Device (n = 35) . | Control (n = 36) . | P-value . |
|---|---|---|---|
| No. (%) . | No. (%) . | ||
| Prolonged ventilation | 1 (3) | 1 (3) | 1.00 |
| Prolonged inotropics or IABP | 2 (6) | 2 (6) | 1.00 |
| In-hospital mortality | 0 (0) | 0 (0) | N.A. |
| Resternotomy | |||
| Bleeding | 1 (3) | 1 (3) | 1.00 |
| Ischaemia | 0 (0) | 1 (3) | 1.00 |
| Cardiac complications | |||
| Infarction | 2 (6) | 2 (6) | 1.00 |
| Atrial fibrillation | 7 (20) | 6 (17) | 0.76 |
| Pulmonary complications | 4 (11) | 3 (8) | 0.71 |
| Renal complications | 2 (6) | 0 (0 | 0.24 |
| Stroke | 0 (0) | 0 (0) | NA |
| Gastrointestinal complications | 0 (0) | 0 (0) | NA |
| Deep wound infection | 1 (3) | 0 (0) | 0.49 |
IABP: intra-aortic balloon pump; NA: not applicable.
In the device group, 6-month follow-up (mean 5.7 ± 0.6 months) was completed in 29 (94%) patients. In addition to the 4 excluded patients with device failures, 1 patient in the device group died 3 months after discharge because of acute mitral valve endocarditis and 1 was unable to visit our clinic due to hospitalisation after carotid surgery. In the control group, 6-month follow-up (mean 5.5 ± 0.5 months) was completed by 35 (97%) patients and 1 voluntary withdrew from the study. In each group, 1 patient suffered from anginal symptoms, due to occlusion of a study vein graft, which required percutaneous coronary reintervention (PCI) of a native coronary artery.
Table 5 shows the CT data at 12-month follow-up. In the device group, 12-month follow-up (mean 11.5 ± 0.9 months) was completed in 29 (94%) patients. In the control group, 12-month follow-up (mean 11.1 ± 0.6 months) was completed by 33 (92%) patients. One patient was immobilized due to a complicated hip surgery. Two patients withdrew from the study due to non-cardiac health problems. In both the device group and the control group, 1 patient underwent PCI because of an occluded LIMA to LAD anastomosis. At the time of follow-up, cases and controls were treated with either acetylsalicylic acid (86 vs 89%, P = 0.78), acenocoumarol (10 vs 11%, P = 1.00) or clopidogrel (3 vs 0%, P = 0.45).
| Variables . | C-Port® Device (n = 29) . | Control (n = 32) . |
|---|---|---|
| No. (%) . | No. (%) . | |
| Patent end-to-side target anastomoses | 86.2 (25/29) | 87.5 (28/32) |
| Patent vein graft anastomoses | 91.7 (66/72) | 94.3 (83/88) |
| Patent LIMA-LAD anastomoses | 96.3 (26/27) | 93.3 (28/30) |
| Variables . | C-Port® Device (n = 29) . | Control (n = 32) . |
|---|---|---|
| No. (%) . | No. (%) . | |
| Patent end-to-side target anastomoses | 86.2 (25/29) | 87.5 (28/32) |
| Patent vein graft anastomoses | 91.7 (66/72) | 94.3 (83/88) |
| Patent LIMA-LAD anastomoses | 96.3 (26/27) | 93.3 (28/30) |
LIMA: left internal mammary artery.
| Variables . | C-Port® Device (n = 29) . | Control (n = 32) . |
|---|---|---|
| No. (%) . | No. (%) . | |
| Patent end-to-side target anastomoses | 86.2 (25/29) | 87.5 (28/32) |
| Patent vein graft anastomoses | 91.7 (66/72) | 94.3 (83/88) |
| Patent LIMA-LAD anastomoses | 96.3 (26/27) | 93.3 (28/30) |
| Variables . | C-Port® Device (n = 29) . | Control (n = 32) . |
|---|---|---|
| No. (%) . | No. (%) . | |
| Patent end-to-side target anastomoses | 86.2 (25/29) | 87.5 (28/32) |
| Patent vein graft anastomoses | 91.7 (66/72) | 94.3 (83/88) |
| Patent LIMA-LAD anastomoses | 96.3 (26/27) | 93.3 (28/30) |
LIMA: left internal mammary artery.
Evaluation of end-to-side distal anastomotic patency with 256-slice CTA was performed in 29 (94%) patients of the device group and in 32 (89%) of the control group. In the control group, 1 patient was excluded for CTA because of permanent atrial fibrillation. The 4 patients who underwent PCI were also evaluated with CTA after 12 months. The patency of end-to-side anastomoses facilitated with the C-Port xA® device was comparable to the patency of end-to-side anastomoses that were performed with the hand-sewn technique. Results are shown in Table 5. Additionally, the patencies of all 160 venous anastomoses, including study anastomoses, and 57 LIMA-LAD anastomoses were studied. Eleven venous anastomoses and three LIMA-LAD anastomoses were occluded, resulting in an overall venous anastomotic patency of 93% and overall LIMA anastomotic patency of 95%.
DISCUSSION
The present study demonstrated that using the C-Port xA® Distal Anastomosis System to perform end-to-side distal coronary artery anastomosis is comparable with the traditional hand-sewn technique with regard to intraoperative flow measurement and 12-month patency. Twelve-month patencies in both groups (86 vs 88%) are higher than those reported in recent literature, which shows 12-month patency rates varying between 73 and 78% [13, 14]. The higher patency rates in this study could be explained by the strict additional intraoperative inclusion criteria. The coronary artery must be free of calcification and must have an internal diameter of >1.25 mm. Therefore, 7 (9%) patients with severely diseased coronary arteries were excluded. This means that the study population included a selected group of coronary artery bypass patients.
To date, no studies have been published that report the 12-month patency of end-to-side distal venous anastomoses facilitated with the C-Port xA® device. Two non-randomized, observational trials have showed a short-term venous graft patency of 92 and 93% determined by angiographic or 64 slice multidetector row CTA [10, 12]. In the multicentre trial of Matschke et al. [10], 119 patients had successful C-Port anastomoses, and patency was evaluated at 6-months in 89 patients (75%). The feasibility trial of Cai et al. [12] reported 50 successful implants, and 3-month patency analysis was determined in 31 patients (62%). Cai et al. compared patencies in 18 study patients who received both treatments incidentally. In our study, 12-month follow-up was completed in 94 vs 92%, in the device group and the control group, respectively.
Our results at 12-month clinical follow-up are supported by the results of the 12-month clinical follow-up reported by Matschke et al. In our study, 4 patients’ (11%) intent-to-treat C-Port xA® anastomoses were converted to hand-sewn anastomoses due to technical failure or inappropriate vessel selection. Device malfunctions were also reported by Matschke et al. and Cai et al. in 11 of 130 (9%) and 8 of 69 (12%) device deployments, respectively.
In our study, additional stitches were necessary in 22 (76%) patients of the device group to obtain haemostasis. Matschke et al. reported the necessity for additional stitches to achieve haemostasis in ‘many’ cases, without reporting an exact number of cases. Cai et al. reported 2 (4%) patients who underwent resternotomy for device-related bleeding and excluded them from further analysis. Two studies on the C-Port xA® device facilitating IMA to LAD anastomoses reported the need for additional stitches in <20% of cases [15, 16]. An explanation for the difference in the need for additional sutures between using the C-Port xA® with saphenous veins or IMAs could be the difference between the mechanical wall properties of venous and arterial conduits. Arteries have a tunica media, which contains more vascular smooth muscle resulting in a thicker wall. Therefore, it could be possible that the wall-squeezing properties of the flanges of the micro clips are not yet optimized for venous conduits.
Though, the need for additional stitches is undesirable, particularly in a device that facilitates sutureless anastomosis. Probably, adjustments to the design are needed before it can be used routinely in everyday practice and particularly when used in minimally invasive surgery.
Prior reports have demonstrated the ability to accurately visualize the patency of distal anastomoses with interrupted clips with 64-slice CTA [12, 17]. To the best of our knowledge, this study is the first that has reported evaluation with 256-slice CTA of distal anastomoses facilitated with an automated anastomotic device. By the use of a customized scan protocol, no artefacts were caused by the stainless steel clips of the device and all distal anastomoses could be assessed in detail. The improved 256-slice CTA combined with the prospective ECG-gated SAS technique, is a potential non-invasive alternative method with a high diagnostic accuracy to evaluate postoperative graft patency instead of the gold standard coronary angiography. An additional finding of this study is the overall venous anastomotic patency of 93%. This result was reported previously by our institution [18] and was less influenced by the exclusion of severely diseased coronary arteries, because the severity of lesions did not determine intraoperative exclusion.
LIMITATIONS
The present study has some limitations. First, due to limited funding, this study has a very small sample size. Therefore, not enough statistical power could be achieved for the desired end-point. With a high probability of committing a type II error, the chance of having the same 12-month patency rates for end-to-side distal venous anastomosis, when using the C-Port xA® device in comparison with hand-sewn distal venous anastomosis, could be overestimated. Second, because of the additional intraoperative inclusion criteria, only highly selected patients are included in this study. The results only relate to this particular subgroup and may not be taken for granted in all patient profiles. The third limitation is that the study covered a long period. Therefore, it could be that the surgeons did not gain enough familiarity with the C-Port xA® system, resulting in increased haemostatic problems in anastomosis facilitated with the device. Finally, the use of sequential grafts instead of single grafts could have influenced patency rates. The anastomotic patency rates are therefore not independent for some anastomosis. However, the impact of sequential vein grafts on graft patency remains controversial [19, 20].
CONCLUSIONS
According to these preliminary results and despite the limited number of patients, the use of the C-Port xA® System is safe enough to perform distal end-to-side vein graft anastomosis, with respect to 12-month end-to-side distal venous anastomotic patency. Although there are some technical challenges with this device, the incidence of complications is comparable to the traditional hand-sewn technique.
Funding
This work was partly supported by LST Europe B.V.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We express our gratitude to the Department of Research and Development of the Cardiothoracic Surgery Department, Catharina Hospital, for coordination of this study. Special gratitude to Esther van Dooren-Putmans, research coordinator and Francine Steerneman-Geurts, research nurse.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr M. Glauber(Massa, Italy): The study design from a methodological point of view was honest and correct. The clinical inclusion and exclusion criteria are clear, restrictive, and even the anatomical intraoperative findings were selective, but probably most of the time are operator-dependent. The study end points were, in acute, measured by flowmeter and, in chronic, assessed by CT angiography at 12 months with a patency rate of the terminal and lateral anastomosis with saphenous vein grafts. The results showed similar patency rates in acute and chronic, and in the C-Port group there were four patients converted to hand sewing and 75 patients who needed additional haemostatic stitches. The pre, intra, and postoperative data did not show any statistical difference and the statistical analysis I found to be correct.
My criticisms are addressed to the fact that the anastomotic device should prove from a theoretical point of view a superiority and not a non-inferiority in terms of patency rate, in terms of time saving, and in terms of reproducibility of the technique that in this study seems to be limited by additional stitches and by anatomical findings. So, due to this limitation, why, in your opinion, are the anastomotic devices not more frequently used in the ORs? Do you really believe that they will enhance minimally invasive CABG? And why did you choose saphenous vein grafts instead of mammary artery grafts for the testing of this device?
Dr Verberkmoes: To answer your first question, why it is not commonly used in the operating theatre, well, I think there are several points to address: one of them is that the device is not very cheap, and we are lacking a device for side-to-side anastomosis and, as you read in our manuscript, we prefer to use jump grafts and not single grafts. So I think that is one of the reasons why it is not used very much, because if you use the C-Port, it only facilitates an end-to-side anastomosis and you will need more proximal anastomosis. That is one point.
The other question was why we did not use the device with LIMA grafts. Well, the point is, we would love to start with the LIMA to LAD using the C-Port device. However, when we began the study, we only had the paper of Jan Gummert reporting vein graft patency, and due to our experience with the CorLink distal device, in which we saw a worse patency of anastomoses facilitated with the CorLink distal device in comparison with the hand-sewn method, we were a little bit anxious about starting with the C-Port on LIMA to LAD. So that is why we chose first to do a prospective trial on the right coronary and not directly on the most important target coronary vessel.
Dr Glauber: And do you believe that they really will enhance minimally invasive surgery?
Dr Verberkmoes: Well, the problem is, as you addressed, that there was a high rate of additional stitches, and besides this, it is necessary to close the insertion site with a stitch. I think, for example, in MIDCAB single-vessel it can help you very much, but we have to develop further. Actually you would need a device or C-Port in which you do not need additional stitches. So there is a long way to go, but I think it will help, yes.
Author notes
Presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.




