-
PDF
- Split View
-
Views
-
Cite
Cite
Tom Verbelen, Toni Lerut, Willy Coosemans, Paul De Leyn, Philippe Nafteux, Dirk Van Raemdonck, Jan Deprest, Herbert Decaluwé, Antireflux surgery after congenital diaphragmatic hernia repair: a plea for a tailored approach, European Journal of Cardio-Thoracic Surgery, Volume 44, Issue 2, August 2013, Pages 263–268, https://doi.org/10.1093/ejcts/ezt001
Close - Share Icon Share
Abstract
Preventive antireflux surgery (ARS) at the moment of congenital diaphragmatic hernia (CDH) repair has been suggested by some authors, particularly in subgroups with a liver herniated in the chest or patch requirement. We evaluated the incidence and associated factors of gastro-oesophageal reflux disease (GERD) and the need for subsequent ARS in our CDH patients.
We retrospectively reviewed our CDH database. Demographics, prenatal assessment of severity, prenatal treatment, type of repair, intraoperative findings and incidences of gastro-oesophageal reflux and ARS were recorded.
CDH repair was performed in 77 infants between July 1993 and November 2009. Eight died after repair. Seven were lost to follow-up. The median follow-up was 4.0 (0.16–14.88) years. Fourteen of these 62 patients were prenatally treated with fetoscopic endoluminal tracheal occlusion (FETO) because of severe pulmonary hypoplasia. After CDH repair, GERD was diagnosed in 31 patients. In all of them, medical antireflux treatment was started. Thirteen (42%) patients needed ARS at a median age of 64 (37–264) days. One year after starting medical treatment, 14 (45%) patients were completely off antireflux medication. In CDH subgroups with patch repair, liver herniated in the chest or previous FETO, the incidences of gastro-oesophageal reflux and ARS were 61 and 32%, 73 and 38% and 71 and 43%, respectively. Univariable analysis of associated potentially predisposing factors shows that patch repair, liver herniated in the chest, pulmonary hypertension, high-frequency oscillatory ventilation and FETO are associated with subsequent ARS. On multivariable analysis, liver herniated in the chest was the only independent predictor for both gastro-oesophageal reflux and ARS.
Of all CDH patients, 50% developed gastro-oesophageal reflux and 21% required ARS. For both, liver in the chest was the only independent predictor. Routine ARS in certain subgroups at the time of CDH repair seems not to be justified. Foetal endoluminal tracheal occlusion creates a new cohort of survivors with an increased risk for undergoing ARS. The surgical group, in particular, reflects a more complex gastro-oesophageal reflux physiopathology.
INTRODUCTION
Advances in perinatal intensive care have resulted in an improved survival of newborns with congenital diaphragmatic hernia (CDH). In the first decade of the 21st century, prenatal treatment regimens have been developed to improve survival chances. Fetoscopic endoluminal tracheal occlusion (FETO) has become a newly developed strategy to increase survival by triggering prenatal lung growth, with an apparent increase in survival compared with historical controls [1].
Infants with CDH also experience long-term morbidity due to the complexity of the disease, the therapeutic modalities and their sequellae. One of these is the development of gastro-oesophageal reflux disease (GERD), reportedly occurring in 28–69% of patients [2–5]. GERD may not only lead to nutritional problems, but as a result of pulmonary aspiration it may also worsen pulmonary function. The exact aetiology of GERD remains unsolved, but anatomical features such as a flat angle of His, oesophageal abnormalities, poor development of the diaphragmatic crura, patch implantation, a partially empty haemithorax secondary to lung hypoplasia and reflux-promoting medication during neonatal intensive therapy may contribute [5–7]. Despite advances in medical treatment, e.g. the development of proton pump inhibitors (PPIs), up to 20% of CDH infants eventually require antireflux surgery (ARS) for their GERD [2–4]. Different predictors for GERD have been described, such as herniation of the liver or the stomach, defect size, patch repair and the use of extracorporeal membrane oxygenation (ECMO) [2–4, 8]. Preventive ARS at the moment of CDH repair has also been suggested by some, particularly in subgroups at high risk for GERD, e.g. intrathoracic liver or those undergoing patch repair [3, 9, 10].
The aim of the present study was to evaluate the incidence and associated factors of GERD and the need for ARS in a consecutive cohort of children treated for congenital CDH at our centre, including in the era of foetal surgery for this condition. We also made an attempt to evaluate whether the need for preventive ARS at the time of CDH repair appears to be justified.
PATIENTS AND METHODS
This is a retrospective chart review on all patients admitted to the Department of Thoracic Surgery of University Hospital Gasthuisberg, Leuven, Belgium, for the surgical treatment of CDH from July 1993 to November 2009. This study was conducted after obtaining institutional review board approval. Data collected from the medical records included, besides GERD-related symptoms, therapy and outcome, foetal ultrasonographic findings, gestational age at birth, birth weight, associated congenital anomalies, duration of artificial ventilation, presence of preoperative pulmonary hypertension or pneumothorax, side of the diaphragmatic defect, type of diaphragmatic repair (patch or primary closure) and operative findings.
The entry criteria for FETO were severe CDH on the basis of sonographic evidence of intrathoracic herniation of the liver and an observed-to-expected lung-to-head ratio of <25%. The technique of FETO has been described in detail previously and has been performed in our centre since 2002 [11].
After stabilizing the haemodynamic and respiratory status of the patients, CDH repair was performed through a low ipsilateral thoracotomy. Patch repair was performed when primary repair without significant tension was impossible, using a polytetrafluoroethylene dual patch of 1-mm thickness (W.L. Gore & Associates, Flagstaff, USA). For diagnosing GERD, pH probe analysis was rarely used, because all infants had clinical symptoms of GERD (in particular excessive regurgitation, feeding refusal/aversia, choking/gaggling/coughing, sleep disturbance and/or abdominal pain). In all patients with clinical symptoms of GERD, upper gastrointestinal contrast studies were performed to assess reflux and/or other upper gastrointestinal pathology, e.g. delayed gastric emptying or pyloric stenosis. ARS (Nissen 360° fundoplication or Lind partial fundoplication) was only performed when patients failed to improve with intensifying medical management (prokinetic medication, H2 blockers and/or PPIs).
Data are presented as mean ± standard deviation or as median (minimum–maximum) data where appropriate. A binary logistic regression model was built for both uni- and multivariable analyses of factors associated with GERD and ARS. In a first step, significant variables from univariable analysis were entered in a model and in a second step, these variables were stepwise entered by the method of conditional forward (entry 0.05 and removal 0.10) to find the best predictors. Statistical analysis was performed using the IBM SPSS 19.0 statistical software. A P–value <0.05 was considered statistically significant.
RESULTS
CDH repair was performed in 77 infants between July 1993 and November 2009. Eight patients died in hospital (7 within 3 weeks after CDH repair and 1 within 3 months), making the postoperative survival rate 90%. Seven patients were lost to follow-up (6 of them returned to their country of origin, after being referred for FETO followed by surgery to our centre). The remaining 62 infants comprised the study group. Demographics and operative data of these patients are displayed in Table 1. The median follow-up was 4.0 (0.16–14.88) years. Fourteen of these 62 (23%) patients were prenatally treated with FETO. GERD was diagnosed in 50% (31 of 62), never in right-sided (n = 4) or bilateral CDH (n = 2). In all of them, medical treatment was started. Because of persistent symptoms and/or failure to thrive in spite of maximal medical antireflux therapy, 13 patients (21% of the overall and 42% of the GERD population) required ARS (12 Nissen and 1 Lind procedure). They all had a sliding hernia and reflux confirmed on upper gastrointestinal contrast studies. Table 2 shows the characteristics of the pre- and peroperative findings and highlights the complex surgical pathology with a short oesophagus in 2 patients and a dilated oesophagus in 5. ARS was performed at a median age of 64 (37–264) days. The median interval between CDH repair and ARS was 61 (35–252) days. A recurrence of GERD symptoms after ARS occurred in 6 (46%) patients. In 4 of them, symptoms were mild and persistent symptomatic control was obtained with PPIs. Two (15%), needed redo surgery, upper gastrointestinal contrast studies confirmed an intrathoracic migration of the Nissen fundoplication. Both of them were prenatally treated with the FETO procedure (Fig. 1).
| Demographics . | (N = 62) . |
|---|---|
| Prenatal data | |
| Prenatal diagnosis | 45 (73%) |
| Herniated liver on prenatal ultrasound | 18 (30%) |
| FETO | 14 (23%) |
| Birth | |
| Male/Female | 38/24 |
| Gestational age at birth (weeks) | 36.9 ± 2.5 |
| Birth weight (g) | 2848 ± 655 |
| Neonatal preoperative morbidity | |
| Associated structural pulmonary anomalies | 4 (6%) |
| Associated structural cardiac anomalies | 28 (45%) |
| Associated non-cardiac anomalies | 24 (39%) |
| Chromosomal | 1 (2%) |
| Others | 24 (37%) |
| HFOV before CDH repair | 33/52 (63%) |
| Days on HFOV before CDH repair | 1.98 ± 2.30 |
| ECMO | 0 (0%) |
| PHT before CDH repair | 33/55 (66%) |
| Pneumothorax before CDH repair | 12 (19%) |
| Operative data CDH repair | (N = 62) |
| Age at repair (days) | 3.4 ± 3.2 |
| Side of defect | |
| Left | 56 (91%) |
| Right | 4 (6%) |
| Bilateral | 2 (3%) |
| Patch repair | 38 (61%) |
| Hernial sac | 11 (18%) |
| Herniated liver | 26 (42%) |
| Demographics . | (N = 62) . |
|---|---|
| Prenatal data | |
| Prenatal diagnosis | 45 (73%) |
| Herniated liver on prenatal ultrasound | 18 (30%) |
| FETO | 14 (23%) |
| Birth | |
| Male/Female | 38/24 |
| Gestational age at birth (weeks) | 36.9 ± 2.5 |
| Birth weight (g) | 2848 ± 655 |
| Neonatal preoperative morbidity | |
| Associated structural pulmonary anomalies | 4 (6%) |
| Associated structural cardiac anomalies | 28 (45%) |
| Associated non-cardiac anomalies | 24 (39%) |
| Chromosomal | 1 (2%) |
| Others | 24 (37%) |
| HFOV before CDH repair | 33/52 (63%) |
| Days on HFOV before CDH repair | 1.98 ± 2.30 |
| ECMO | 0 (0%) |
| PHT before CDH repair | 33/55 (66%) |
| Pneumothorax before CDH repair | 12 (19%) |
| Operative data CDH repair | (N = 62) |
| Age at repair (days) | 3.4 ± 3.2 |
| Side of defect | |
| Left | 56 (91%) |
| Right | 4 (6%) |
| Bilateral | 2 (3%) |
| Patch repair | 38 (61%) |
| Hernial sac | 11 (18%) |
| Herniated liver | 26 (42%) |
FETO: foetal endoluminal tracheal occlusion; HFOV: high-frequency oscillatory ventilation; CDH: congenital diagphragmatic hernia; ECMO: extracorporeal membrane oxygenation; PHT: pulmonary hypertension.
| Demographics . | (N = 62) . |
|---|---|
| Prenatal data | |
| Prenatal diagnosis | 45 (73%) |
| Herniated liver on prenatal ultrasound | 18 (30%) |
| FETO | 14 (23%) |
| Birth | |
| Male/Female | 38/24 |
| Gestational age at birth (weeks) | 36.9 ± 2.5 |
| Birth weight (g) | 2848 ± 655 |
| Neonatal preoperative morbidity | |
| Associated structural pulmonary anomalies | 4 (6%) |
| Associated structural cardiac anomalies | 28 (45%) |
| Associated non-cardiac anomalies | 24 (39%) |
| Chromosomal | 1 (2%) |
| Others | 24 (37%) |
| HFOV before CDH repair | 33/52 (63%) |
| Days on HFOV before CDH repair | 1.98 ± 2.30 |
| ECMO | 0 (0%) |
| PHT before CDH repair | 33/55 (66%) |
| Pneumothorax before CDH repair | 12 (19%) |
| Operative data CDH repair | (N = 62) |
| Age at repair (days) | 3.4 ± 3.2 |
| Side of defect | |
| Left | 56 (91%) |
| Right | 4 (6%) |
| Bilateral | 2 (3%) |
| Patch repair | 38 (61%) |
| Hernial sac | 11 (18%) |
| Herniated liver | 26 (42%) |
| Demographics . | (N = 62) . |
|---|---|
| Prenatal data | |
| Prenatal diagnosis | 45 (73%) |
| Herniated liver on prenatal ultrasound | 18 (30%) |
| FETO | 14 (23%) |
| Birth | |
| Male/Female | 38/24 |
| Gestational age at birth (weeks) | 36.9 ± 2.5 |
| Birth weight (g) | 2848 ± 655 |
| Neonatal preoperative morbidity | |
| Associated structural pulmonary anomalies | 4 (6%) |
| Associated structural cardiac anomalies | 28 (45%) |
| Associated non-cardiac anomalies | 24 (39%) |
| Chromosomal | 1 (2%) |
| Others | 24 (37%) |
| HFOV before CDH repair | 33/52 (63%) |
| Days on HFOV before CDH repair | 1.98 ± 2.30 |
| ECMO | 0 (0%) |
| PHT before CDH repair | 33/55 (66%) |
| Pneumothorax before CDH repair | 12 (19%) |
| Operative data CDH repair | (N = 62) |
| Age at repair (days) | 3.4 ± 3.2 |
| Side of defect | |
| Left | 56 (91%) |
| Right | 4 (6%) |
| Bilateral | 2 (3%) |
| Patch repair | 38 (61%) |
| Hernial sac | 11 (18%) |
| Herniated liver | 26 (42%) |
FETO: foetal endoluminal tracheal occlusion; HFOV: high-frequency oscillatory ventilation; CDH: congenital diagphragmatic hernia; ECMO: extracorporeal membrane oxygenation; PHT: pulmonary hypertension.
| Patient . | UGI contrast study . | Other operative findings . | Patch . | Liver . | FETO . | CDH repair–ARS (days) . |
|---|---|---|---|---|---|---|
| 1 | Sliding hernia | Very thin weak crura. Concommitant obstructive adhesions below Treitz | Yes | Yes | No | 65 |
| 2 | Sliding hernia | Dilated oesophagus, no phreno-oesophageal ligament | Yes | Yes | No | 59 |
| 3 | Sliding hernia | No | No | No | 98 | |
| 4 | Sliding hernia | Short oesophagus | Yes | Yes | No | 57 |
| 5 | MD | MD | Yes | Yes | Yes | 55 |
| 6 | Sliding hernia | Difficult dissection of oesophagus in hiatus hernia, short oesophagus | Yes | Yes | No | 63 |
| 7 | Sliding hernia | Yes | No | No | 41 | |
| 8 | Sliding hernia | Yes | No | No | 35 | |
| 9 | Sliding hernia | Dilated oesophagus: difficult fundoplication. Concomitant pylorplasty | Yes | Yes | Yes | 45 |
| 10 | Sliding hernia with sacculary dilatation of oesophagus | Marked dilatation of oesophagus | Yes | Yes | Yes | 82 |
| 11 | Sliding hernia | Yes | Yes | Yes | 53 | |
| 12 | Sliding hernia | Yes | Yes | Yes | 136 | |
| 13 | Dilatation of lower oesophagus with intermittent herniation of the stomach | Dilated oesophagus | Yes | Yes | Yes | 252 |
| Total | 92% | 77% | 46% |
| Patient . | UGI contrast study . | Other operative findings . | Patch . | Liver . | FETO . | CDH repair–ARS (days) . |
|---|---|---|---|---|---|---|
| 1 | Sliding hernia | Very thin weak crura. Concommitant obstructive adhesions below Treitz | Yes | Yes | No | 65 |
| 2 | Sliding hernia | Dilated oesophagus, no phreno-oesophageal ligament | Yes | Yes | No | 59 |
| 3 | Sliding hernia | No | No | No | 98 | |
| 4 | Sliding hernia | Short oesophagus | Yes | Yes | No | 57 |
| 5 | MD | MD | Yes | Yes | Yes | 55 |
| 6 | Sliding hernia | Difficult dissection of oesophagus in hiatus hernia, short oesophagus | Yes | Yes | No | 63 |
| 7 | Sliding hernia | Yes | No | No | 41 | |
| 8 | Sliding hernia | Yes | No | No | 35 | |
| 9 | Sliding hernia | Dilated oesophagus: difficult fundoplication. Concomitant pylorplasty | Yes | Yes | Yes | 45 |
| 10 | Sliding hernia with sacculary dilatation of oesophagus | Marked dilatation of oesophagus | Yes | Yes | Yes | 82 |
| 11 | Sliding hernia | Yes | Yes | Yes | 53 | |
| 12 | Sliding hernia | Yes | Yes | Yes | 136 | |
| 13 | Dilatation of lower oesophagus with intermittent herniation of the stomach | Dilated oesophagus | Yes | Yes | Yes | 252 |
| Total | 92% | 77% | 46% |
UGI: upper gastro-intestinal; MD: missing data; CT: computed tomography; FETO: foetal endoluminal tracheal occlusion; CDH: congenital diagphragmatic hernia.
| Patient . | UGI contrast study . | Other operative findings . | Patch . | Liver . | FETO . | CDH repair–ARS (days) . |
|---|---|---|---|---|---|---|
| 1 | Sliding hernia | Very thin weak crura. Concommitant obstructive adhesions below Treitz | Yes | Yes | No | 65 |
| 2 | Sliding hernia | Dilated oesophagus, no phreno-oesophageal ligament | Yes | Yes | No | 59 |
| 3 | Sliding hernia | No | No | No | 98 | |
| 4 | Sliding hernia | Short oesophagus | Yes | Yes | No | 57 |
| 5 | MD | MD | Yes | Yes | Yes | 55 |
| 6 | Sliding hernia | Difficult dissection of oesophagus in hiatus hernia, short oesophagus | Yes | Yes | No | 63 |
| 7 | Sliding hernia | Yes | No | No | 41 | |
| 8 | Sliding hernia | Yes | No | No | 35 | |
| 9 | Sliding hernia | Dilated oesophagus: difficult fundoplication. Concomitant pylorplasty | Yes | Yes | Yes | 45 |
| 10 | Sliding hernia with sacculary dilatation of oesophagus | Marked dilatation of oesophagus | Yes | Yes | Yes | 82 |
| 11 | Sliding hernia | Yes | Yes | Yes | 53 | |
| 12 | Sliding hernia | Yes | Yes | Yes | 136 | |
| 13 | Dilatation of lower oesophagus with intermittent herniation of the stomach | Dilated oesophagus | Yes | Yes | Yes | 252 |
| Total | 92% | 77% | 46% |
| Patient . | UGI contrast study . | Other operative findings . | Patch . | Liver . | FETO . | CDH repair–ARS (days) . |
|---|---|---|---|---|---|---|
| 1 | Sliding hernia | Very thin weak crura. Concommitant obstructive adhesions below Treitz | Yes | Yes | No | 65 |
| 2 | Sliding hernia | Dilated oesophagus, no phreno-oesophageal ligament | Yes | Yes | No | 59 |
| 3 | Sliding hernia | No | No | No | 98 | |
| 4 | Sliding hernia | Short oesophagus | Yes | Yes | No | 57 |
| 5 | MD | MD | Yes | Yes | Yes | 55 |
| 6 | Sliding hernia | Difficult dissection of oesophagus in hiatus hernia, short oesophagus | Yes | Yes | No | 63 |
| 7 | Sliding hernia | Yes | No | No | 41 | |
| 8 | Sliding hernia | Yes | No | No | 35 | |
| 9 | Sliding hernia | Dilated oesophagus: difficult fundoplication. Concomitant pylorplasty | Yes | Yes | Yes | 45 |
| 10 | Sliding hernia with sacculary dilatation of oesophagus | Marked dilatation of oesophagus | Yes | Yes | Yes | 82 |
| 11 | Sliding hernia | Yes | Yes | Yes | 53 | |
| 12 | Sliding hernia | Yes | Yes | Yes | 136 | |
| 13 | Dilatation of lower oesophagus with intermittent herniation of the stomach | Dilated oesophagus | Yes | Yes | Yes | 252 |
| Total | 92% | 77% | 46% |
UGI: upper gastro-intestinal; MD: missing data; CT: computed tomography; FETO: foetal endoluminal tracheal occlusion; CDH: congenital diagphragmatic hernia.
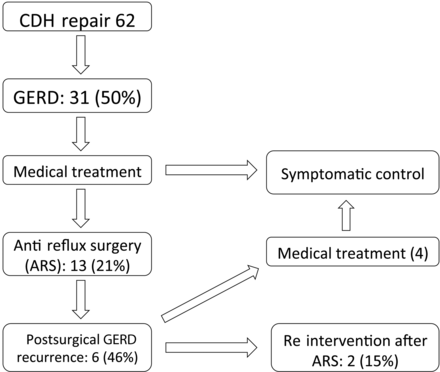
One year after the start of GERD treatment, 5 of the 18 medically treated GERD patients were completely off medication. The remaining 13 had persistent symptom control. The 7 successful ARS patients were completely off medication, and the 4 medically treated recurrences after ARS had persistent symptom control. The two redo surgeries were completely off medication. As a result, at 1 year, 14 of 31 (45%) of the GERD patients or 45 of 62 (73%) of the CDH patients were completely off antireflux medication.
The incidences of GERD and ARS in the CDH subgroup with patch repair were 61 and 32%, respectively, in the CDH subgroup with herniated liver 73 and 38%, respectively, and in the CDH subgroup who underwent FETO 71 and 43%, respectively.
Univariable analysis of associated factors of GERD and ARS in Table 3 shows that, days on high-frequency oscillatory ventilation before CDH repair, pulmonary hypertension before CDH repair, patch repair, herniated liver, but also FETO are associated with ARS. Two multivariable logistic regression models were built, with GERD and ARS as dependent variables. The entered covariates for both models were: intraoperative liver herniation, type of repair, FETO and days on high-frequency oscillatory ventilation before surgery. For GERD, entering all above variables in the model showed a χ² of 9.93(4); sig. 0.042. Goodness of fit was assessed by the Hosmer and Lemeshow test: χ² of 6.571(7); sig. 0.475. Entering the covariates by the method of forward conditional, the model showed a χ² of 8.74(1); sig. 0.003. Intraoperative liver herniation was the only significant covariate withheld in the model (sig. 0.004; hazard ratio 6.11; 95% confidence interval, CI, for HR 1.79–20.78). The classification accuracy rate computed was 70.6%. For ARS, entering all variables in the model showed a χ² of 11.31(4); sig. 0.023. Goodness of fit was assessed by the Hosmer and Lemeshow test: χ² of 2.58(7); sig. 0.921. Entering the covariates by the method of forward conditional, the model showed a χ² of 6.10(1); sig. 0.013. Intraoperative liver herniation was the only significant covariate withheld in the model (sig. 0.02; hazard ratio 7.25; 95% CI for HR 1.33–39.53). The classification accuracy rate computed was 82.4%.
| . | GERD (n = 31) . | No GERD (n = 31) . | P-value . | ARS (n = 13) . | No ARS (n = 49) . | P-value . |
|---|---|---|---|---|---|---|
| Prenatal diagnosis (45) | 26 | 19 | 0.047 | 12 | 33 | 0.075 |
| Herniated liver on prenatal ultrasound (18) | 13 | 5 | 0.011 | 6 | 8 | 0.056 |
| FETO (14) | 10 | 4 | 0.070 | 6 | 8 | 0.022 |
| Gestational age at birth (weeks) | 37.0 (31.3–39.0) | 38.0 (27.5–40.2) | 0.11 | 37.5 (32–39) | 38 (27.5–40.2) | 0.37 |
| Birth weight (g) | 2770 (1430–3650) | 3245 (1204–4200) | 0.027 | 2600 (1430–3345) | 3040 (1204–4200) | 0.060 |
| Days on HFOV before CDH repair | 2 (0–11) | 2 (0–8) | 0.024 | 2 (0–11) | 1 (0–6) | 0.022 |
| PHT before CDH repair (33) | 20 | 13 | 0.037 | 10 | 23 | 0.049 |
| Patch repair (38) | 23 | 15 | 0.037 | 12 | 26 | 0.0093 |
| Intraoperative liver herniation (26) | 19 | 7 | 0.0022 | 10 | 16 | 0.0043 |
| . | GERD (n = 31) . | No GERD (n = 31) . | P-value . | ARS (n = 13) . | No ARS (n = 49) . | P-value . |
|---|---|---|---|---|---|---|
| Prenatal diagnosis (45) | 26 | 19 | 0.047 | 12 | 33 | 0.075 |
| Herniated liver on prenatal ultrasound (18) | 13 | 5 | 0.011 | 6 | 8 | 0.056 |
| FETO (14) | 10 | 4 | 0.070 | 6 | 8 | 0.022 |
| Gestational age at birth (weeks) | 37.0 (31.3–39.0) | 38.0 (27.5–40.2) | 0.11 | 37.5 (32–39) | 38 (27.5–40.2) | 0.37 |
| Birth weight (g) | 2770 (1430–3650) | 3245 (1204–4200) | 0.027 | 2600 (1430–3345) | 3040 (1204–4200) | 0.060 |
| Days on HFOV before CDH repair | 2 (0–11) | 2 (0–8) | 0.024 | 2 (0–11) | 1 (0–6) | 0.022 |
| PHT before CDH repair (33) | 20 | 13 | 0.037 | 10 | 23 | 0.049 |
| Patch repair (38) | 23 | 15 | 0.037 | 12 | 26 | 0.0093 |
| Intraoperative liver herniation (26) | 19 | 7 | 0.0022 | 10 | 16 | 0.0043 |
FETO: foetal endoluminal tracheal occlusion; HFOV: high-frequency oscillatory ventilation; CDH: congenital diagphragmatic hernia; PHT: pulmonary hypertension; GERD: gastro-oesophageal reflux disease; ARS: antireflux surgery.
| . | GERD (n = 31) . | No GERD (n = 31) . | P-value . | ARS (n = 13) . | No ARS (n = 49) . | P-value . |
|---|---|---|---|---|---|---|
| Prenatal diagnosis (45) | 26 | 19 | 0.047 | 12 | 33 | 0.075 |
| Herniated liver on prenatal ultrasound (18) | 13 | 5 | 0.011 | 6 | 8 | 0.056 |
| FETO (14) | 10 | 4 | 0.070 | 6 | 8 | 0.022 |
| Gestational age at birth (weeks) | 37.0 (31.3–39.0) | 38.0 (27.5–40.2) | 0.11 | 37.5 (32–39) | 38 (27.5–40.2) | 0.37 |
| Birth weight (g) | 2770 (1430–3650) | 3245 (1204–4200) | 0.027 | 2600 (1430–3345) | 3040 (1204–4200) | 0.060 |
| Days on HFOV before CDH repair | 2 (0–11) | 2 (0–8) | 0.024 | 2 (0–11) | 1 (0–6) | 0.022 |
| PHT before CDH repair (33) | 20 | 13 | 0.037 | 10 | 23 | 0.049 |
| Patch repair (38) | 23 | 15 | 0.037 | 12 | 26 | 0.0093 |
| Intraoperative liver herniation (26) | 19 | 7 | 0.0022 | 10 | 16 | 0.0043 |
| . | GERD (n = 31) . | No GERD (n = 31) . | P-value . | ARS (n = 13) . | No ARS (n = 49) . | P-value . |
|---|---|---|---|---|---|---|
| Prenatal diagnosis (45) | 26 | 19 | 0.047 | 12 | 33 | 0.075 |
| Herniated liver on prenatal ultrasound (18) | 13 | 5 | 0.011 | 6 | 8 | 0.056 |
| FETO (14) | 10 | 4 | 0.070 | 6 | 8 | 0.022 |
| Gestational age at birth (weeks) | 37.0 (31.3–39.0) | 38.0 (27.5–40.2) | 0.11 | 37.5 (32–39) | 38 (27.5–40.2) | 0.37 |
| Birth weight (g) | 2770 (1430–3650) | 3245 (1204–4200) | 0.027 | 2600 (1430–3345) | 3040 (1204–4200) | 0.060 |
| Days on HFOV before CDH repair | 2 (0–11) | 2 (0–8) | 0.024 | 2 (0–11) | 1 (0–6) | 0.022 |
| PHT before CDH repair (33) | 20 | 13 | 0.037 | 10 | 23 | 0.049 |
| Patch repair (38) | 23 | 15 | 0.037 | 12 | 26 | 0.0093 |
| Intraoperative liver herniation (26) | 19 | 7 | 0.0022 | 10 | 16 | 0.0043 |
FETO: foetal endoluminal tracheal occlusion; HFOV: high-frequency oscillatory ventilation; CDH: congenital diagphragmatic hernia; PHT: pulmonary hypertension; GERD: gastro-oesophageal reflux disease; ARS: antireflux surgery.
Thus, multivariable logistic regression identified the presence of intraoperative liver in the chest as the single-independent predictor for both GERD and ARS.
DISCUSSION
The overall incidence of GERD (50%) in this series is consistent with what has previously been reported [2–5, 10, 12]. Weakened crura, abnormalities of the gastro-oesophageal junction, disruption of the angle of His, oesophageal lumen widening, increased intra-abdominal pressure as a result of loss of abdominal domain, pulmonary hypoplasia, a shortened abdominal oesophagus, delayed maturation of the lower oesophageal sphincter and intrinsic/motility disorders of the oesophageal body all may contribute to the development of GERD in the CDH population [5, 6, 13]. The adverse effect of GERD on failure to thrive and on the respiratory state may potentially be life-threatening. The long-term consequences of GERD should also not be underestimated: Vanamo et al. [14] observed oesophagitis in 54% and Barrett oesophagus in 12% of patients 20 years after CDH repair.
Because all operated patients were intolerant to feeds, clinically vomiting and/or regurgitating and their diagnosis was confirmed by upper gastrointestinal contrast study, we found pH monitoring and endoscopy not relevant to further document GERD. In all patients with GERD, antireflux medication was first started and ARS was only performed when medical management failed to control symptoms or when persistent regurgitation ± aspiration was documented on contrast studies.
Reportedly, ARS for GERD after CDH repair is required in 8–21% patients [2–4, 13, 15, 16]. The 21% of ARS incidence in our series seems to be in the higher range.
Predictive factors for the development of GERD and subsequent need for ARS in the CDH population have been examined by several authors. Although not consistent in all studies, these include patch repair, thoracic position of the stomach or liver, prenatal diagnosis of CDH, use of ECMO, advanced respiratory support and related medication with negative impact on lower oesophageal sphincter tonus and/or impairing oesophageal motility [2–4, 8, 10, 12, 17]. Several of these factors are interlinked and coincide with larger diaphragmatic defects and more severe forms of pulmonary hypoplasia. It seems that GERD and the subsequent need for ARS are associated with the presence of CDH of increased complexity. In our series, GERD was associated with herniated liver on prenatal ultrasound, lower birth weight, number of days on high-frequency oscillatory ventilation before CDH repair, pulmonary hypertension before CDH repair, patch repair and intraoperative finding of liver in the chest. ARS was associated with FETO, high-frequency oscillatory ventilation, pulmonary hypertension, patch repair and intraoperative liver herniation. Earlier on, Diamond et al. [10] identified patch closure and liver within the chest as being independent risk factors for ARS. However, subgroup analysis on left-sided cases only showed patch closure and non-conventional ventilation to be independently predictive for ARS, but not liver in the chest. In our series, only left-sided CDH had GERD, but only liver in the chest was an independent predictor of GERD and the subsequent need for ARS.
An important and new surgical subgroup is composed of those children having undergone FETO in the prenatal period. Given the selection criteria used for FETO, these are babies who are more likely not to survive, and who have per definition factors predictive of severe reflux, such as liver in the chest, and hypoplastic lungs. To our knowledge, there are no data on GERD related to this subgroup in the literature. There was a trend to FETO babies more frequently having GERD (FETO: 71 vs non-FETO: 44%; P = 0.070). On univariable analysis, there was also an association between ARS and having undergone FETO (15 of the non-FETO group vs 43% in the FETO group; P = 0.022). This likely explains the rate of ARS being at a higher proportion in our series. Since liver in the chest is an entry criterion for being offered FETO, the FETO group is not a per se new risk group for ARS, but rather a new cohort within the known risk group of liver in the chest.
Data on reintervention rates for ARS after CDH in the literature are scarce. In the study of Maier et al. [15], 16% of the ARS patients had undergone a redo ARS after 2 years of follow-up. We had a similar 15% reintervention rate, i.e. 2 patients past FETO. Moreover, the indications for ARS as well as the peroperative findings, as shown in Table 2, illustrate the often complex GERD physiopathology and the resulting more complicated aspects of this surgery when compared with the classic ARS in childhood. Hoffman et al. [18] analysed a series of 174 children who underwent ARS for GERD at our institution. They all underwent a Ba contrast study. None of them showed an anatomic abnormality (i.e. no sliding hernia). Even in patients with refractory GERD, no anatomical abnormalities were found when performing a Ba swallow [19]. This is in sharp contrast with the findings in this group in which sliding hernia was always present, short oesophagus was seen in 2 patients and a dilated oesophagus, as expression of severe motility disorders, was seen in 4.
Given the high incidence of GERD in the CDH population, preventive ARS at the moment of CDH repair has been suggested by some authors, especially in subgroups with a liver in the chest or patch requirement [9, 10, 12, 20–22]. If we had applied this prophylactic strategy, we would have performed ARS in as much as 51 or 68% of our patients, instead of the current 21%. Selective preventive ARS in FETO patients (who all have a patch repair and a liver in the chest) would lead to prophylactic ARS performed in as much as 41% of our patients instead of the current 21% (22 ARS on 54 calculated from the start of FETO in 2002). Moreover, Maier et al. compared CDH repair with and without the concomitant fundoplication in a single-blinded prospective study. They report that the patients with ARS marginally benefit from ARS, and this only in the first year. This decreasing GERD with increasing age correlates to the normal maturation of the lower oesophageal sphincter in babies and is in our series reflected by 5 of 18 asymptomatic patients who are completely off medication at 1 year [23]. This, in our opinion, justifies an expecting attitude.
Preventive ARS in risk groups would double or even triple our antireflux surgeries in CDH patients. Moreover, also given the possible adverse effects and complications, the substantial recurrence rate of fundoplication and need for redo ARS, our policy, as suggested by others, remains to first control the reflux symptoms with medications as much as possible and to only perform ARS when medical management fails [24, 25].
CONCLUSIONS
Of our CDH patients, 50% develops GERD and 21% needs ARS. We identified liver in the chest, patch repair, pulmonary hypertension and high-frequency oscillatory ventilation as factors associated with a higher risk of developing GERD and needing subsequent ARS. FETO creates a new cohort of CDH survivors who have the same risk factors and, as a result, have an increased risk of ARS. Liver in the chest appeared to be the only independent predictor for both GERD and ARS. The indication for ARS is based on the failure of medical management. Preventive ARS at the time of CDH repair seems not justified, despite raised incidences of GERD and ARS in certain risk groups. The characteristics of the patients who underwent ARS reflects the more complex physiopathology of reflux in CDH patients.
ACKNOWLEDGEMENT
The authors thank J. Moons, for his valuable help regarding the statistical analysis of the data.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr S. Mattioli(Bologna, Italy): This is a very interesting presentation. I have a question. As far as I know, the lower esophageal sphincter is mature after one year from birth. Therefore you cannot really evaluate the anti-reflux barrier until the lower oesophageal sphincter is completely functioning, 13–14 months after birth. That could be supportive of not operating stat in those patients. Did you consider this physiological factor?
Dr Verbelen: That is, of course, the reason why we only operate when medical management fails. Dr Susan Maier from the University of Heidelberg presented a paper in which she performed a single blinded prospective study. She concluded that CDH patients only marginally benefit from fundoplication, and this only within the first year. So I think that indeed the decrease of GERD together with increasing age correlates with the normal maturation of the lower esophageal sphincter, and that is also the reason why we only perform a fundoplication when medical management fails.
Dr Y. Colson(Boston, MA, USA): Do you think that that maturation, so to speak, over time also accounts for why the four recurrences who initially failed medical management suddenly could be treated medically after their recurrence? Do you think it has anything to do with maturation as they get older that they can be treated with medicine, or does that not make sense?
Dr Verbelen: Concerning the recurrences in our series, the two that were reoperated were both FETO patients. In other words, it is a totally new group with probably more factors inducing the development of GERD. So it is hard to form conclusions on that. And as for the medical therapy, it is important to continue this therapy as long as necessary.
Author notes
Presented at the 20th European Conference on General Thoracic Surgery, Essen, Germany, 10–13 June 2012.
- pulmonary hypertension
- antireflux surgery
- gastroesophageal reflux disease
- demography
- disease susceptibility
- fetus
- follow-up
- objective (goal)
- infant
- intraoperative care
- surgical procedures, operative
- survivors
- liver
- prenatal care
- chest
- trachea
- pulmonary hypoplasia
- hernia, congenital diaphragmatic
- high frequency oscillatory ventilation
- repair of neonatal diaphragmatic hernia
- lost to follow-up
- prevention
- medical management
- fetal endoscopic tracheal occlusion




