-
PDF
- Split View
-
Views
-
Cite
Cite
Nawwar Al-Attar, Ulrik Hvass, Right papillary muscle sling: proof of concept and pilot clinical experience, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages e187–e189, https://doi.org/10.1093/ejcts/ezt100
Close - Share Icon Share
Abstract
Left-sided intraventricular remodelling by papillary muscle approximation associated with annuloplasty of the mitral valve improved outcomes for severe functional mitral regurgitation compared with annuloplasty alone. We conceived of, and studied, a papillary muscle sling on the right side of the heart associated with annuloplasty, seeking to reduce tricuspid valve tethering and right ventricular volumes and to preserve ventricular function.
An experimental model on ex vivo porcine hearts established the anatomical feasibility of the procedure. A first-in-man clinical series of 5 patients (3 men) with a mean age of 63.3 years (51–73) had mean right ventricular volumes of 320 ml (280–350) and 200 ml (155–250) in diastole and systole, respectively, and an ejection fraction of 30% (25–40). The mean pulmonary artery pressure was 60 mmHg (55–70), and all had Grade IV/IV tricuspid regurgitation (TR).
There was no operative mortality. Post-repair, magnetic resonance imaging and echocardiographic studies showed mean right ventricle volumes of 165 ml (155–180) and 124 ml (110–140) in diastole and systole, respectively, and an ejection fraction of 28% (25–35) (P = 0.03). TR was <2, gradient across tricuspid valve was ≤4 mmHg and there was no right ventricular outflow tract obstruction. All patients were in New York Heart Association Class ≤2.
Intraventricular remodelling with a papillary muscle sling is safe and feasible on the right heart. Short-term follow-up shows that it ameliorates clinical functional status and improves valve competency through reduced tension and tethering of tricuspid leaflets.
INTRODUCTION
Tricuspid regurgitation (TR) is the most common pathology of the tricuspid valve. In the majority of cases, it is secondary to diseases on the left side of the heart or pulmonary hypertension [1]. Right ventricle (RV) dysfunction and remodelling are associated with annular dilatation and displacement of papillary muscles. Reducing TR by treating the mitral valve pathology alone is controversial. The latter would reduce the afterload without the correction of thepreload or right ventricular, or tricuspid valve, dilatation. Thus, when the tricuspid annulus is dilated, the size of the RV does not regress, but may continue to increase independently even though the mitral pathology was treated. This has been observed regardless of the aetiology of the mitral pathology, but is particularly true in rheumatic mitral disease [2–4]. When TR was not addressed during mitral valve surgery, significant TR appeared after a mean of 10 years [5]. Late functional TR carries an independent prognostic impact that is correlated with the degree of TR. An increase in symptoms, degradation of the functional status and poorer survival are observed with greater TR [6]. TR, per se, has been shown to reduce survival regardless of the presence of pulmonary hypertension and/or left ventricular dysfunction [7]. Even when tricuspid annuloplasty is incorporated with mitral valve surgery, 10% of patients have residual or recurrent moderate-to-severe TR on follow-up [8]. By analogy to the left-sided papillary muscle sling technique for mitral valve tethering, we report our experimental and early clinical data on right-sided intraventricular remodelling by papillary muscle approximation associated with annuloplasty of the tricuspid valve.
MATERIALS AND METHODS
Experimental model (feasibility study)
Five adult pig hearts were used for the experiments in an ex vivo setting. Through a right atriotomy, a size 4 polytetrafluoroethylene (Goretex®) tube was advanced using a right-angle forcep through the base of the papillary muscles of the tricuspid valve in the RV. Traction on the tube provided an approximation of the papillary muscles. This was considered sufficient when the papillary muscles came into contact; the ends of the Goretex tube were then tailored down to size and were fixed by two 4/0 polypropylene sutures to close the loop, producing the ‘sling’. To prevent the sling from slipping and becoming loose, it was placed as close as possible to the ventricular wall.
Clinical experience
Local Institutional Review Board approval and patient consent were obtained. Five patients (men) aged 46–72 years with TR secondary to chronic mitral disease were included. All patients were severely symptomatic [New York Heart Association (NYHA) Class III or IV] and in chronic atrial fibrillation. Mitral valve disease was rheumatic in 3 (with previous balloon valvuloplasty in all patients), and degenerative in 2. Mean pulmonary artery pressure was 60 (55–70 mmHg) with dilated RVs and Grade IV/IV TR. The patients underwent surgery primarily with the indication of mitral valve replacement and tricuspid annuloplasty or tricuspid valve replacement according to the tethering and right ventricular dilatation. No coronary surgery was needed. All patients had peroperative transesophageal echocardiography (TEE). Magnetic resonance imaging (MRI) study of the RVs showed mean volumes of 320 (280–350 ml) and 200 (155–250 ml) in diastole and systole, respectively, and an ejection fraction of 30 (25–40%).
Surgical technique
After replacing the mitral valve, the aortic cross-clamp was removed, the heart defibrillated and the tricuspid valve inspected on a beating heart. The size and position of the papillary muscles and the surrounding muscular bands were then inspected. Papillary muscle sling employed a 4-mm Goretex tube that was passed through the trabecular muscles and/or the moderator band surrounding the base of the anterior papillary muscle. The tube was then placed through the trabeculae at the base of the small posterior papillary muscle. When the ventricular wall was too thin without adequate trabeculae, the RV wall was pierced and the tube passed outside the RV and re-entered 2 cm further. When tightened, the sling leaves a gap of approximately 1 cm between the anterior and posterior ventricular wall, thus considerably reducing the intracavity anterior-to-posterior end-diastolic diameter. A size 36 MC3 tricuspid annuloplasty ring (Edwards Lifesciences, Irvin, CA, USA) was then used to remodel the tricuspid annulus (Supplementary Video 1).
Statistical analysis
Data were expressed as mean (range). The Mann–Whitney U-test was used to compare continuous variables. A P-value <0.05 was considered to indicate a statistically significant difference. Statistical analysis was performed using statistical software Statview version 5.0 (Abacus Corporation, Baltimore, MD, USA).
RESULTS
In the experimental model, the right ventricular cavity was explored through an anterior ventriculotomy, and the right ventricular outflow tract was assessed for patency. In all five hearts, there was no impingement of the outflow tract. The RV musculature was carefully examined, and there were no tears related to the sling (Fig. 1).
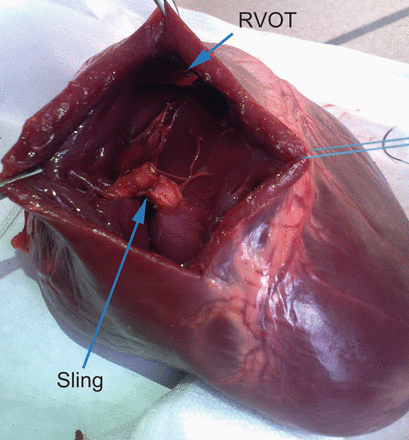
Porcine heart—sling approximating papillary muscles of the tricuspid valve. Note the non-compromised right ventricular outflow tract (RVOT).
In the clinical series, placing a sling was possible in all cases. Mean age was 63.3 (51–73 years). There were 3 male patients. The tricuspid annular distension facilitated access to the ventricular cavity and was associated with an augmented distance between the anterior-to-posterior commissures ranging between 65 and 80 mm. The tricuspid leaflets appeared normal, albeit restricted, in their movements. Following weaning from cardiopulmonary bypass, restricted expansion of the RV was visibly observed by a hollow on the anterior and inferior surfaces of the ventricle. Post-repair TEE in the operating room showed mild TR (Grade ≤2). Postoperative recovery was uneventful, with patients requiring only low doses of inotropic support in the intensive care unit. One patient, with a history of pneumonectomy for lung cancer, died from pulmonary infection 3 weeks after the procedure, in the absence of adverse cardiac events. Predischarge transthoracic echocardiography (TTE) and TEE in all patients demonstrated a TR <2, gradient across tricuspid valve ≤3 mmHg and the absence of right ventricular outflow tract obstruction/stenosis. Cardiac MRI showed mean RV volumes of 165 (155–180 ml) and 124 (110–140 ml) in diastole and systole, respectively, and an ejection fraction of 28 (25–35%). Follow-up TTE in 2 patients at 6 months showed that the satisfactory results persisted. The sling was visible between the inflow and trabecular portions of the RV with no breakdown ofthe ventricular walls. All patients were in NYHA Class ≤2.
DISCUSSION
Severe TR presents clinically with anasarca that is extremely difficult to manage and often associated with very poor outcomes [9]. The main therapeutic options are either conservative, namely diuretics with limited effectiveness, or operative in a risky redo context with high perioperative morbi-mortality [10, 11]. Our experience with the papillary muscle sling in patients with ischaemic mitral regurgitation in severely impaired left ventricles [12] was the basis to determine the feasibility of a combined ventricular and annular correction of functional TR in severely impaired RVs. Given the anatomical specificities of the RV, the pig heart model allowed us to overcome our concerns regarding the stability of the sling and the structural strength and risk of avulsion of the papillary muscles. Recently, approximation of the head of the anterior papillary muscle arising from the RV free wall to the ventricular septum was studied by three-dimensional echocardiography in an ex vivo porcine heart model. TR was significantly reduced by RV papillary-muscle approximation alone than by annuloplasty alone. Combined RV papillary-muscle approximation and annuloplasty resulted in the least regurgitation. Moreover, RV papillary-muscle approximation reduced tricuspid septolateral diameter, annular area, RV sphericity index and tricuspid tethering height [13]. In this pilot clinical series, we performed the procedure without aortic cross-clamping to minimize ischaemic time in these diseased hearts. The RV diameters were significantly reduced after repair (P = 0.03). The transmural passage of the right-angle forceps in the distended thin-walled RV was easily repaired by sutures reinforced with small Teflon patches. Although follow-up is limited to 6 months, we believe that double-staged repair on the RV has contributed to maintaining tricuspid valve competency and improved short-term outcome in this small clinical series.
In conclusion, experimental data and the first clinical experience in man demonstrate the feasibility and safety of direct right-sided intraventricular remodelling with a papillary muscle sling.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Video 1. Operative technique showing slinging of papillary muscles with 4 mm polytetrafluoroethylene tube. Final montage with tricuspid ring implantation.
Conflict of interest: none declared.
REFERENCES
- papillary muscle
- magnetic resonance imaging
- tricuspid valve insufficiency
- echocardiography
- ventricular function
- mitral valve
- tricuspid valve
- right ventricle
- diastole
- follow-up
- objective (goal)
- heart ventricle
- suidae
- systole
- heart
- sling
- right ventricular outflow obstruction
- ejection fraction
- functional mitral regurgitation
- right side of heart
- functional status
- spinal tethering
- tricuspid valve leaflet
- surgical mortality
- tissue approximation
- pulmonary artery mean pressure




