-
PDF
- Split View
-
Views
-
Cite
Cite
Yoshiyuki Tokuda, Hideki Oshima, Yoshimori Araki, Yuji Narita, Masato Mutsuga, Katsuhiko Kato, Akihiko Usui, Detection of thoracic aortic prosthetic graft infection with 18F-fluorodeoxyglucose positron emission tomography/computed tomography, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1183–1187, https://doi.org/10.1093/ejcts/ezs693
Close - Share Icon Share
Abstract
To investigate the diagnostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) in detecting thoracic aortic prosthetic graft infection.
Nine patients with clinically suspected thoracic aortic graft infection underwent FDG-PET/CT scanning. In these patients, the diagnoses could not be confirmed using conventional modalities. The patients' clinical courses were retrospectively reviewed.
On the basis of surgical, microbiological and clinical follow-up findings, the aortic grafts were considered infected in 4 patients and not infected in 5. All 4 patients with graft infection (root: 2 cases, arch: 1 case and descending: 1 case) eventually underwent in situ re-replacement. Two of the 4 patients also had abdominal grafts; however, only the thoracic grafts were replaced because uptake was low around the abdominal grafts. The maximal standardized uptake value (SUVmax) in the perigraft area was higher in the infected group than in the non-infected group (11.4 ± 4.5 vs 6.9 ± 6.4), although the difference was not statistically significant. According to the receiver operating characteristic analysis, SUVmax >8 appeared to be the cut-off value in distinguishing the two groups (sensitivity: 1.0 and specificity: 0.8).
FDG-PET/CT is useful for confirming the presence of graft infection by detecting high uptake around grafts and excluding other causes of inflammation. An SUVmax value greater than 8 around a graft suggests the presence of graft infection. In addition, FDG-PET/CT can be used to clarify the precise extent of infection. This is especially useful if multiple separated prosthetic grafts have been implanted.
INTRODUCTION
Aortic prosthetic graft infection, particularly thoracic aortic graft infection, is associated with very high morbidity and mortality. Surgical intervention without delay is usually necessary [1]. Therefore, making a prompt and precise diagnosis is essential in order to improve surgical outcomes. It is, however, generally not easy to obtain a definite diagnosis of graft infection. Computed tomography (CT) has been used as a good imaging approach for the assessment of graft infection because its high spatial resolution provides details of anatomical changes in perigraft areas. However, haematomas and seromas around grafts often appear similar to abscesses, thus making it difficult to distinguish between non-infected and infected prosthetic grafts on CT scans [2]. In most of the cases of thoracic prosthetic graft infection, patients are required to undergo very high-risk reoperations. The anatomical information obtained on CT is sometimes too ‘inconclusive’ to indicate whether patients should undergo such high-risk reoperations. In addition to confirming the presence of infection, it is necessary to obtain information regarding the extent of infection in order to select a proper strategy for reoperation. Therefore, developing a more reliable physiological approach to detect infected prostheses is required.
18F-fluorodeoxyglucose positron emission tomography (FDG-PET) has drawn attention to its use in the functional imaging of tissues with elevated levels of intracellular glucose metabolism. FDG-PET is also becoming important for diagnosis and staging in clinical oncology [3].
The standardized uptake value (SUV) is often used in the semiquantitative analysis of increased uptake observed on FDG-PET [4]. Its value in the diagnosis of infectious diseases with elevated levels of intracellular glucose metabolism has also been reported. Moreover, the fusion of FDG-PET and CT images acquired in a single session, called FDG-PET/CT, has been developed to enable the acquisition of more precise anatomical and metabolic information [5]. The aim of this study was to investigate the diagnostic value of FDG-PET/CT and SUV in detecting thoracic aortic prosthetic graft infection.
PATIENTS AND METHODS
This study is an institutional review board-approved retrospective observational study of patients who underwent FDG-PET/CT for the diagnosis of thoracic aortic prosthetic graft infection between 2008 and 2011. The suspicion of prosthetic graft infection was based on the presence of undefined fevers, elevated infectious variables in laboratory analyses and undefined malaise in patients with previously implanted thoracic prosthetic grafts. If graft infection was apparent on conventional investigations, including contrast-enhanced CT, blood cultures and laboratory analyses, FDG-PET/CT was not required and thus not performed. Otherwise, FDG-PET/CT was usually chosen as the next modality used to gain additional information. A total of 9 patients under suspicion of thoracic prosthetic graft infection who underwent FDG-PET/CT were included in this study. The patients' clinical courses and radiological findings were reviewed retrospectively.
As described above, in the period between 2008 and 2011, a total of 9 patients under suspicion of thoracic prosthetic graft infection underwent FDG-PET/CT after the results of other anatomical imaging modalities proved inconclusive. There were six males and three females with a mean age of 65.4 ± 14.3 years. Two patients had previously undergone root replacement, 1, ascending aortic replacement, 4, arch replacement (1 of these patients had also previously undergone root replacement and 1, descending aortic replacement) and 2, thoracic endovascular aortic repair (TEVAR) with stent grafting.
On the basis of the surgical, microbiological and clinical follow-up findings, the aortic grafts were considered to be infected in 4 patients (infected group) and not infected in 5 (non-infected group). The process used to obtain a final diagnosis of graft infection was as follows. First, a graft was confirmed to be infected if the culture specimen obtained from the graft or the tissue area around the graft was positive for infection (n = 4). Secondly, if the specimen obtained was negative for infection (n = 0) or a specimen was not obtained (n = 5), the final diagnosis was then made based on a clinical follow-up period of >6 months. After 6 months, the inflammation findings were settled with conservative management in 5 patients. Therefore, based on the clinical courses, all of the 5 patients were ultimately considered to be negative for infection. Conversely, in the 4 patients with infection, the diagnoses were confirmed with the culture specimens.
As per the scanning protocol, the patients were instructed to fast for at least 6 h before undergoing examination. The blood sugar levels were checked prior to examination in order to confirm that they were within normal limits. Unenhanced CT and PET images were acquired consecutively 60 min after injection of 4–6 MBq of 18F-FDG per kilogram using a PET/CT system (Biograph 16; Siemens Medical Solutions) combining a multislice spiral CT scanner with a full-ring PET scanner. Matching PET and CT slices were fused, and an image of 18F-FDG activity overlying the corresponding anatomical plane was reconstructed. Areas with maximal focal 18F-FDG uptake were visually detected, and the maximal standardized uptake value (SUVmax) in each area, particularly focusing on the tissues around each graft (the perigraft area), was measured.
The values of continuous variables are expressed as the mean ± SD, unless otherwise specified. The optimal cut-off values of the SUVmax of the perigraft area to predict graft infection were determined by means of the receiver operating characteristic (ROC) curve analysis. The area under the curve, sensitivity and specificity were also calculated. The optimal cut-off value was defined as that providing maximal sensitivity and specificity.
RESULTS
The clinical courses of these patients are outlined in Table 1. In this study, only patients with thoracic prosthetic grafts were included; however, 4 of the 9 patients also had abdominal prosthetic grafts. The mean maximal C reactive protein (CRP) value prior to scanning was 11.7 ± 7.9 mg/dl, and the mean maximal white blood cell count was 9900 ± 3600/ μml in the blood analyses. Patients underwent FDG-PET/CT 33.1 ± 76.7 months (range 1.6–236 months and median: 4 months) after initially undergoing surgery.
| Case no. . | Age . | Previous operation . | Months after the operation . | Maximal CRP (mg/dl) . | SUVmax . | Graft infection . | Identified organism . | Reoperation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 83 | Arch replacement + frozen elephant trunk | 2.7 | 12.1 | 18 | No | ||
| 2 | 67 | Arch replacement + elephant trunk | 1.6 | 20.0 | 6.4 | No | ||
| 3 | 42 | Bentall operation + abdominal Ao replacement | 4.0 | 2.4 | 8.5 | Yes | Staphylococcus aureus | Bentall operation |
| 4 | 78 | TEVAR (descending Ao) | 4.1 | 5.7 | 2.8 | No | ||
| 5 | 76 | Ascending | 31.3 | 2.9 | 3.2 | No | ||
| 6 | 46 | Redo Bentall operation + arch replacement | 10.6 | 12.4 | 10.9 | Yes | Propionibacterium acnes | Arch replacement |
| 7 | 61 | Bentall operation | 236.1 | 11.3 | 8.27 | Yes | Edwardsiella tarda | Bentall operation |
| 8 | 63 | TEVAR (descending Ao) + abdominal Ao replacement | 2.2 | 26.8 | 18 | Yes | Gram-positive cocci | Descending Ao replacement |
| 9 | 75 | Arch replacement + descending Ao replacement | 5.0 | 11.9 | 4.06 | No |
| Case no. . | Age . | Previous operation . | Months after the operation . | Maximal CRP (mg/dl) . | SUVmax . | Graft infection . | Identified organism . | Reoperation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 83 | Arch replacement + frozen elephant trunk | 2.7 | 12.1 | 18 | No | ||
| 2 | 67 | Arch replacement + elephant trunk | 1.6 | 20.0 | 6.4 | No | ||
| 3 | 42 | Bentall operation + abdominal Ao replacement | 4.0 | 2.4 | 8.5 | Yes | Staphylococcus aureus | Bentall operation |
| 4 | 78 | TEVAR (descending Ao) | 4.1 | 5.7 | 2.8 | No | ||
| 5 | 76 | Ascending | 31.3 | 2.9 | 3.2 | No | ||
| 6 | 46 | Redo Bentall operation + arch replacement | 10.6 | 12.4 | 10.9 | Yes | Propionibacterium acnes | Arch replacement |
| 7 | 61 | Bentall operation | 236.1 | 11.3 | 8.27 | Yes | Edwardsiella tarda | Bentall operation |
| 8 | 63 | TEVAR (descending Ao) + abdominal Ao replacement | 2.2 | 26.8 | 18 | Yes | Gram-positive cocci | Descending Ao replacement |
| 9 | 75 | Arch replacement + descending Ao replacement | 5.0 | 11.9 | 4.06 | No |
Ao: aorta; CRP: C reactive protein; TEVAR: thoracic endovascular aortic repair.
| Case no. . | Age . | Previous operation . | Months after the operation . | Maximal CRP (mg/dl) . | SUVmax . | Graft infection . | Identified organism . | Reoperation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 83 | Arch replacement + frozen elephant trunk | 2.7 | 12.1 | 18 | No | ||
| 2 | 67 | Arch replacement + elephant trunk | 1.6 | 20.0 | 6.4 | No | ||
| 3 | 42 | Bentall operation + abdominal Ao replacement | 4.0 | 2.4 | 8.5 | Yes | Staphylococcus aureus | Bentall operation |
| 4 | 78 | TEVAR (descending Ao) | 4.1 | 5.7 | 2.8 | No | ||
| 5 | 76 | Ascending | 31.3 | 2.9 | 3.2 | No | ||
| 6 | 46 | Redo Bentall operation + arch replacement | 10.6 | 12.4 | 10.9 | Yes | Propionibacterium acnes | Arch replacement |
| 7 | 61 | Bentall operation | 236.1 | 11.3 | 8.27 | Yes | Edwardsiella tarda | Bentall operation |
| 8 | 63 | TEVAR (descending Ao) + abdominal Ao replacement | 2.2 | 26.8 | 18 | Yes | Gram-positive cocci | Descending Ao replacement |
| 9 | 75 | Arch replacement + descending Ao replacement | 5.0 | 11.9 | 4.06 | No |
| Case no. . | Age . | Previous operation . | Months after the operation . | Maximal CRP (mg/dl) . | SUVmax . | Graft infection . | Identified organism . | Reoperation . |
|---|---|---|---|---|---|---|---|---|
| 1 | 83 | Arch replacement + frozen elephant trunk | 2.7 | 12.1 | 18 | No | ||
| 2 | 67 | Arch replacement + elephant trunk | 1.6 | 20.0 | 6.4 | No | ||
| 3 | 42 | Bentall operation + abdominal Ao replacement | 4.0 | 2.4 | 8.5 | Yes | Staphylococcus aureus | Bentall operation |
| 4 | 78 | TEVAR (descending Ao) | 4.1 | 5.7 | 2.8 | No | ||
| 5 | 76 | Ascending | 31.3 | 2.9 | 3.2 | No | ||
| 6 | 46 | Redo Bentall operation + arch replacement | 10.6 | 12.4 | 10.9 | Yes | Propionibacterium acnes | Arch replacement |
| 7 | 61 | Bentall operation | 236.1 | 11.3 | 8.27 | Yes | Edwardsiella tarda | Bentall operation |
| 8 | 63 | TEVAR (descending Ao) + abdominal Ao replacement | 2.2 | 26.8 | 18 | Yes | Gram-positive cocci | Descending Ao replacement |
| 9 | 75 | Arch replacement + descending Ao replacement | 5.0 | 11.9 | 4.06 | No |
Ao: aorta; CRP: C reactive protein; TEVAR: thoracic endovascular aortic repair.
On the FDG-PET/CT scans, the mean SUVmax value around the grafts was 8.9 ± 5.8. Two patients (Cases 3 and 7) also exhibited uptake in near by lymph nodes. After undergoing scanning, 4 of the 9 patients were considered to require surgical intervention and underwent in situ re-replacement with rifampicin-soaked gelatin-coated grafts (Gelweave, Terumo Cardiovascular Systems, Tokyo, Japan) followed by omental flap coverage if applicable. All 4 patients survived reoperation without a recurrence of infection during the follow-up period. Two of these 4 patients also had abdominal grafts; however, only the thoracic grafts were replaced because uptake was low around the abdominal grafts (Cases 3 and 8). An FDG-PET/CT image obtained in Case 3 is shown in Fig. 1.
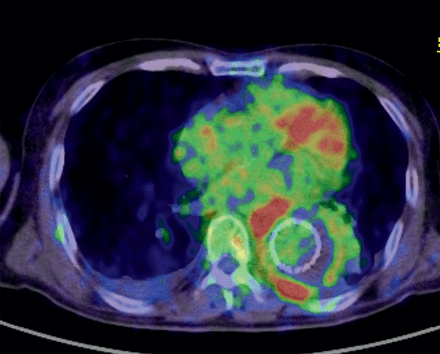
Image of graft infection. High uptake lesions were seen around the stent graft in the descending aorta. The SUVmax was 18. This patient also had abdominal grafts; however, only the graft of the descending aorta was re-replaced in the reoperation because the uptake around the abdominal graft was low. Gram-positive cocci were identified in the infected graft. Note that the uptake in the heart was not abnormal.
In 2 patients without infection (Cases 1 and 5), the other causes of inflammation were identified (cancer and multiple arthritis) using FDG-PET/CT in conjunction with further investigations. The patient of Case 1 finally died of cancer 2 years after undergoing FDG-PET/CT scanning. The FDG-PET/CT image obtained in Case 5 is shown in Fig. 2.
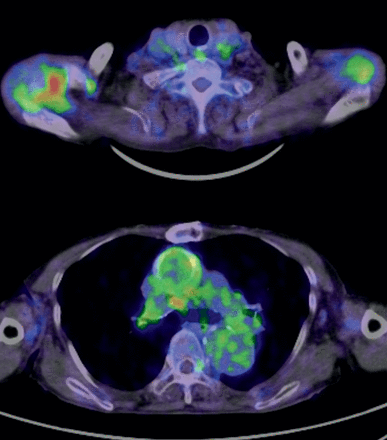
Images of a patient without graft infection. This patient had previously undergone ascending aorta replacement and suffered from a persistent fever. The SUVmax around the graft was 3.2, and the uptake was not high. Instead, a high uptake was seen in the right shoulder joint. Multiple arthritis (elderly-onset rheumatoid arthritis) was confirmed to be the cause of the fever.
The mean SUVmax in the perigraft areas was higher in the infected group than in the non-infected group (11.4 ± 4.5 vs 6.9 ± 6.4), although the difference was not statistically significant. According to the ROC analysis, the SUVmax of 8.27 or greater appeared to be the cut-off value for distinguishing the two groups (sensitivity: 1.0 and specificity: 0.8). Although the ROC curve formed a zigzag shape and was angular due to the small sample size, the area under the curve was 0.825, which indicated that the SUVmax is a good diagnostic test for distinguishing graft infection. As another example of a infected case, an image obtained in Case 6 is shown in Fig. 3.
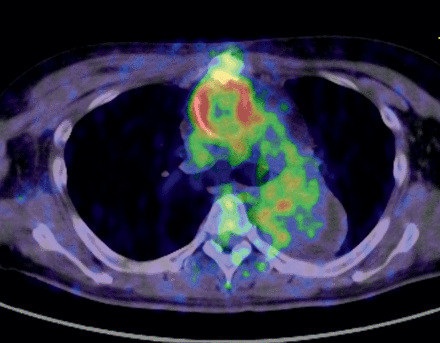
Image of graft infection. High uptake lesions were seen around the ascending aorta graft. The SUVmax was 10.9. Note that the uptake in the thrombosed false lumen in the descending aorta was low.
DISCUSSION
Despite the high mortality of the disease, the clinical presentation of prosthetic vascular infection is often non-specific. When vascular graft tissue is surrounded by typical abscesses on CT, making a diagnosis of prosthetic vascular graft infection is relatively easy. However, in most of the cases, conventional imaging findings are non-specific, and making a diagnosis can be difficult. The value of magnetic resonance imaging (MRI) for detecting soft tissue infection has also been recognized, however, the sensitivity of MRI for the detection of perigraft infection remains unclear [2]. MRI should be sensitive for demonstrating small amounts of perigraft fluid, but would be limited because of motion artefact from the great vessels; therefore, it probably has a similar diagnostic value as CT scanning. With MRI, it is similarly difficult to distinguish ‘normal’ perigraft fluid in the postoperative period from an infected perigraft fluid collection.
The use of FDG-PET/CT to identify the location of inflammation is attracting interest. Orthopaedic infections, particularly those related to implanted prostheses and osteomyelitis, can be successfully detected with the use of FDG-PET. Increasingly, this technique is being used to detect infection in soft tissues, including those representing the sources of fever of unknown origin [5]. Recently, FDG-PET/CT has been reported to be a good tool to diagnose vascular prosthetic infections; however, these studies mostly evaluated patients with prostheses in the abdominal aorta or lower extremities, and clear cut-off values for SUV to distinguish the presence of infection were not reported [6, 7].
The present study adds that FDG-PET/CT can be applied to evaluate thoracic prosthetic graft infections, which are one of the most critical clinical situations. The present study also showed that the SUVmax >8 in the perigraft area appears to be the cut-off value for distinguishing infected grafts from non-infected grafts. This clear cut-off criterion will be useful for clinical practice. Of course, one should note that the specificity is not 100%; hence, there is a chance of high uptake, even without infection. The present study also suggests that FDG-PET/CT not only can confirm the presence of prosthetic infection according to high uptake, but also may possibly reveal other causes of inflammation, such as cancer, other sites of infection or autoimmune processes.
In addition to confirming the presence of infection, FDG-PET/CT is useful for obtaining information about the extent of infection needed to select the strategy for reoperation. The present study also showed that FDG-PET/CT can be used to determine which grafts should be replaced. In this study, 2 patients had thoracic grafts and abdominal grafts; however, only the thoracic grafts were replaced because uptake was low around the abdominal grafts. In such cases, clarifying the extent of infection is important in order to select an operative strategy. Indeed, it is not unusual for patients with aortic aneurysms to have multiple grafts in different locations. We think that the use of FDG-PET/CT is particularly indicated for the identification of infected grafts in cases where multiple separated prosthetic grafts have been implanted.
There are, however, a few issues that need to be resolved in order to make more precise interpretations possible. First, information must be obtained regarding normal uptake due to postoperative inflammation. Since FDG-PET/CT reflects glucose metabolism, various types of inflammation, not only infection, can cause high uptake on scans. In the present study, the minimum interval between surgery and scanning was one and one half months. In our experience, there are little adverse effects of postoperative inflammation on scan interpretation beyond 4–6 weeks after surgery. However, a shortage of experience with normal postoperative scanning is certainly a limiting factor in evaluation, especially in the early postoperative period. Further accumulation of normal images obtained immediately after surgery is needed.
The second issue to be considered is the uptake of the native aorta itself. Atherosclerosis is a dynamic inflammatory disorder. FDG uptake in large arteries has been frequently observed and is associated with cardiovascular risk factors [8]. FDG accumulates in plaque macrophages, and uptake is correlated with macrophage density.
In addition to atherosclerotic inflammation, aortic dissection and acute aortic syndrome are associated with high uptake [9]. Prosthetic grafts are implanted in patients with aortic aneurysms, an advanced form of atherosclerosis. This is especially the case in patients who undergo TEVAR because the aortic wall of the aneurysm is always present and is not removed even after repair. In addition, during the process of aortic remodelling following TEVAR, the development of some inflammation in the aortic wall and thrombus is expected [10]. Therefore, the uptake of the aorta may possibly make the detection of infection difficult. According to previous reports, however, uptake caused by atherosclerosis or dissection is generally not as high as that due to graft infection of the present study [9]. The diagnosis of prosthetic graft infection using FDG-PET/CT must be made under a complex set of circumstances, including postoperative inflammation and the uptake of the native aorta.
In addition, there is an obvious limitation in the present study. The study was a retrospective review of a very small number of patients. This is, however, expected because graft infection in the thoracic aorta is quite rare and not all patients with suspected graft infections undergo FDG-PET/CT scanning.
Moreover, according to our protocol, if the specimen obtained was negative for infection or a specimen was not obtained, the final diagnosis was made based on a clinical follow-up period of >6 months. The certainty of non-infection is therefore not complete, although a 6-month period should be a sufficient time to confirm a lack of infection. This is another limitation of the study design.
In spite of these unsolved issues and limitation, the ability of FDG-PET/CT to detect prosthetic vascular graft infection, especially when other anatomical imaging modalities are inconclusive, has the potential to identify the sites of infection with high precision. In the near future, this modality can be employed on a routine basis for detecting, characterizing and monitoring patients with suspected and proven infections. Further possible applications of FDG-PET/CT scanning in the future may include (i) follow-up scanning to confirm the control of infection after reoperation for a graft infection and (ii) serial scanning to determine the optimal timing of a reoperation for a graft infection.
In conclusion, FDG-PET/CT is a useful tool for confirming the presence of thoracic aortic graft infection by detecting the high uptake around grafts and excluding other causes of inflammation. A value of SUVmax >8 around a graft suggests the presence of graft infection. In addition, FDG-PET/CT can be used to clarify the precise extent of infection. This is especially useful if multiple separated prosthetic grafts have been implanted.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr S. Leontyev(Leipzig, Germany): I will be brief. Do you have experience with patients early postoperatively? How sensitive are your methods in the event of a non-specific inflammatory reaction in the pericardium or around the aortic graft?
Dr Tokuda: I think that is a good point. The minimum period between operation and the PET/CT is six weeks in our experience. In the early postoperative period you may have a high uptake all around the graft or heart, so you can't see anything. We need to have more normal postoperative images, definitely.
Dr Leontyev: And did you have any experience in the diagnostics of embolization in a patient with acute endocarditis?
Dr Tokuda: We had a root abscess case, which I showed. Usually there is some uptake in the heart, so it's very difficult to distinguish whether this is heart uptake or infection uptake. In the valve area, I think it is very difficult to use this.
Dr M. Glauber(Massa, Italy): I have just one brief question. Do you think that this diagnostic tool will really change your surgical strategy, and did you have cases in which the sensitivity of the test changed due to antibiotic therapy?
Dr Tokuda: That's possible, yes. It is hard to say, actually. I'm sorry.
Dr Glauber: I never saw a response changing even after some weeks of antibiotic therapy in very complex cases, and, in any case, in my personal experience, it never changed the surgical strategy. So I really don't believe in that test anymore. The test only confirms the clinical findings that you have and does not change the surgical strategy for the patients.
Dr Tokuda: That might be true, but, like I said, for multiple grafts or excluding other causes of inflammation, it may help you.
Dr M. Nawaz(Glasgow, UK): We understand that imaging in the postop period would be difficult because of the inflammation going on, and also when there is abscess it would be quite sensitive, but how early could this actually detect? If a patient is subclinical, would you still be able to get good sensitivity results from this FDG-PET?
Dr Tokuda: In the future, if we accumulate more numbers with normal patients, that may be possible. But at this stage, we only see usefulness of FDG-PET/CT for cases in which we strongly suspect infection. Therefore, at this stage, it is very difficult to detect subclinical infection in the postoperative period, but in the future, I think it may be possible.
Author notes
Presented at the 26th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Barcelona, Spain, 27–31 October 2012.




