-
PDF
- Split View
-
Views
-
Cite
Cite
Nawid Khaladj, Ute Pichlmaier, Arne Stachmann, Sven Peterss, Angela Reichelt, Christian Hagl, Axel Haverich, Maximilian Pichlmaier, Cryopreserved human allografts (homografts) for the management of graft infections in the ascending aortic position extending to the arch, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1170–1175, https://doi.org/10.1093/ejcts/ezs572
Close - Share Icon Share
Abstract
The management of infected Dacron grafts in the ascending aortic position involving the aortic arch and aortic root remains a technical challenge. Total replacement of the infected graft material with cryopreserved homografts appears to be an effective treatment strategy for these patients.
Seventeen consecutive patients were operated on for infection of their ascending aortic graft where the aortic arch was also involved 26 ± 33 months after initial surgery, in 70%, for type A aortic dissection (12 acute aortic dissection type A and 1 chronic aortic dissection type A). The aortic root was additionally affected in 14 patients. Computed tomography and echocardiography follow-up was performed in all patients.
Hospital mortality was 24% (n = 4). Cardiopulmonary bypass and aortic cross-clamp times were 288 ± 128 and 165 ± 78 min, respectively. In 14 patients, the aortic root was replaced. Hypothermic circulatory arrest was necessary in all patients (41 ± 25 min) with additional cold selective antegrade cerebral perfusion in 14 (41 ± 30 min). During follow-up, 1 patient died due to a cerebral haemorrhage at 3 months and another at 4 years, of pulmonary embolism. Two patients were successfully reoperated on for degeneration of the aortic valve at 16 and 94 months; 1 patient had an early degeneration of the homograft and needed re-replacement with a homograft. In 2 other patients, a stent graft had to be placed to cover the distal anastomosis between the homograft and native aorta. In all others, recent follow-up revealed no degeneration of the implanted homografts.
Surgery for infected Dacron grafts in the ascending aortic position with involvement of the arch can be performed with an acceptable perioperative mortality. In case of degeneration of the valve, reoperations can be performed with good results in experienced hands. Therefore, we consider the concept of complete explantation of all infected material and replacement with homografts a successful treatment strategy. Nevertheless, close follow-up of the patients is mandatory so as not to miss any degeneration or reinfection of the implanted grafts.
INTRODUCTION
Bacterial infection of Dacron grafts in the ascending aortic position is an uncommonly described complication in thoracic aortic surgery [1, 2]. This is in contrast to graft infection in the descending or abdominal aortic segments, and regarding these, surgical treatment strategies have also been published more frequently [3]. Whether infection is indeed less frequently in the earlier-mentioned part of the aorta or patients are not brought up for redo surgery and/or die of the sequels of this catastrophic complication cannot be determined from the literature. A possible explanation may be the clearly much-smaller number of ascending aortic replacements generally performed. A possible role may be played by potential contaminations from the lung during descending aortic surgery or by the vicinity of the bowel during abdominal aortic replacement.
Currently, different treatment strategies for patients with infected grafts exist. In marked contrast to valvular or even valvular prosthetic infection, the replacement of infected vascular grafts involving the aortic root using conventional artificial grafts is associated with an exorbitantly high mortality both due to the procedure as such or subsequent reinfection [4]. As classical extra-anatomical re-routing options are not applicable at the site of the aortic root and within the arch, replacement of the infected graft with biological tissue such as cryopreserved human allografts (homografts) or alternatively, self-made pericardial tube grafts become a more convincing treatment strategy [5, 6].
Accordingly, the experience and follow-up in patients with infected ascending aortic grafts involving at least the proximal aortic arch after previous elective or emergency surgery are reported.
MATERIALS AND METHODS
This study was approved by the institutional review board. All patients gave informed consent to the use of their data for scientific research.
During an 11-year period, 17 patients were reoperated at Hannover Medical School on for an infection of their Dacron graft 26 ± 33 months following previous ascending aortic surgery (Table 1).
| Variable . | N . | % . |
|---|---|---|
| No. of patients | 17 | |
| Male | 13 | 77 |
| Female | 4 | 23 |
| Age (years) | 55 (40–71) | |
| Previous AVR | 8 | 47 |
| Previous ARR | 5 | 29 |
| Supracommissural replacement | 4 | 24 |
| Re-thoracotomy after Dacron graft implantation | 9 | 53 |
| AADA | 12 | 71 |
| CADA | 1 | 6 |
| Preoperative symptomatology (leading) | ||
| Embolic | 2 | 12 |
| Fever | 12 | 71 |
| Infection parameters | 3 | 18 |
| Asymptomatic | 1 | 6 |
| Variable . | N . | % . |
|---|---|---|
| No. of patients | 17 | |
| Male | 13 | 77 |
| Female | 4 | 23 |
| Age (years) | 55 (40–71) | |
| Previous AVR | 8 | 47 |
| Previous ARR | 5 | 29 |
| Supracommissural replacement | 4 | 24 |
| Re-thoracotomy after Dacron graft implantation | 9 | 53 |
| AADA | 12 | 71 |
| CADA | 1 | 6 |
| Preoperative symptomatology (leading) | ||
| Embolic | 2 | 12 |
| Fever | 12 | 71 |
| Infection parameters | 3 | 18 |
| Asymptomatic | 1 | 6 |
AVR: aortic valve replacement; ARR: aortic root reconstruction; AADA: acute aortic dissection type A; CADA: chronic aortic dissection type A.
| Variable . | N . | % . |
|---|---|---|
| No. of patients | 17 | |
| Male | 13 | 77 |
| Female | 4 | 23 |
| Age (years) | 55 (40–71) | |
| Previous AVR | 8 | 47 |
| Previous ARR | 5 | 29 |
| Supracommissural replacement | 4 | 24 |
| Re-thoracotomy after Dacron graft implantation | 9 | 53 |
| AADA | 12 | 71 |
| CADA | 1 | 6 |
| Preoperative symptomatology (leading) | ||
| Embolic | 2 | 12 |
| Fever | 12 | 71 |
| Infection parameters | 3 | 18 |
| Asymptomatic | 1 | 6 |
| Variable . | N . | % . |
|---|---|---|
| No. of patients | 17 | |
| Male | 13 | 77 |
| Female | 4 | 23 |
| Age (years) | 55 (40–71) | |
| Previous AVR | 8 | 47 |
| Previous ARR | 5 | 29 |
| Supracommissural replacement | 4 | 24 |
| Re-thoracotomy after Dacron graft implantation | 9 | 53 |
| AADA | 12 | 71 |
| CADA | 1 | 6 |
| Preoperative symptomatology (leading) | ||
| Embolic | 2 | 12 |
| Fever | 12 | 71 |
| Infection parameters | 3 | 18 |
| Asymptomatic | 1 | 6 |
AVR: aortic valve replacement; ARR: aortic root reconstruction; AADA: acute aortic dissection type A; CADA: chronic aortic dissection type A.
Preoperative work-up
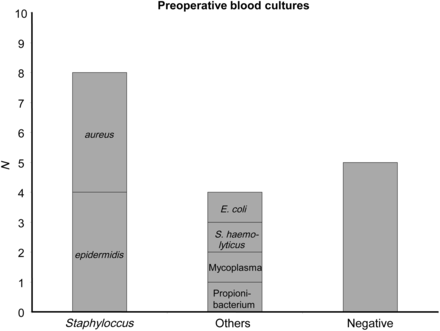
In most of the cases, the diagnosis of graft infection was made by a combination of positive blood cultures, elevated serum markers for systemic infection and typical changes in the computed tomography (CT) scans. The spectrum of bacteria found in the blood cultures is depicted in Figure 1. In 5 patients, no germ could be identified preoperatively. Lately, position emissions tomography CT (PET-CT) has supplemented the diagnostic work-up. All patients were treated preoperatively with high-dose intravenous antibiotics according to the antibiotic sensitivities or the guidelines for infective endocarditis in cases of unknown organisms (triple therapy with vancomycin, gentamycin, rifampicin).
A cryopreserved homograft was ordered from a European or German Homograft Bank. In cases in which the aortic root had been previously replaced (n = 13) or an implanted mechanical valve was involved (n = 1), an ascending aortic graft including the aortic valve was requested.
Surgery
Median re-sternotomy was performed with discontinued ventilation and the patient in Trendelenburg position to decrease the right ventricular preload. Whenever damage of the structures lying directly behind the sternum was anticipated, patients received the full dose of heparin and on occasions cardiopulmonary bypass (CBP) was established prior to sternotomy (n = 6). The femoral artery (n = 7) or the right subclavian artery (n = 6) was used for arterial access. In 4 cases, it was possible to cannulate the arch distal to the infectious process.
In the majority of cases, venous return was established via a two-stage cannula placed in the right atrium. Alternatively, a three-stage femoral cannula was used. CBP was initiated, and the patient cooled to a target temperature of 24–28°C whereby target body temperature depended on the method employed for cerebral protection (antegrade cerebral perfusion [ACP] during recent years) and the expected duration of hypothermic circulatory arrest (HCA) for aortic arch replacement, according to our institutional policy [7].
A vent catheter was placed in the left heart through the right superior pulmonary vein. Myocardial protection was achieved by repeated antegrade administration of cold blood cardioplegia (Buckberg, 6–8°C) into the aortic root or into the graft via a combined vent/cardioplegic cannula and selective administration directly into the coronary ostia thereafter. CO2 was continuously insufflated into the surgical field at 2–3 l/min as soon as the aorta was incised until declamping.
Dissection of the surgical field including the aortic root, the arch and the supra-aortic vessels was performed to the necessary extent in order to replace all previously introduced artificial material. Extensive dissection of the heart as such was, on the contrary, avoided as much as possible. The pleural cavities were also left closed if possible to avoid disseminating the infectious process. Depending on the site and the extent of the infection, circulatory arrest was occasionally installed without prior clamping of the aorta. In most cases, it proved possible to expose the graft sufficiently in order to clamp it and start with the reconstruction proximally. Complete removal of all artificial material including felt strips, pledgets and graft remnants around the reimplanted coronary ostia was felt to be mandatory and achieved in all cases. The valvular homograft was then implanted into the root using up to 24 single 4/0 or 3/0 Prolene sutures with the complete parachute technique. Coronary ostia were reinserted into the homograft with the inlay technique utilizing the native ostia of the graft in most cases and using a running 5/0 Prolene. In the cases where no valve replacement was required, the proximal anastomosis was performed using a 3/0 or 4/0 Prolene running suture. The suture lines were impregnated following knotting with neomycin-enriched fibrin glue.
Circulatory arrest was then established in all patients and the arch inspected after establishment of ACP (n = 14). Again, extensive debridement was performed including complete removal of all foreign material. Aortic tissue was resected until a non-infected wall of presumed sufficient quality for suturing was reached. This included removal of four elephant trunks where the distal anastomosis then became a technical challenge and was essentially to adventitia. The homograft was used in one piece wherever possible in order to save an additional suture line that has the potential of bleeding or later degeneration. To avoid bleeding in areas that thus become less exposable during the procedure interrupted 4/0 Prolene sutures—occasionally >20 were used for the distal anastomosis with the parachute technique and neomycin-enriched fibrin glue applied generously for haemostasis and infection control. The supra-aortic vessels were re-attached to the homograft as peninsula as a part of the distal anastomosis or a single island wherever possible. Again, generous resection of material previously involved in the anastomosis or showing changes following the previous use of GRF glue was performed. In 8 cases, this led to the abandonment of the patch technique with a subsequent necessity of anastomosing the supra-aortic vessels individually to the corresponding branches of the homograft. Clamping was performed using a dressed soft Crawford bowel clamp. De-airing was performed using active suction via a combined cardioplegia/vent cannula inserted via the proximal anastomosis or if unavoidable into the ascending aortic homograft. During weaning from CPB and, thereafter, haemostatic sutures were exclusively armed with homograft pledgets instead of artificial material (Teflon). Generally, only monofilament sutures were used. The operative field was extensively drained, but no postoperative irrigation installed. Homografts were covered with neomycin-enriched fibrin glue at the end of the procedure prior to closing the thorax.
Following surgery, patients were treated with intravenous antibiotics and antimycotics for at least 6 weeks.
Follow-up
Follow-up was done 3 months after surgery and thereafter in a yearly fashion in our outpatient clinic. Examination included, in addition to an interview, transthoracic echocardiography and CT angiography.
Statistical analysis
Due to the limited number of patients, only descriptive statistics are presented. Data are shown as mean ± standard deviation, or median and range, or percentages, as appropriate.
RESULTS
The operative procedures proved to be technically challenging, which is reflected by the details shown in Table 2.
| . | Mean ± standard deviation (range)/n . | % . |
|---|---|---|
| Operation time [min] | 416 ± 136 (271–834) | |
| CPB time [min] | 288 ± 128 (142–701) | |
| Cross-clamp time [min] | 165 ± 78 (68–383) | |
| HCA time [min] (n = 17) | 41 ± 25 (10–102) | |
| SACP time [min] (n = 14) | 41 ± 31 (7–98) | |
| Aortic root replacement | 14 | 82 |
| Arch replacement | ||
| Proximal | 7 | 41 |
| Subtotal | 2 | 12 |
| Total | 8 | 47 |
| Arterial cannulation | ||
| Central | 4 | 24 |
| Subclavia | 6 | 35 |
| Femoral | 7 | 41 |
| IABP | 1 | 6 |
| TAH | 1 | 6 |
| . | Mean ± standard deviation (range)/n . | % . |
|---|---|---|
| Operation time [min] | 416 ± 136 (271–834) | |
| CPB time [min] | 288 ± 128 (142–701) | |
| Cross-clamp time [min] | 165 ± 78 (68–383) | |
| HCA time [min] (n = 17) | 41 ± 25 (10–102) | |
| SACP time [min] (n = 14) | 41 ± 31 (7–98) | |
| Aortic root replacement | 14 | 82 |
| Arch replacement | ||
| Proximal | 7 | 41 |
| Subtotal | 2 | 12 |
| Total | 8 | 47 |
| Arterial cannulation | ||
| Central | 4 | 24 |
| Subclavia | 6 | 35 |
| Femoral | 7 | 41 |
| IABP | 1 | 6 |
| TAH | 1 | 6 |
CPB: cardiopulmonary bypass; HCA: hypothermic circulatory arrest; SACP: selective antegrade cerebral perfusion; IABP: intra-aortic balloon pump; TAH: temporary artificial heart.
| . | Mean ± standard deviation (range)/n . | % . |
|---|---|---|
| Operation time [min] | 416 ± 136 (271–834) | |
| CPB time [min] | 288 ± 128 (142–701) | |
| Cross-clamp time [min] | 165 ± 78 (68–383) | |
| HCA time [min] (n = 17) | 41 ± 25 (10–102) | |
| SACP time [min] (n = 14) | 41 ± 31 (7–98) | |
| Aortic root replacement | 14 | 82 |
| Arch replacement | ||
| Proximal | 7 | 41 |
| Subtotal | 2 | 12 |
| Total | 8 | 47 |
| Arterial cannulation | ||
| Central | 4 | 24 |
| Subclavia | 6 | 35 |
| Femoral | 7 | 41 |
| IABP | 1 | 6 |
| TAH | 1 | 6 |
| . | Mean ± standard deviation (range)/n . | % . |
|---|---|---|
| Operation time [min] | 416 ± 136 (271–834) | |
| CPB time [min] | 288 ± 128 (142–701) | |
| Cross-clamp time [min] | 165 ± 78 (68–383) | |
| HCA time [min] (n = 17) | 41 ± 25 (10–102) | |
| SACP time [min] (n = 14) | 41 ± 31 (7–98) | |
| Aortic root replacement | 14 | 82 |
| Arch replacement | ||
| Proximal | 7 | 41 |
| Subtotal | 2 | 12 |
| Total | 8 | 47 |
| Arterial cannulation | ||
| Central | 4 | 24 |
| Subclavia | 6 | 35 |
| Femoral | 7 | 41 |
| IABP | 1 | 6 |
| TAH | 1 | 6 |
CPB: cardiopulmonary bypass; HCA: hypothermic circulatory arrest; SACP: selective antegrade cerebral perfusion; IABP: intra-aortic balloon pump; TAH: temporary artificial heart.
Replacement of the infected grafts with a homograft was successful in all patients. Three patients died. In 2 patients, weaning from CPB was not possible. The first received a biventricular assist, but died several days following surgery due to profound bleeding. In the second, weaning from CPB was impossible despite intra-aortic balloon counterpulsation and catecholamine therapy. The implantation of an additional extracorporeal assist system was declined due to advanced age and infection status.
The third patient was admitted to our institution intubated, under inotropic support and acute renal failure requiring haemodialysis. Previous aortic valve replacement and arch replacement (6 weeks before) was complicated by idiopathic thrombocytopenia postoperatively, requiring high doses of intravenous immunglobulins and cortisone. The patient died 6 days after emergency surgery of multiorgan failure due to a massive mycosis.
Re-thoracotomy for bleeding was necessary in 3 patients. In 2 patients, a stent graft was placed in the descending aorta. In 1 patient, it was due to a rapid enlargement in the region of the distal anastomosis 10 days after homograft implantation. The other patient developed a leakage 3 days after arch replacement within the region of the distal anastomosis (Fig. 2). Both patients had previously had an elephant trunk so that the distal anastomosis had to be performed on a weakened proximal descending aorta.
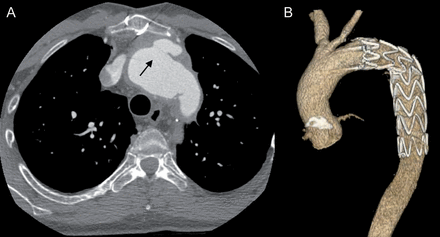
A 39-year old patient developed a leak of the distal anastomis (arrow) 3 days after homograft operation (A). This was treated with a endovascular stent graft implantation (B). The left sublclavian artery was not reinserated during the homograft implantation.
The postoperative course of all the other patients was uneventful. The intra- and postoperative data are reported in Table 2. One patient died 3 months after surgery due to a cerebral haemorrhage, while he was on coumadin for intermittent atrial fibrillation. Another died 4 years postoperatively due to a pulmonary embolism.
One patient needed rehomograft replacement of the aortic root and arch 21 month after previous homograft implantation due to early degeneration. Since an infected component for this could not be definitively excluded preoperatively (PET-CT), another homograft was implanted instead of an artificial graft (Fig. 3).
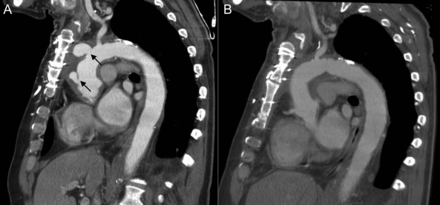
A 64-year old patient needed re-homograft replacement of the aortic root, ascending aorta and aortic arch due to homograft degeneration (arrows) 2 years after surgery (A). The initial follow-up CT scan was without pathological findings (B). Again a homograft was implanted since an infected component could not be excluded.
Two patients were successfully reoperated for degeneration of the aortic valve 16 and 94 months after implantation without any suggestion of recurrence of infection. Mechanical valves were implanted given the patient's age and preferences.
In all others, latest follow-up (mean 35 months) revealed no degeneration of the implanted homograft, as judged by echocardiography and CT scans.
DISCUSSION
Surgical procedures involving the ascending aorta and the arch are associated with a mortality and morbidity, particularly in redo cases [8, 9]. The additional impact of bacterial infection of the Dacron grafts in this context has not been evaluated in detail and moreover, there are no confirmed treatment recommendations for the ascending aorta. Generally, surgery for patients with infected grafts is associated by itself with a high morbidity and mortality [10].
In the presented series, a hospital mortality of 24% was observed. Given that 2 patients were extremely sick when they went into the operation, and no patients were turned down for operation during this time interval, this appears acceptable.
There are a number of generally accepted techniques to deal with infected grafts that refer mostly to the infradiaphragmatic aortic segment. However, exclusion and bypass are of little or no value in the region concerned in this series. Furthermore, autologous materials for in situ reconstruction such as the deep femoral veins have not been tested and are most probably not suitable for numerous reasons [11]. Silver grafts, besides being not available as knitted grafts and in the required sizes, have again not been tested and their performance is limited in frank infection in most people's hands [12]. There may be a place for self-made pericardial tube grafts as an alternative to homografts as recently reported [6]. This applies at least to the ascending aortic position although this technique has long been abandoned in abdominal aortic reconstruction for infection. Replacement of the graft with another artificial graft, whether soaked in antibiotics or not has not proved to be successful—neither abdominally nor in the thorax [4]. This stands in marked contrast to the experience in valvular endocarditis or prosthetic valvular endocarditis. Here, reconstruction is in fact often performed using artificial valves as homografts, which has not conclusively shown better results [13]. There are other groups, however, that do favour the implantation of homografts in this setting, particularly if a perianular abscess is present [14]. This discussion leaves homografts for the treatment of infected grafts in the ascending aortic position, particularly if the aortic arch is also involved [5].
Homografts are well known for their excellent haemodynamics, resistance to infection, decreased thromboembolic event rate, ease of handling and lack of the need for anticoagulation [15, 16].
Our institution has extensive experience in the treatment of patients with homografts for either infected native vessels or infected grafts, as well as for vascular reconstruction following resection of large thoracic malignancies [10, 12, 17, 18]. Due to this previous experience, the standard treatment for patients with infected grafts involving the ascending aorta/aortic arch has also been with homografts as presented here.
Prior to the surgical procedures, careful planning including three-dimensional high-resolution computer tomography of the entire aorta is mandatory. Then, a suitable homograft can be selected according to the patient's anatomy [19].
The impact of preoperative PET-CT examinations for the diagnosis of graft infections has been controversially discussed. Synthetic vascular grafts have a high uptake of markers used for this kind of examinations (18F-FDG), even a long time after surgery, leading to the risk of false-positive results [20]. In our own experience, we have also seen negative PET-CT results in patients who then yielded positive microbiology intraoperatively. The value of PET-CT thus remains controversial.
In general, well-established principles for the definitive management of prosthetic vascular graft or indeed any artificial graft infection are the complete removal of the infected material and the subsequent avoidance of recurrence. No patient in this cohort suffered from early reinfection of the graft within the observation period. Only 1 patient needed a reoperation due to rapid degeneration of his graft, where, despite being unlikely, it cannot be excluded that this may have been triggered by an infective component.
Prevention of recurrence is the second mainstay of the treatment strategy. Covering the new graft with viable tissue (e.g. omentum) is feasible but not commonly performed due to the additional trauma of the laparotomy and the risk of introducing infection into the abdomen. Whereas, it has also been proved useful for the treatment of deep sternal wound infections, the use of the omentum has not been evaluated for ascending aortic graft infections. Generous drainage of the infected site and stringent antibiotic treatment are important aspects of the treatment strategy as has been shown in analogy for other types of infected cardiovascular implants [21].
Homografts may even allow for limited debridement, which is sometimes necessary in the mediastinum due to the surrounding vital structures, since they are more resistant to reinfection in experimental models and clinical experience [10, 15].
Neomycin-impregnated fibrin glue was used in all cases to impregnate the suture lines and to cover the homografts. Its value has been shown in vivo for valvular endocarditis as well as in vitro [22, 23]. Whether imprignation is of significance in the patient population described here cannot be answered from the data.
One of the major drawbacks of homografts is degeneration, particularly of the implanted valve. In cases of infected endocarditis, 2 of the patients needed reoperation due to degeneration of the valve within the observation period. Interestingly, this phenomenon is not present in patients after heart transplantation. Heart valves in these patients with the exception of the tricuspid valve are functionally and structurally preserved, even more than 10 years after transplantation. Immunosuppression might thus be expected to preserve the homograft valve, but in the setting of graft infection, it is obviously not a feasible option [24]. Therefore, modification of homografts by means of tissue engineering to reduce the immunogenicity might be an interesting option in the future [25].
LIMITATIONS
The number of patients presented in this study is low. However, to the best of our knowledge, this is the largest single-centre experience treating patients with cryopreserved homografts in the aortic root and aortic arch position published so far. Due to our institutional policy, all patients with infected grafts were treated with homografts. Therefore, there is no control group that we were also unable to recruit from the literature. The morbidity of the treatment advocated here is also not inconsiderable. Long-term follow-up is lacking so far.
CONCLUSION
Surgery for infected Dacron graft can be performed with an acceptable perioperative mortality in the ascending aortic position, even and especially if the root and/or arch is involved. Close follow-up is necessary to identify patients with degeneration and enlargement early. Stent graft implantation does seem a successful treatment option in such cases. In case of degeneration of the valve, reoperations can be performed with good results in experienced hands. Therefore, we consider the concept of complete explantation of all infected material and replacement by homografts as a successful treatment strategy in this special subset of high-risk patients.
Conflict of interest: none declared.
REFERENCES
Author notes
The first two authors contributed equally to this work.
- aorta
- aortic arch
- computed tomography
- anastomosis, surgical
- cryopreservation
- follow-up
- repeat surgery
- surgical procedures, operative
- transplantation, homologous
- tissue transplants
- infections
- surgery specialty
- endoluminal grafts
- graft infections
- allografting
- supraaortic valve area
- tissue degeneration
- reinfection
- circulatory arrest




