-
PDF
- Split View
-
Views
-
Cite
Cite
Kirsti Berg, Mette Langaas, Madelene Ericsson, Hilde Pleym, Samar Basu, Ivar Skjåk Nordrum, Nicola Vitale, Rune Haaverstad, Acetylsalicylic acid treatment until surgery reduces oxidative stress and inflammation in patients undergoing coronary artery bypass grafting, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1154–1163, https://doi.org/10.1093/ejcts/ezs591
Close - Share Icon Share
Abstract
Acetylsalicylic acid (ASA) is a cornerstone in the treatment of coronary artery disease (CAD) due to its antiplatelet effect. Cessation of aspirin before coronary artery bypass grafting (CABG) is often recommended to avoid bleeding, but the practice is controversial because it is suggested to worsen the underlying CAD. The aims of the present prospective, randomized study were to assess if ASA administration until the day before CABG decreases the oxidative load through a reduction of inflammation and myocardial damage, compared with patients with preoperative discontinuation of ASA.
Twenty patients scheduled for CABG were randomly assigned to either routine ASA-treatment (160 mg daily) until the time of surgery (ASA), or to ASA-withdrawal 7 days before surgery (No-ASA). Blood-samples were taken from a radial artery and coronary sinus, during and after surgery and analysed for 8-iso-prostaglandin (PG) F2α; a major F2-isoprostane, high-sensitivity C-reactive protein (hs-CRP), cytokines and troponin T. Left ventricle Tru-Cut biopsies were taken from viable myocardium close to the left anterior descending artery just after connection to cardiopulmonary bypass, and before cardioplegia were established for gene analysis (Illumina HT-12) and immunohistochemistry (CD45).
8-Iso-PGF2α at baseline (t1) were 111 (277) pmol/l and 221 (490) pmol/l for ASA and No-ASA, respectively (P = 0.065). Area under the curve showed a significantly lower level in plasma concentration of 8-iso-PGF2α and hsCRP in the ASA group compared with the No-ASA group with (158 pM vs 297 pM, P = 0.035) and hsCRP (8.4 mg/l vs 10.1 mg/l, P = 0.013). All cytokines increased during surgery, but no significant differences between the two groups were observed. Nine genes (10 transcripts) were found with a false discovery rate (FDR) <0.1 between the ASA and No-ASA groups.
Continued ASA treatment until the time of CABG reduced oxidative and inflammatory responses. Also, a likely beneficial effect upon myocardial injury was noticed. Although none of the genes known to be involved in oxidative stress or inflammation took a different expression in myocardial tissue, the genetic analysis showed interesting differences in the mRNA level. Further research in this field is necessary to understand the role of the genes.
INTRODUCTION
Antiplatelet treatment prevents platelet aggregation, and acetyl salicylic acid (ASA) is the most widely prescribed drug in this category. ASA acts by irreversible acetylation of a serine residue of cyclooxygenase 1 (COX-1), thereby inhibiting the release of thromboxane A2 (TXA2), a potent mediator of platelet aggregation. This clinically important effect has given ASA a primary role in the prevention of cardiovascular diseases. However, the beneficial effects of ASA are not confined just to its platelet inhibition, as other mechanisms like inhibition of TXA2-induced vasoconstriction [1], reduced inflammation and prostaglandin (PG) F2α formation [2] are well known. The beneficial effects of ASA on the reduction of oxidative stress may also be extended to both vasoconstriction and inflammation [3]; hence, these multiple interactions have generated a growing interest in this drug [4].
Coronary artery bypass grafting (CABG) with the use of cardiopulmonary bypass (CPB) and ischaemic cardiac arrest mediates oxidative stress [5]. Reactive oxygen species (ROS) are generated by specific enzymes, including the multi-enzyme system NADPH oxidase [6], or through leakage in the mitochondrial respiratory chain during reperfusion. The latter mechanism contributes to the post-ischaemic reperfusion injury, such as inflammation. The process of CPB may also produce ROS due to the inflammation generated by the exposure of blood to non-physiological surfaces. In addition, surgical trauma is another cause of induction of inflammation of different magnitudes [5].
As ASA-treatment until surgery may increase postoperative blood loss in patients undergoing CABG [7], preoperative discontinuation of ASA- has still a grade B recommendation, based on multiple-level 2a and 2b studies [8]. There is no general consensus on the timing of ASA discontinuation, hence patients are recommended to stop aspirin intake 2–10 days before elective cardiac surgery. However, patients undergoing urgent cardiac surgery with an acute coronary syndrome should continue aspirin therapy up to the day of surgery [8]. Moreover, a recent retrospective study including 10 000 CABG patients showed that ASA therapy did not increase blood loss [9]. Even reduced overall mortality has been reported in other studies [10]. The practice of ASA withdrawal 1 week before surgery is therefore controversial and it is recommended that the surgeon should assess the advantages and disadvantages of ASA treatment prior to CABG in view of the patient's risk profile. [11]. Furthermore, there is experimental evidence that ASA can interfere with ROS production and inactivation processes, thereby lowering oxidative stress [12, 13]. However, to the best of our knowledge, the relationship between ASA and ROS has not previously been evaluated in open heart surgery.
The primary objectives of this prospective, randomized study were to assess whether ASA administration until the day before surgery exerts any effect on ROS production, inflammation and myocardial damage, compared with patients with preoperative discontinuation of ASA 7 days before surgery. Secondly, we aimed to identify the underlying mechanisms of reduced oxidative stress by evaluation of the patients' gene profile from left ventricular myocardial biopsies obtained during surgery.
MATERIALS AND METHODS
Study design
This prospective randomized study was carried out on 20 patients referred for first-time CABG during a period of 11 months. Inclusion criteria were: (i) stable angina with double or triple-vessel disease; (ii) left ventricular ejection fraction >40%; (iii) age >18 years. Patients with diabetes, renal failure (plasma creatinine >140 µmol/l) or other systemic diseases, use of NSAID or antithrombotic drugs other than ASA or systemic glucocorticoids were excluded. Patients undergoing reoperations, combined procedures or emergency surgery were also excluded. Patients' clinical characteristics are presented in Table 1.
| Group . | No-ASA (n = 7) . | ASA (n = 11) . | P . |
|---|---|---|---|
| Gender (M/F) | 6/1 | 9/2 | 1.000 |
| Age (years) | 58 (20) | 65 (23) | 0.035 |
| BMI | 26.4 (9.4) | 27.0 (11.0) | 0.791 |
| EuroSCORE1 | 1 (2) | 3 (4) | 0.044 |
| Postoperative hospital stay (days) | 7 (14) | 6 (5) | 0.246 |
| Left ventricular ejection fraction (%) | 57 (33) | 55 (35) | 0.607 |
| Previous myocardial infarction, n (%) | 2 (29) | 4 (36) | 0.614 |
| Present smoker2, n (%) | 3 (43) | 2 (18) | 0.280 |
| Hypertension, n (%)3 | 7 (100) | 7 (64) | 0.224 |
| Aortic cross-clamp time (min) | 41 (36) | 37 (19) | 0.724 |
| CPB time (min) | 67 (36) | 68 (49) | 0.930 |
| Operation time (min) | 145 (32) | 142 (72) | 0.860 |
| β-Blocker, n (%) | 5 (71) | 10 (91) | 0.536 |
| Calcium channel blocker, n (%) | 3 (43) | 3 (27) | 0.596 |
| Clopidogrel, n (%) | 7 (100) | 11 (100) | 1.000 |
| ACE inhibitors, n (%) | 2 (29) | 3 (27) | 1.000 |
| Diuretics, n (%) | 2 (29) | 3 (27) | 1.000 |
| Statins, n (%) | 7 (100) | 11 (100) | 1.000 |
| Group . | No-ASA (n = 7) . | ASA (n = 11) . | P . |
|---|---|---|---|
| Gender (M/F) | 6/1 | 9/2 | 1.000 |
| Age (years) | 58 (20) | 65 (23) | 0.035 |
| BMI | 26.4 (9.4) | 27.0 (11.0) | 0.791 |
| EuroSCORE1 | 1 (2) | 3 (4) | 0.044 |
| Postoperative hospital stay (days) | 7 (14) | 6 (5) | 0.246 |
| Left ventricular ejection fraction (%) | 57 (33) | 55 (35) | 0.607 |
| Previous myocardial infarction, n (%) | 2 (29) | 4 (36) | 0.614 |
| Present smoker2, n (%) | 3 (43) | 2 (18) | 0.280 |
| Hypertension, n (%)3 | 7 (100) | 7 (64) | 0.224 |
| Aortic cross-clamp time (min) | 41 (36) | 37 (19) | 0.724 |
| CPB time (min) | 67 (36) | 68 (49) | 0.930 |
| Operation time (min) | 145 (32) | 142 (72) | 0.860 |
| β-Blocker, n (%) | 5 (71) | 10 (91) | 0.536 |
| Calcium channel blocker, n (%) | 3 (43) | 3 (27) | 0.596 |
| Clopidogrel, n (%) | 7 (100) | 11 (100) | 1.000 |
| ACE inhibitors, n (%) | 2 (29) | 3 (27) | 1.000 |
| Diuretics, n (%) | 2 (29) | 3 (27) | 1.000 |
| Statins, n (%) | 7 (100) | 11 (100) | 1.000 |
Results are presented as frequencies (percentage) for dichotomous variables and median (range) for non-dichotomous variables (continuous).
1EuroSCORE: European System for Cardiac Operative Risk Evaluation.
2Present smoker or quit <2 months ago.
3BT >140 mmHg or on anti-hypertensive treatment.
| Group . | No-ASA (n = 7) . | ASA (n = 11) . | P . |
|---|---|---|---|
| Gender (M/F) | 6/1 | 9/2 | 1.000 |
| Age (years) | 58 (20) | 65 (23) | 0.035 |
| BMI | 26.4 (9.4) | 27.0 (11.0) | 0.791 |
| EuroSCORE1 | 1 (2) | 3 (4) | 0.044 |
| Postoperative hospital stay (days) | 7 (14) | 6 (5) | 0.246 |
| Left ventricular ejection fraction (%) | 57 (33) | 55 (35) | 0.607 |
| Previous myocardial infarction, n (%) | 2 (29) | 4 (36) | 0.614 |
| Present smoker2, n (%) | 3 (43) | 2 (18) | 0.280 |
| Hypertension, n (%)3 | 7 (100) | 7 (64) | 0.224 |
| Aortic cross-clamp time (min) | 41 (36) | 37 (19) | 0.724 |
| CPB time (min) | 67 (36) | 68 (49) | 0.930 |
| Operation time (min) | 145 (32) | 142 (72) | 0.860 |
| β-Blocker, n (%) | 5 (71) | 10 (91) | 0.536 |
| Calcium channel blocker, n (%) | 3 (43) | 3 (27) | 0.596 |
| Clopidogrel, n (%) | 7 (100) | 11 (100) | 1.000 |
| ACE inhibitors, n (%) | 2 (29) | 3 (27) | 1.000 |
| Diuretics, n (%) | 2 (29) | 3 (27) | 1.000 |
| Statins, n (%) | 7 (100) | 11 (100) | 1.000 |
| Group . | No-ASA (n = 7) . | ASA (n = 11) . | P . |
|---|---|---|---|
| Gender (M/F) | 6/1 | 9/2 | 1.000 |
| Age (years) | 58 (20) | 65 (23) | 0.035 |
| BMI | 26.4 (9.4) | 27.0 (11.0) | 0.791 |
| EuroSCORE1 | 1 (2) | 3 (4) | 0.044 |
| Postoperative hospital stay (days) | 7 (14) | 6 (5) | 0.246 |
| Left ventricular ejection fraction (%) | 57 (33) | 55 (35) | 0.607 |
| Previous myocardial infarction, n (%) | 2 (29) | 4 (36) | 0.614 |
| Present smoker2, n (%) | 3 (43) | 2 (18) | 0.280 |
| Hypertension, n (%)3 | 7 (100) | 7 (64) | 0.224 |
| Aortic cross-clamp time (min) | 41 (36) | 37 (19) | 0.724 |
| CPB time (min) | 67 (36) | 68 (49) | 0.930 |
| Operation time (min) | 145 (32) | 142 (72) | 0.860 |
| β-Blocker, n (%) | 5 (71) | 10 (91) | 0.536 |
| Calcium channel blocker, n (%) | 3 (43) | 3 (27) | 0.596 |
| Clopidogrel, n (%) | 7 (100) | 11 (100) | 1.000 |
| ACE inhibitors, n (%) | 2 (29) | 3 (27) | 1.000 |
| Diuretics, n (%) | 2 (29) | 3 (27) | 1.000 |
| Statins, n (%) | 7 (100) | 11 (100) | 1.000 |
Results are presented as frequencies (percentage) for dichotomous variables and median (range) for non-dichotomous variables (continuous).
1EuroSCORE: European System for Cardiac Operative Risk Evaluation.
2Present smoker or quit <2 months ago.
3BT >140 mmHg or on anti-hypertensive treatment.
The patients were randomized by block size four and stratified by gender and age (below 63 years, or equal or above 63 years) to either routine ASA-treatment (160 mg daily) until the day before surgery (ASA, n = 12) or to ASA withdrawal 7 days before surgery (No-ASA, n = 8). Randomization was carried out at the Unit for Applied Clinical Research, Norwegian University of Science and Technology, Trondheim.
The study was performed according to the Helsinki declaration. The Regional Medical Research Ethics Committee, Central Norway, approved the protocol (Approval number 2006.686) and written informed consent was obtained from all patients before enrolment.
Procedures
The overall procedures identifying main events and sampling time points are presented in Fig. 1.
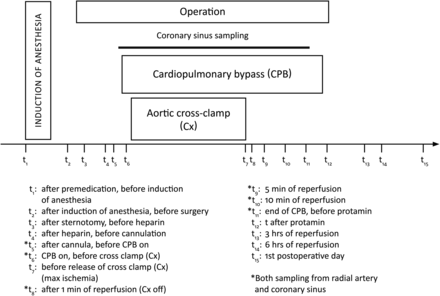
Anaesthesia
All patients received premedication with morphine (10 mg) and scopolamine (0.4 mg). Prior to anaesthesia, the left radial artery was cannulated for arterial pressure monitoring and blood sampling, and a catheter was introduced into the urinary bladder. Anaesthesia was induced with diazepam, fentanyl, thiopental and pancuronium, and was maintained with isoflurane and fentanyl. Inotropic agents (dobutamine), vasodilators (nitroprusside) or vasoconstrictors (phenylephrine) were applied according to the clinical situation. Intra-arterial blood pressure and central venous pressure were monitored continuously. Before and after CPB, the lungs were ventilated mechanically with oxygen-enriched air and isoflurane, adjusted to keep the end-tidal pCO2 at 4.66 kPa. Cephalotin was used as perioperative antibiotic prophylaxis.
Surgery
The heart was approached through a median sternotomy; thereafter, the left internal thoracic artery and the saphenous vein were harvested. An initial dose of 300 IU/kg heparin was given prior to cannulation of the ascending aorta and the right atrium. Additional heparin was given when necessary to maintain the activated clotting time (ACT) >480 s during CPB. A membrane-oxygenator with biocompatible surfaces (Maxima, Medtronic, Minneapolis, MN, USA) was used in all operations. The pump prime consisted of 1800-ml Ringer solution and 7500 IU heparin. Arterial perfusion with mild hypothermia (34°C) was performed with non-pulsatile flow at 2.4 l/min/m2 body surface area with a roller pump. After aortic cross-clamping, cardiac arrest was induced with delivery of the St. Thomas' Hospital 2 cardioplegic solution [14] in the aortic root. At the end of CPB, heparin was neutralized with protamine sulphate.
In order to sample coronary venous blood, a retrograde cardioplegia catheter (Edwards Lifesciences Corp., Irvine, CA, USA) was inserted into the coronary sinus before CPB and removed when weaning off CPB. Correct position of the catheter was ascertained by transoesophageal echocardiography, and blood was slowly withdrawn to avoid haemolysis and aspiration of right atrial blood. Blood loss was recorded hourly until drain removal on the first postoperative day.
Sampling and handling of blood and urine samples
Blood samples were drawn from the radial artery at 15 time points before, during and after surgery (t1–t15), as well as from the coronary sinus during surgery at six time points (t5, t6, t8–t11) as shown in Fig. 1. All blood samples were further collected into pre-cooled tubes with K3EDTA and kept on ice before centrifugation (10 min, 4°C, 3000 g) within 30 min. Urine samples were collected from the urinary catheter at three time points: baseline (after induction of anaesthesia), during operation and during 24 h following surgery. After collection, all blood samples were frozen and stored at −80°C until analysis.
Biopsies
During surgery, one biopsy by Tru-Cut needle (BioPince™) was taken from each patient from the viable left ventricular myocardium just after cannulation for cardiopulmonary bypass, and prior to aortic cross-clamping. All biopsies were snap-frozen in liquid nitrogen and further frozen at −80°C before processing.
Biochemical assessments
8-Iso-prostaglandin (PG)F2α in plasma and urine was measured by a validated radioimmunoassay (RIA) method without any extraction procedure as previously described by Basu [15]. The detection limit of the assay was 23 pmol/l, with an intra-assay coefficient variation (CV) of 12–15%.
The high sensitive (hs)-CRP (mg/l) in plasma was measured by latex-enhanced immuno-turbidimetry in a Hitachi 917 analyzer (Latex HS, Roche Diagnostics, Mannheim, Germany). The assay detection limit was 0.03 mg/l (analytical sensitive).
Troponin T (µg/l) in plasma was measured by electrochemiluminescence in a Roche Modular E analyzer (Roche Diagnostics, Mannheim, Germany). The assay detection limit was 0.01 µg/l.
Albumin (g/l) in plasma was measured by a colorimetric end-point assay in a Roche Modular P analyzer (Roche Diagnostics, Mannheim, Germany).
For 8-iso-PGF2α, hs-CRP and Troponin T values below the detection limit were set to the detection limit. All biochemical values in plasma (8-iso-PGF2α, hs-CRP and Troponin T) were adjusted with concentration of albumin at the same sample points in plasma [adjusted value = measured value × (albumin actual/albumin baseline)].
Cytokines
The following cytokines, chemokines and growth factors were measured in plasma samples: interleukin 1ra (IL-1ra), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-9, IL-10, IL-12p70, IL-13, IL-15, IL-17, tumour necrosis factor-α (TNF-α), interferon-gamma (IFN-γ), macrophage inhibitory protein-1α (MIP-1α), MIP-1β, eotaxin, monocyte chemotactic protein-1 (MCP-1), granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), fibroblast growth factor (basic FGF), vascular endothelial growth factor (VEGF), interferon-inducible protein (IP-10), regulated upon activation, normal T-cell expressed and secreted (RANTES), and platelet derived growth factor bb (PDGFbb). Measurements were carried out in a Bioplex Array Reader (LUMINEX 100, Bio-Rad Laboratories, Hercules, CA) using Bio-Plex Human Cytokine 27-plex panel (Bio-Rad Laboratories, Hercules, CA). Values below the detection limit are replaced with zero since robust inference was performed.
Gene expression in left ventricle tissue
Total RNA was isolated from left ventricle tissue with the use of RNeasy Micro Kit (Qiagen®), according to the manufacturer's protocol. Briefly, 10 mg (wet weight) frozen tissue was homogenized by knife (Rotor Stator, 10 strokes/40 s) and lysed in RLT lysis buffer with β-mercaptoethanol. Homogenization was performed on ice. Proteinase K or DNAase were not used during isolation, to minimize contamination. RNA concentration and quality were determined using a NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA), which calculates an RNA integrity number (RIN). The average RIN value of RNA samples used for cRNA amplification was 9.2 (8.3–9.9), indicating the good quality of RNA samples. One sample was excluded due to poor RNA quality (RIN value 6.7).
The Illumina TotalPrep RNA amplification Kit (Ambion Inc., Austin, TX) was used to amplify RNA for hybridization on Illumina BeadChips. To synthesize first strand cDNA by reverse transcription, total RNA was used from each sample collected. Following the second strand cDNA synthesis and cDNA purification steps, the in vitro transcription to synthesize cRNA was prepared overnight for 12 h. The gene expression profiles were measured on biopsies using Illumina HT-12 Expression BeadChip (Illumina, San Diego, CA), which enables genome-wide expression analysis (48 803 transcripts).
Data analysis
Raw data were exported from the Illumina Genome studio software to R using the Bioconductor package ‘Lumi’. Probes that were detected for less than half of the samples (the function detection-Call was used) were removed from the analysis, leaving 13 671 transcripts for further evaluation. The data were log2 transformed and quantile-quantile normalized. The groups were compared using a moderated t-test from the Bioconductor package ‘Limma’ [16]. Fold change was used to demonstrate the rate of changes in average gene expressions between the No-ASA and ASA groups.
Statistical analyses were performed using the Benjamini–Hochberg step-up algorithm, controlling the false discovery rate (FDR) at level 0.1. Conversion between Illumina ProbeID, Entrez GeneID and Gene Symbol is performed using the ReMOAT tool for re-annotation and mapping.
cDNA synthesis and real-time PCR quantification
RNA-containing solution was applied directly to obtain a first-strand complementary DNA (cDNA) using the iScript cDNA Synthesis Kit with oligo(dT) (Bio-Rad, CA, USA). Reactions were performed and monitored using the Applied Biosystems StepOne Plus™ Real-Time PCR System (ABI, Foster City, CA, USA) and the 2X iQ SYBR Green Supermix, based on iTaq DNA polymerase (Bio-Rad, Hercules, CA, USA). cDNA samples were analysed in triplicate both for the genes of interest and reference/housekeeping gene (ACTB/β-actin). The Ct value was measured for each sample, and arbitrary units were calculated using standard curves that consisted of serial dilutions of cDNA from a pool of samples. Fold change was estimated as the ratio of means of concentrations. P values were calculated using a two-sample one-sided unequal variances t-test on concentrations.
Immunohistochemistry
Serial cryosections (5–8 μm) from left ventricular biopsies were used for haematoxylin-erythrosin staining and immunohistochemically examination. The leucocyte common antigen marker, CD45 (abcam 10558, Cambridge, UK) was used as a general marker of inflammation. P47-phox (C20) and p67-phox (N-19) (SantaCruz Biotechnology Inc, CA, USA), and 8-iso PGF2α, (abcam, ab2280, Cambridge, UK), were used as markers of oxidative stress. Primary antibodies were diluted (CD45,1:750; p47, 1:250; p67, 1:100; 8-iso PGF2α, 1:250) in PBS with 1% BSA and incubated for 60 min in room temperature. Sections stained for CD45 were detected with Envision+ (Dako Cytomation, Denmark) in accordance with the manufacturer's protocol. Visualizing antibodies for oxidative stress used Alexa Fluor®488 (Invitrogen, CA, USA) donkey anti-goat or goat anti-rabbit. For CD45, endogenous peroxidase was blocked with 0.3% H2O2 for 10 min. Nuclear staining used DAPI (Invitrogen, CA, USA) for fluorescent markers or haematoxylin for CD45. Positive control for oxidative stress used myocardium and spleen from CCl4-treated rats.
Statistical analysis
Non-parametric tests were applied unless otherwise described, because of non-normal distribution of most data. Results are presented as median level with range (max–min). The Wilcoxon Mann–Whitney test was applied for comparison of the two independent groups (ASA vs No-ASA) and Wilcoxon signed rank for pairwise data. One-sided tests were applied for 8-iso-PGF2α, hs-CRP and Troponin T since the biological hypothesis was that the amount of these markers was lower in the ASA group. Fisher's exact test was used for dichotomous variables.
Area under the curve (AUC) was calculated using the trapezoidal rule. Missing time points for each patient were imputed using the mean of the corresponding time point(s) for the other patients. Missing values of 8-iso-PGF2α, hs-CRP and Troponin T were imputed using the last observation carried forward rule.
Friedman's test with Bonferroni corrections was used to investigate effect over time (comparison of dependent groups). Spearman's correlation coefficient (ρ) was applied for correlation analyses.
Ordering of the heat-maps was based on hierarchical clustering, with average linkage and one minus the Pearson correlation coefficient as the distance measure. Dendrograms are drawn when this ordering is used. In the heat-maps, light squares depict high response values, and dark squares, low response values.
Statistical analysis was performed using SPSS version PASW Statistics 18 (SPSS Inc., Chicago, IL, USA) and in R. A significance level of 0.05 was used.
RESULTS
Of the 20 patients originally enrolled, 2 were later excluded: 1 in the ASA group who was reopened for bleeding, and 1 in the No-ASA group who suffered a postoperative AMI (pronounced ST-elevation during surgery with Troponin T = 2.9 µg/l on postoperative day 1) and subsequent cerebral ischaemia. A total of 18 patients concluded the study, 11 in the ASA group and 7 in the No-ASA group. All these patients underwent an uneventful CABG operation and postoperative recovery.
Postoperative bleeding
Median (range) levels of postoperative bleeding up to 18 h after surgery were 540 (655) and 670 (415) ml for ASA and No-ASA patients, respectively (P = 0.211).
Oxidative stress detected by 8-iso-PGF2α
Median (range) plasma levels of 8-iso-PGF2α at baseline (t1) were 111 (277) pmol/l and 221 (490) pmol/l for ASA and without-ASA patients, respectively (P = 0.065). Area under the curve showed a significantly lower level in plasma concentration of 8-iso-PGF2α in the ASA group compared with the No-ASA group (Table 2). Furthermore, as shown in Fig. 2A, the plasma levels of 8-iso-PGF2α in the No-ASA group began to increase in the post-sternotomy sample (t3). The maximum level (median over peaks: 592/969 pmol/l) in the No-ASA group occurred at t4, after heparin administration but before cannulation. On the other hand, the maximum level in the ASA-group occurred at t6 after CPB circulation (391/690 pmol/l) (P = 0.104). Plasma concentrations of 8-iso-PGF2α fell gradually and were below baseline on postoperative day 1 in both groups.
| Group . | No-ASA . | ASA . | P . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | ||
| Troponin T | 0.790 | 1.762 | 0.690 | 0.840 | 0.239 |
| 8-iso-PGF2α | 257.6 | 380.8 | 87.2 | 380.8 | 0.043 |
| HsCRP | 45.6 | 96.6 | 38.9 | 96.6 | 0.022 |
| Group . | No-ASA . | ASA . | P . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | ||
| Troponin T | 0.790 | 1.762 | 0.690 | 0.840 | 0.239 |
| 8-iso-PGF2α | 257.6 | 380.8 | 87.2 | 380.8 | 0.043 |
| HsCRP | 45.6 | 96.6 | 38.9 | 96.6 | 0.022 |
PG: prostaglandin; HsCRP: high-sensitivity C-reactive protein.
| Group . | No-ASA . | ASA . | P . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | ||
| Troponin T | 0.790 | 1.762 | 0.690 | 0.840 | 0.239 |
| 8-iso-PGF2α | 257.6 | 380.8 | 87.2 | 380.8 | 0.043 |
| HsCRP | 45.6 | 96.6 | 38.9 | 96.6 | 0.022 |
| Group . | No-ASA . | ASA . | P . | ||
|---|---|---|---|---|---|
| Median . | Range . | Median . | Range . | ||
| Troponin T | 0.790 | 1.762 | 0.690 | 0.840 | 0.239 |
| 8-iso-PGF2α | 257.6 | 380.8 | 87.2 | 380.8 | 0.043 |
| HsCRP | 45.6 | 96.6 | 38.9 | 96.6 | 0.022 |
PG: prostaglandin; HsCRP: high-sensitivity C-reactive protein.
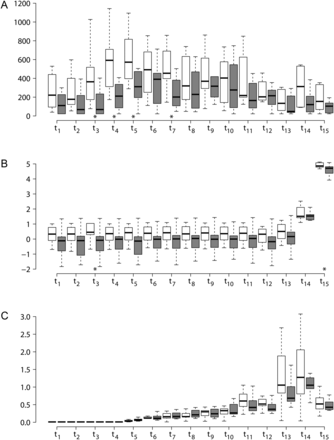
(A) Boxplots for 8-iso-PGF2α over the time points for the No-ASA (open boxes) and ASA (filled boxes) group. Significant differences between the ASA and No-ASA group are found at t3, t4, t5 and t7. (B) Boxplots for hs-CRP on log-scale over the time points for the No-ASA (open boxes) and ASA (filled boxes) group. Significant differences between the ASA and No-ASA group are found at t3 and t15. (C) Boxplots for Troponin T over the time points for the No-ASA (open boxes) and ASA (filled boxes) group. No significant differences between the ASA and No-ASA group are found at any time points.
Urinary level of 8-iso-PGF2α at baseline was 0.38 (0.72) and 0.50 (1.26) pmol/mmol creatinine for No-ASA and ASA patients, respectively (P = 0.659 between groups). A non-significant increase was observed in urine during operation: 0.46 (1.00) (P = 0.078) and 0.54 (2.38) (P = 0.563) pmol/mmol creatinine for No-ASA and ASA patients, respectively (P = 0.717 between groups). The urine concentrations of 8-iso-PGF2α during the first 24 h after surgery were 0.44 (4.03) (P = 0.375) and 0.50 (0.99) (P = 0.240) pmol/mmol creatinine for No-ASA and ASA patients, respectively (P = 0.751 between groups).
Inflammation detected by hs-CRP
Median (range) basal levels (t1) of hs-CRP were 1.39 (9.6) and 0.89 (3.68) mg/l for No-ASA and ASA patients, respectively (P = 0.105). Fig. 2B presents a time-based profile; no hs-CRP increase developed in any of the groups until t14, 6 h after reperfusion. The highest level was measured at t15 on the first postoperative day, with a significant higher value among the No-ASA patients with 129.5 (229.6) mg/l compared with the ASA group with 111.1 (111.5) mg/l, P = 0.022. The area under the curve for hs-CRP also shows a significantly lower increase in plasma concentration of ASA vs No-ASA (P = 0.022) (Table 2).
Myocardial injury detected by troponin T
Median (basal) Troponin T levels were below the detection limit (<0.01 µg/l) for all patients at baseline except for one ASA patient with 0.018 µg/l. Troponin T increased gradually in both groups immediately after cannulation (t5) to 0.032 (0.072) and 0.066 (0.117) µg/l (P = 0.975 between groups) (Fig. 2C). The highest level was measured 6 h after reperfusion (t14) with median level 1.27 (3.03) µg/l and 1.05 (1.56) µg/l in No-ASA and ASA, respectively (P = 0.430 between groups). The area under the curve shows no differences between ASA and No-ASA (P = 0.239).
Correlations
The comparisons of AUC between 8-iso-PGF2α, hs-CRP and Troponin T for each patient did not produce any significant correlations; Troponin T vs 8-iso-PGF2α (ρ = 0.36, P = 0.138), Troponin T vs hs-CRP (ρ = −0.22, P = 0.375), 8-iso-PGF2α vs hs-CRP (ρ = 0.45, P = 0.065).
Coronary sinus vs radial artery
Using Friedman's test (time points t5, t6, t8, t9 and t10), we found a significant difference between paired measurements of Troponin T from the coronary sinus and the radial artery (P < 0.005), while no significant differences were found for 8-iso-PGF2α and hs-CRP. In detail, significant differences of Troponin T between the coronary sinus and the radial artery measurements were found for all time points (P values below 0.02) but t6 (P = 0.24). The median (range) paired difference between the coronary sinus and radial artery measurements over all patients and time points was 0.016 (1.023).
Immunohistochemistry
CD45 was not expressed in any groups, but was positive in the positive control (spleen). The samples were negative for all other antibodies including positive control (myocardium).
Cytokines
No significant differences between the two groups were observed for any cytokine at any time. The results are therefore presented based on both groups together in a heat-map (Fig. 3). All cytokines did increase during the study, with one peak observed for each cytokine. Maximum level was observed at t1 for IL5, at t12 (end of operation) for 19 cytokines, at t13 (reperfusion) for four cytokines and t15 for three cytokines.
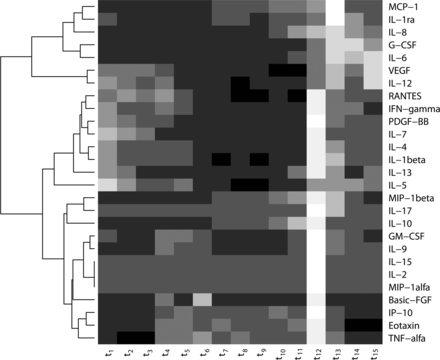
Heat map of 27 different cytokines. For each time point the median value over all 18 patients is shown for each cytokine. Each row shows one cytokine and each column shows one time point. The cytokines are ordered using hierarchical clustering. Light values mean high expression values and dark values low expression values.
Microarray and real-time PCR
Nine genes (10 transcripts) were found with a FDR < 0.1 between the ASA and No-ASA groups (Table 3). The microarray results were confirmed with real-time PCR for two of the top differentially expressed genes (CES2: estimated fold change 1.50, P value = 0.02, and ME2: estimated fold change 0.82, P = 0.06). Due to limited material (biopsies), the remaining seven top differentially expressed genes were not tested with real-time PCR. The top 31 differentially expressed transcripts (FDR ≤ 0.44) are presented in a heat-map in Fig. 4.
The nine genes (10 transcripts) found to be differentially expressed between the ASA and No-ASA group (FDR <0.1)
| Illumina ID . | Gene symbol . | Entrez . | Unigene . | FC . | AE . | FDR . |
|---|---|---|---|---|---|---|
| ILMN_1661491 | SH3GL2 | 6456 | Hs.715611 | 2.43 | 7.88 | 0.0086 |
| ILMN_1698680 | ARL17P1 | 51326 | Hs.579108 | 1.45 | 6.68 | 0.0086 |
| ILMN_1670218 | EXOSC6 | 118460 | Hs.660633 | 1.36 | 8.39 | 0.0239 |
| ILMN_1730229 | CGNL1 | 84952 | Hs.148989 | 1.80 | 10.18 | 0.0361 |
| ILMN_1786278 | FAM149A | 25854 | Hs.357025 | 0.75 | 7.32 | 0.0361 |
| ILMN_1696675 | CES2 | 8824 | Hs.282975 | 1.41 | 9.49 | 0.0361 |
| ILMN_2061435 | FP504 | 55384 | Hs.525589 | 1.43 | 7.71 | 0.0710 |
| ILMN_2048636 | ME2 | 4200 | Hs.233119 | 0.77 | 9.20 | 0.0710 |
| ILMN_2390544 | FAM149A | 25854 | Hs.357025 | 0.82 | 6.80 | 0.0710 |
| ILMN_2049642 | RPA1 | 6117 | Hs.461925 | 1.33 | 9.45 | 0.0710 |
| Illumina ID . | Gene symbol . | Entrez . | Unigene . | FC . | AE . | FDR . |
|---|---|---|---|---|---|---|
| ILMN_1661491 | SH3GL2 | 6456 | Hs.715611 | 2.43 | 7.88 | 0.0086 |
| ILMN_1698680 | ARL17P1 | 51326 | Hs.579108 | 1.45 | 6.68 | 0.0086 |
| ILMN_1670218 | EXOSC6 | 118460 | Hs.660633 | 1.36 | 8.39 | 0.0239 |
| ILMN_1730229 | CGNL1 | 84952 | Hs.148989 | 1.80 | 10.18 | 0.0361 |
| ILMN_1786278 | FAM149A | 25854 | Hs.357025 | 0.75 | 7.32 | 0.0361 |
| ILMN_1696675 | CES2 | 8824 | Hs.282975 | 1.41 | 9.49 | 0.0361 |
| ILMN_2061435 | FP504 | 55384 | Hs.525589 | 1.43 | 7.71 | 0.0710 |
| ILMN_2048636 | ME2 | 4200 | Hs.233119 | 0.77 | 9.20 | 0.0710 |
| ILMN_2390544 | FAM149A | 25854 | Hs.357025 | 0.82 | 6.80 | 0.0710 |
| ILMN_2049642 | RPA1 | 6117 | Hs.461925 | 1.33 | 9.45 | 0.0710 |
Illumina ProbeID, Gene Symbol and Entrez GeneID are presented for all transcripts, together with estimated fold change (FC) for the ASA group compared to the No-ASA group, average log2 expression for each transcript and estimated false discovery rate (FDR). A FC larger than one, means that the ASA group is up-regulated when compared with the No-ASA group. FC <1, means that the ASA group is down-regulated when compared with the No-ASA group. Two transcripts (ILMN_1786278 and ILMN_2301544) are found to be part of the FAM149A gene, and both show a down-regulation in the ASA group when compared with the No-ASA group.
The nine genes (10 transcripts) found to be differentially expressed between the ASA and No-ASA group (FDR <0.1)
| Illumina ID . | Gene symbol . | Entrez . | Unigene . | FC . | AE . | FDR . |
|---|---|---|---|---|---|---|
| ILMN_1661491 | SH3GL2 | 6456 | Hs.715611 | 2.43 | 7.88 | 0.0086 |
| ILMN_1698680 | ARL17P1 | 51326 | Hs.579108 | 1.45 | 6.68 | 0.0086 |
| ILMN_1670218 | EXOSC6 | 118460 | Hs.660633 | 1.36 | 8.39 | 0.0239 |
| ILMN_1730229 | CGNL1 | 84952 | Hs.148989 | 1.80 | 10.18 | 0.0361 |
| ILMN_1786278 | FAM149A | 25854 | Hs.357025 | 0.75 | 7.32 | 0.0361 |
| ILMN_1696675 | CES2 | 8824 | Hs.282975 | 1.41 | 9.49 | 0.0361 |
| ILMN_2061435 | FP504 | 55384 | Hs.525589 | 1.43 | 7.71 | 0.0710 |
| ILMN_2048636 | ME2 | 4200 | Hs.233119 | 0.77 | 9.20 | 0.0710 |
| ILMN_2390544 | FAM149A | 25854 | Hs.357025 | 0.82 | 6.80 | 0.0710 |
| ILMN_2049642 | RPA1 | 6117 | Hs.461925 | 1.33 | 9.45 | 0.0710 |
| Illumina ID . | Gene symbol . | Entrez . | Unigene . | FC . | AE . | FDR . |
|---|---|---|---|---|---|---|
| ILMN_1661491 | SH3GL2 | 6456 | Hs.715611 | 2.43 | 7.88 | 0.0086 |
| ILMN_1698680 | ARL17P1 | 51326 | Hs.579108 | 1.45 | 6.68 | 0.0086 |
| ILMN_1670218 | EXOSC6 | 118460 | Hs.660633 | 1.36 | 8.39 | 0.0239 |
| ILMN_1730229 | CGNL1 | 84952 | Hs.148989 | 1.80 | 10.18 | 0.0361 |
| ILMN_1786278 | FAM149A | 25854 | Hs.357025 | 0.75 | 7.32 | 0.0361 |
| ILMN_1696675 | CES2 | 8824 | Hs.282975 | 1.41 | 9.49 | 0.0361 |
| ILMN_2061435 | FP504 | 55384 | Hs.525589 | 1.43 | 7.71 | 0.0710 |
| ILMN_2048636 | ME2 | 4200 | Hs.233119 | 0.77 | 9.20 | 0.0710 |
| ILMN_2390544 | FAM149A | 25854 | Hs.357025 | 0.82 | 6.80 | 0.0710 |
| ILMN_2049642 | RPA1 | 6117 | Hs.461925 | 1.33 | 9.45 | 0.0710 |
Illumina ProbeID, Gene Symbol and Entrez GeneID are presented for all transcripts, together with estimated fold change (FC) for the ASA group compared to the No-ASA group, average log2 expression for each transcript and estimated false discovery rate (FDR). A FC larger than one, means that the ASA group is up-regulated when compared with the No-ASA group. FC <1, means that the ASA group is down-regulated when compared with the No-ASA group. Two transcripts (ILMN_1786278 and ILMN_2301544) are found to be part of the FAM149A gene, and both show a down-regulation in the ASA group when compared with the No-ASA group.
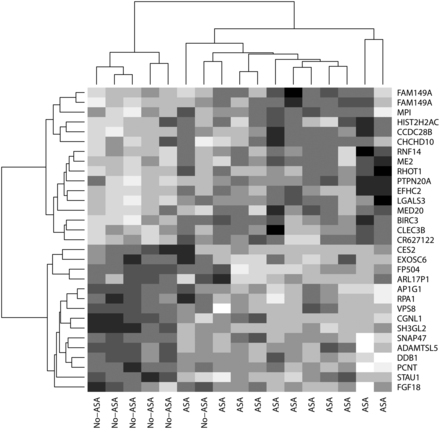
Heat map of the top 31 differentially expressed genes between the ASA and No-ASA group. The heat map is a two-dimensional presentation of the gene expression data (normalized data on log2 scale) where the rows and columns are ordered using hierarchical clustering. Each row shows one gene transcript and each column shows one patient. Values are presented on a scale from white to black: white is for high expression and black for low expression values.
DISCUSSION
This prospective, randomized study evaluated the effects of continued ASA intake until the day prior to CABG on oxidative stress, inflammation and myocardial injury, compared with preoperative withdrawal of ASA 7 days before surgery. The results in the two groups revealed the following major effects of continued ASA therapy: (i) lower and delayed increase of plasma levels of 8-iso-PGF2α, indicating reduced oxidative stress; (ii) lower plasma levels of hs-CRP indicating a weaker inflammatory response; (iii) a tendency towards reduced myocardial injury according to the levels of Troponin T.
Oxidative stress and inflammation
Lower and delayed increase of 8-iso-PGF2α plasma levels occurred in the ASA patients compared with No-ASA subjects. These findings are in line with our previous observations [5] indicating lower plasma levels of 8-iso-PGF2α in patients undergoing CABG who had stopped ASA medication ≤4 days before surgery. The same study also identified a predominant release of a major F2-isoprostane, namely 8-iso-PGF2α in plasma during the initial stages of surgery, triggered by the surgical trauma. As a result of this key finding, an importance of the non-enzymatic ROS-mediated oxidation of arachidonic acid inducing oxidative stress during and following CABG was elucidated. The findings of the present study are also supported by an animal and in vitro study reporting reduced oxidative load upon ASA treatment [13]. Several COX-independent mechanisms have been proposed to contribute to the cardiovascular beneficial effects of ASA, many of them focusing on the potential role of ASA as a redox-modulating agent. Firstly, the ASA-metabolite salicylic acid can directly scavenge hydroxyl radicals to form 2,3- and 2,5-dihydroxybenzoate derivatives [17]. Secondly, ASA can also acetylate proteins and inhibit (as for COX) or stimulate cellular processes [18]. Specifically, it has been demonstrated that ASA, due to acetylation of endothelial nitric oxide (NO) synthase (eNOS) proteins, elicits NO release from the vascular endothelium [19]. A potential inactivation of NADPH oxidase was evaluated by Wu et al. [13], who observed a markedly reduced vascular superoxide-production due to ASA treatment. The inhibitory effects, however, were not found after short-term ASA treatment in animals, indicating the direct inhibition of NADPH oxidase. Thirdly, ASA is shown to interfere with the redox-sensitive nuclear factor-κB (NF-κB), which in turn influences gene expression [3, 12].
In the present study, ASA patients consistently demonstrated lower plasma levels of hs-CRP than the No-ASA group at all time points. Several inflammatory pathways are activated during CABG, causing oxidative stress through the activation of leucocytes [3]. However, since ASA is both an anti-inflammatory drug and COX-1- inhibitor that may have effects on ROS production, it is difficult to distinguish between anti-oxidative and anti-inflammatory effects from this study. In support of our findings suggesting an additional anti-oxidative effect of ASA, an experimental study on normotensive and spontaneously hypertensive rats demonstrated that ASA only, among all the non-steroidal anti-inflammatory drugs and the non-selective COX inhibitor ibuprofen, reduced ROS production in vascular tissues [13]. The reduced oxidative stress and inflammation was not supported by reduced levels of cytokines, as there were no differences in cytokines levels between the ASA and No-ASA groups. The observed variation in cytokine production within the treatment groups was large compared with the variation between the groups. Further investigations using a larger sample size could be helpful in revealing any underlying mechanisms connecting oxidative stress, inflammation and cytokine production in this ASA vs No-ASA population.
There was a significantly (FDR < 0.1) different expression of 10 transcripts (nine genes) between the ASA- and No-ASA groups in the biopsies taken from the viable left ventricle ( see Table 3). The SH3GL2 gene shows the highest degree of up-regulation in ASA patients as compared with No-ASA patients. The SH3GL2 gene, also called Src homology 3 (SH3) domain protein 2A and endophilin 1, is known to be involved in cell communication and signal transduction [20]. The SH3 domain is known to inactivate NADPH oxidase by preventing phosphorylation of the subunits p47phox and p67phox [20]. NADPH oxidase is supposed to be one of the most important sources for superoxide generation [6]. Superoxide is further described as an important molecule in signal transduction, but in excess, it is shown to be cytotoxic. It should therefore be of interest to reduce the superoxide production during surgery. The malic enzyme 2 or ME2 was up-regulated in the ASA group compared with the No -ASA group. This gene is involved in oxido-reductase activity. The CGNL1 cingulin-like 1 gene, up-regulated in the ASA-group, is, in the Gene Ontology cell component system, classified as being associated with cell junctions. The activity of cell junctions is affected by redox status [21] and may also be of interest in this context. Finally, the gene FAM149 is presented by two of the top 10 transcripts, and is found to be down-regulated in ASA compared with No-ASA. To the best of our knowledge, the down-regulation of FAM 149 in the ASA group has not previously been reported. Furthermore, the function of this gene is still unknown. Unfortunately, we are unable to provide any possible explanations for our observations on the behaviour of genes. Further investigations should be carried out to highlight the underlying mechanisms of these gene regulations.
Myocardial injury
There was a trend towards lower release of Troponin T in the ASA group compared with the No-ASA patients. However, this trend was not found to be significant. Furthermore, we found a significantly higher level of Troponin T in blood samples collected from the coronary sinus vs the radial artery. There are only few previous studies on the role of the myocardium in oxidative stress during CABG [22]. A likely explanation for the higher levels of Troponin T in the samples from coronary sinus compared with samples from the radial artery could be the concomitant association of temporary myocardial ischaemia and manipulation of the heart during construction of the anastomoses. Immunohistochemistry for the leucocyte common antigen marker, CD45, was negative in all biopsies, demonstrating similar myocardial conditions regarding inflammation, in the ASA and the No-ASA group before CABG was performed.
The outcome of immunohistochemistry for oxidative stress did not demonstrate positive staining in the myocardium of the left ventricle. The result does not exclude the presence of the examined proteins since a disadvantage of immunohistochemistry is that the specificity and sensitivity of the primary antibody is crucial for the results. Unfortunately, few antibodies are available for the detection of oxidative stress in tissue sections. An interesting finding in this study is the time aspect of observed variables in the blood samples (Fig. 5). The first observed peaks were 8-iso-PGF2α at t5 followed by the cytokines at t12, Troponin T at t14 and CRP at t15. The surgical trauma may trigger a release of the superoxide anion, or another ROS, starting a ROS-catalysed arachidonic oxidation, following an increase of 8-iso-PGF2α with further damage to the myocardium through its vasoconstrictive and COX-activation properties [23]. Many transcription factors are redox sensitive (e.g. NFκB), with expression of genes and subsequent translation of several pro- and anti-inflammatory cytokines and CRP as results. We therefore suggest that increased oxidative stress is responsible for the release of cytokines. As cytokines are not known to induce myocardial damage, we have reason to believe that the increase of oxidative stress also is partly responsible for the increase of Troponin T. This conclusion is also supported by one study reporting a change in the oxidative status [24]. Whether the increase of CRP is caused by ROS or cytokines per se or the local ischaemic injury measured by Troponin T is still unknown.
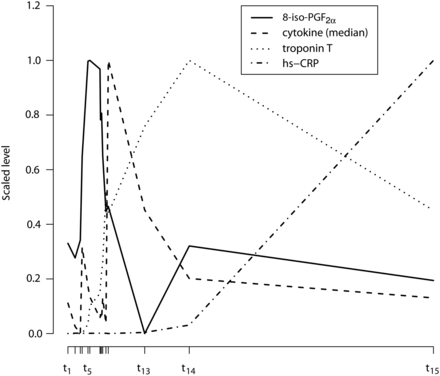
Time course behaviour of 8-iso-PGF2α, Troponin T, hs-CRP and median cytokine level. On the x-axis the median time for the different clinical events t1, t2,…, t15 are given. The median times are calculated over all patients on the original scale, times are calculated as relative difference from t1. On the y-axis, values of 8-iso-PGF2α, Troponin T, and hs-CRP are shown as a median for overall patients. Values for the cytokines at each time point are calculated as follows: the median on overall patients is obtained for each of the 27 cytokines, subsequently the median is calculated over all 27 cytokines. Scaled values (over the medians of all time points) are presented by subtracting the minimum value and dividing with the range.
Postoperative bleeding
No differences regarding bleeding during the first 18 h after the end of surgery were observed between the two groups. The relation between preoperative ASA-treatment on clinical outcome and bleeding complications remains an unsolved issue [20–22]. Increased postoperative bleeding is the only clinically relevant negative effect of ASA therapy until the day of surgery. The review by Gulbins et al. [9] outlines that preoperative aspirin therapy does not seem to influence the operative outcome of isolated CABG, thus there is weak support for a recommendation to discontinue this therapy prior to an elective CABG procedure. In an experimental study on rats, it was found that COX-2 inhibition produced by small residual ASA is the probable cause of ischaemic accidents and drug-eluting stents thrombosis a few days after ASA withdrawal [25].
Study limitations and strengths
Limitations of the present study include a potentially non-homogeneous and small patient population, resulting in low power to detect differences between the ASA and No-ASA population. The results of this study must be confirmed in a larger study population. A particular strength of the study is the frequent sampling by the same technicians, which made it possible to follow the exact time course of oxidative and inflammation processes and handling of experimental procedures.
CONCLUSION
The present results indicate that continued ASA treatment until the time of CABG systemically reduced oxidative and inflammatory responses. Also, a likely beneficial effect on myocardial injury was noticed. The observed effects may be related to inhibition of COX-1, but a redox-modulating activity of ASA may represent an additional mechanism. The biopsies from the viable left ventricle revealed no basal inflammation in the myocardium in any of the patients. Due to the frequent sampling, we were also able to demonstrate the timing of occurrence of biochemical and physiological events during CABG. Our findings provide new insight into the beneficial cardiovascular effects of ASA, and indicate that ASA may reduce the oxidative processes due to surgical trauma. Thus, the present study suggests a protective role of continued ASA treatment until the time of surgery. Although none of the genes known to be involved in oxidative stress or inflammation took a different expression in myocardial tissue, the genetic analysis showed interesting differences in mRNA levels, which suggests the need for further research in this field.
Funding
The work was supported by grants from Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
The Illumnia Bead Analyses were provided by NMC at the national technology platform, and supported by the functional genomics program (FUGE) in the Research Council of Norway. The authors acknowledge the help by Heidi Brurok in the planning and design of the study, Tom Even Wheeler for isolating the RNA from biopsies, Kamilla Stunes for help with the RNA-PCR analyses and Liv Ryan for help with the cytokine analyses.
REFERENCES
- aspirin
- cytokine
- myocardium
- oxidative stress
- prostaglandins
- coronary artery bypass surgery
- hemorrhage
- immunohistochemistry
- inflammation
- left ventricle
- biopsy
- cd45 antigens
- area under curve
- genes
- preoperative care
- radial artery
- surgical procedures, operative
- troponin t
- surgery specialty
- myocardial injury
- coronary sinus
- international organization for standardization




