-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher M. Humphries, Jeffery L. Anderson, Jean H. Flores, John R. Doty, Cardiac magnetic resonance imaging for perioperative evaluation of sternal eversion for pectus excavatum, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 6, June 2013, Pages 1110–1113, https://doi.org/10.1093/ejcts/ezs662
Close - Share Icon Share
Abstract
Pectus excavatum is associated with varying degrees of exercise intolerance and symptomatology. Various forms of evaluation have been inconsistent in identifying objective data for correlation with symptoms. Cardiac magnetic resonance (CMR) imaging provides a promising method for delineating the anatomical and physiological components of pectus excavatum as well as measuring the results of surgical repair.
Six patients with symptomatic pectus excavatum underwent preoperative evaluation with CMR. All patients had successful, uncomplicated repair of pectus excavatum using the sternal eversion technique. At the first postoperative visit, all patients underwent postoperative evaluation with CMR. Pre- and postoperative CMR measurements were compared for each patient.
Preoperative CMR demonstrated evidence of anatomical and dynamical compression of the heart in all patients. After surgery, all patients showed improvement on postoperative CMR. Five of the 6 (83%) patients had complete relief of right ventricular compression, and 5 of the 6 (83%) patients had relief of left atrial compression. The degree of antero-posterior chest wall narrowing was also markedly improved, with an average postoperative vs preoperative Haller index of 3.2 (range, 2.7–3.8) vs 5.0 (range, 4.0–5.9).
After surgical correction of pectus excavatum with the sternal eversion technique, CMR demonstrates improvement in both anatomical chest wall contour and cardiac performance. Sternal eversion provides the most complete anatomical correction and greatest relief of internal cardiac compression. We recommend CMR as the definitive modality for evaluation of patients with pectus excavatum, as this modality shows that the primary underlying physiological abnormality in pectus excavatum is cardiac compression.
INTRODUCTION
Surgical repair of pectus excavatum has yielded excellent cosmetic and functional results, with the majority of the patients having improved chest wall contour and exercise tolerance. Multiple operations have been described to address the anatomical deformities associated with pectus excavatum, including sub-perichondrial resection of costal cartilages, transverse sternal osteotomy and elevation of the sternum [1–8]. Modifications have been developed to maintain the repositioned sternum, including support with prosthetic materials, metal struts and autologous tissue.
The technique of sternal eversion was first described by Wada et al. in 1961 [9] and has become the procedure of choice for the treatment of pectus excavatum at our institution since 1983. Sternal eversion forms an excellent biological contour of the chest wall and provides anatomical relief of compression of the underlying structures. The addition of the internal mammary artery pedicle was reported by us in 1984 and provides a reliable blood supply to the sternal plate [10].
The development of cardiac magnetic resonance (CMR) imaging allows for simultaneous evaluation of both anatomy and function of the heart and great vessels. This new approach to cardiac imaging is particularly accurate in assessing the effects of chest wall deformities on valvular and ventricular function. We sought to investigate whether the use of CMR would provide objective data about the relief of cardiac compression after repair of pectus excavatum with the sternal eversion technique.
METHODS
Evaluation of pectus excavatum with cardiac magnetic resonance
Magnetic resonance imaging can display static, cine or multiplanar reconstructions. Spin echo, gradient echo, echo-planar imaging and steady-state free precession are the most commonly used signal sequences for CMR imaging. Spin echo sequences are commonly used for multislice anatomical imaging. Gradient echo and steady-state free precession sequences are used for the assessment of physiological function via cine imaging. Electrocardiographic gating is also required for most CMR scans to coordinate the correct phases of the cardiac cycle. As a result, CMR offers both anatomical and physiological evaluation of the heart and great vessels.
CMR at Intermountain Medical Center, in routine practice since 1983, was used to evaluate patients with pectus excavatum before and after surgical repair by sternal eversion. CMR imaging parameters included end diastolic volume, end systolic volume, wall thickness, mass index, stroke volume index, cardiac index and segmental and global function of the left and right ventricles. Function of all four cardiac valves was assessed for degree of insufficiency. The left and right atria were measured in antero-posterior diameter. Displacement of the heart from the midline and degree of apposition of the sternum to the heart were noted. The ratio of internal transverse (right to left) to antero-posterior dimension (Haller index) was recorded.
Operative technique
The surgical repair is performed through a midline or sub-mammary incision. Bilateral pectoralis major muscle flaps are elevated to expose the anterior chest wall laterally out to the level of rib angulation. The rectus abdominus and diaphragmatic muscle attachments are freed from the xyphoid process and costal margins. The sternum is divided transversely in the second or third inter-costal space at a point above the origin of the sternal deformity. The costal cartilages and inter-costal muscles are transected vertically near the junction of the osseous and cartilaginous ribs.
The sternum and costal cartilages are separated posteriorly from the pericardium and anterior mediastinal tissues beginning inferiorly at the xiphoid and costal margins. The sternal place is elevated, while both the internal mammary vascular pedicles are preserved. One of the internal mammary pedicles is chosen (usually the left) and mobilized proximally to its origin from the sub-clavian vessels. The internal mammary vessels on the opposite side are ligated and divided, or mobilized if the primary vascular pedicle has been damaged.
The sternum and attached cartilages are then everted by turning the graft 180° axially, taking care to avoid excessive tension on the mammary artery pedicle. Patency of the preserved internal mammary artery in its new position is confirmed by Doppler ultrasound velocity probe. The sternum is stabilized vertically using two or three loops of no. 6 stainless steel wire. The costal cartilages on the graft are shortened to correct length and angles to ensure proper position and contour of the new anterior chest wall. The costal cartilages are reattached to the lateral ribs using figure-of-eight no. 5 wire.
A scalpel or oscillating saw may be used to shave thin layers of cartilage or bone off protruding areas to produce a smooth anterior wall contour. The rectus abdominus muscles and fascia are reattached to the costal margin using continuous size 0 polyglycolic acid suture. The pectoralis major muscles are reattached in the midline using interrupted size 0 polyglycolic acid suture. The sub-cutaneous tissues are then closed, and the skin is reapproximated by sub-cuticular suture.
Study population
Six patients with symptomatic pectus excavatum underwent preoperative CMR to evaluate cardiac physiology and function according to the above parameters. All patients underwent successful, uncomplicated repair of pectus excavatum using the sternal eversion technique. Postoperative CMR scans were performed 6 weeks after surgery and compared with the preoperative scans.
RESULTS
Preoperative CMR demonstrated evidence of anatomical and dynamical compression of the heart in all patients (Table 1). The most pronounced finding was compression of the right ventricle and left atrium. In addition, most patients had significant narrowing of the antero-posterior chest wall dimension, with an average Haller index of 5.0 (range, 4.0–5.9; normal, <3). Figure 1 demonstrates representative static CMR images of a patient prior to operation.
Preoperative cardiac magnetic resonance findings for patients with pectus excavatum
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 60 | Mild | Mild | 5.9 | Yes | No | Yes | Yes |
| 2 | 67 | 54 | No | Mild | 4 | Yes | No | No | Yes |
| 3 | 61 | 45 | Mild | Mild | 5 | Yes | No | No | Yes |
| 4 | 60 | 54 | Mild | No | 5 | Yes | No | No | Yes |
| 5 | 56 | 34 | Mild | Mild | 5 | Yes | No | No | Yes |
| 6 | 65 | 59 | Mild | No | 5.2 | Yes | No | No | Yes |
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 60 | Mild | Mild | 5.9 | Yes | No | Yes | Yes |
| 2 | 67 | 54 | No | Mild | 4 | Yes | No | No | Yes |
| 3 | 61 | 45 | Mild | Mild | 5 | Yes | No | No | Yes |
| 4 | 60 | 54 | Mild | No | 5 | Yes | No | No | Yes |
| 5 | 56 | 34 | Mild | Mild | 5 | Yes | No | No | Yes |
| 6 | 65 | 59 | Mild | No | 5.2 | Yes | No | No | Yes |
EF: ejection fraction; comp.: compression; LV: left ventricular; RV: right ventricular; LA: left atrial; RA: right atrial.
Preoperative cardiac magnetic resonance findings for patients with pectus excavatum
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 60 | Mild | Mild | 5.9 | Yes | No | Yes | Yes |
| 2 | 67 | 54 | No | Mild | 4 | Yes | No | No | Yes |
| 3 | 61 | 45 | Mild | Mild | 5 | Yes | No | No | Yes |
| 4 | 60 | 54 | Mild | No | 5 | Yes | No | No | Yes |
| 5 | 56 | 34 | Mild | Mild | 5 | Yes | No | No | Yes |
| 6 | 65 | 59 | Mild | No | 5.2 | Yes | No | No | Yes |
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 61 | 60 | Mild | Mild | 5.9 | Yes | No | Yes | Yes |
| 2 | 67 | 54 | No | Mild | 4 | Yes | No | No | Yes |
| 3 | 61 | 45 | Mild | Mild | 5 | Yes | No | No | Yes |
| 4 | 60 | 54 | Mild | No | 5 | Yes | No | No | Yes |
| 5 | 56 | 34 | Mild | Mild | 5 | Yes | No | No | Yes |
| 6 | 65 | 59 | Mild | No | 5.2 | Yes | No | No | Yes |
EF: ejection fraction; comp.: compression; LV: left ventricular; RV: right ventricular; LA: left atrial; RA: right atrial.
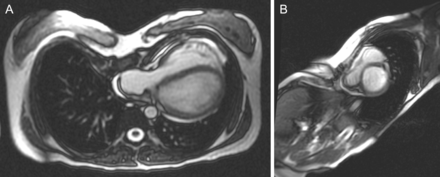
(A and B) Preoperative axial and sagittal CMR imaging views of pectus excavatum. The right ventricle and right and left atria are markedly compressed and ‘entrapped’ between the sternum and the vertebral column.
After surgery, all patients showed improvement on postoperative CMR (Table 2). Five of 6 (83%) patients had complete relief of right ventricular (RV) compression and 1 had very mild residual compression. Five of the 6 (83%) patients had relief of left atrial compression and 1 had very mild residual compression. Figure 2 demonstrates representative static CMR images of a patient after surgical repair. RV ejection fraction after surgery was improved in 1 patient, unchanged in 4 and diminished in 1. Left ventricular (LV) ejection fraction was normal in all patients prior to operation and unchanged after surgery.
Postoperative cardiac magnetic resonance findings for patients with pectus excavatum
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 45 | No | Mild | 2.7 | No | No | No | No |
| 2 | 68 | 52 | No | No | 3 | No | No | No | No |
| 3 | 62 | 46 | Mild | No | 3.2 | No | No | No | No |
| 4 | 58 | 55 | Mild | No | 3.8 | No | No | No | Yes |
| 5 | 55 | 64 | Mild | Mild | 3.5 | Minimal | No | No | No |
| 6 | 67 | 59 | Mild | No | 3.6 | Very mild | No | No | Very mild |
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 45 | No | Mild | 2.7 | No | No | No | No |
| 2 | 68 | 52 | No | No | 3 | No | No | No | No |
| 3 | 62 | 46 | Mild | No | 3.2 | No | No | No | No |
| 4 | 58 | 55 | Mild | No | 3.8 | No | No | No | Yes |
| 5 | 55 | 64 | Mild | Mild | 3.5 | Minimal | No | No | No |
| 6 | 67 | 59 | Mild | No | 3.6 | Very mild | No | No | Very mild |
EF: ejection fraction; comp.: compression; LV: left ventricular; RV; right ventricular; LA: left atrial; RA: right atrial.
Postoperative cardiac magnetic resonance findings for patients with pectus excavatum
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 45 | No | Mild | 2.7 | No | No | No | No |
| 2 | 68 | 52 | No | No | 3 | No | No | No | No |
| 3 | 62 | 46 | Mild | No | 3.2 | No | No | No | No |
| 4 | 58 | 55 | Mild | No | 3.8 | No | No | No | Yes |
| 5 | 55 | 64 | Mild | Mild | 3.5 | Minimal | No | No | No |
| 6 | 67 | 59 | Mild | No | 3.6 | Very mild | No | No | Very mild |
| Patient no. . | LV EF (%) . | RV EF (%) . | Mitral prolapse . | Tricuspid prolapse . | Haller index . | RV comp. . | LV comp. . | RA comp. . | LA comp. . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 60 | 45 | No | Mild | 2.7 | No | No | No | No |
| 2 | 68 | 52 | No | No | 3 | No | No | No | No |
| 3 | 62 | 46 | Mild | No | 3.2 | No | No | No | No |
| 4 | 58 | 55 | Mild | No | 3.8 | No | No | No | Yes |
| 5 | 55 | 64 | Mild | Mild | 3.5 | Minimal | No | No | No |
| 6 | 67 | 59 | Mild | No | 3.6 | Very mild | No | No | Very mild |
EF: ejection fraction; comp.: compression; LV: left ventricular; RV; right ventricular; LA: left atrial; RA: right atrial.
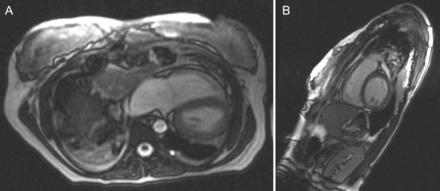
(A and B) Postoperative axial and sagittal CRMI views of pectus excavatum. There is relief of compression of both the right ventricle and the right atrium and (less well shown in B) left atrium with restoration of a more normal antero-posterior dimension.
The degree of antero-posterior chest wall narrowing was also markedly improved, with an average postoperative Haller index of 3.2 (range, 2.7–3.8; P = 0.0029). Of the 4 patients who had tricuspid regurgitation prior to operation, 2 (50%) showed improvement and had no residual tricuspid regurgitation on postoperative CMR.
DISCUSSION
Multiple non-invasive modalities have been used to evaluate the effects of surgical repair of pectus excavatum. Pulmonary function testing with spirometry provides measurement of tidal volume and total lung capacity. Pulmonary function testing, however, does not have any anatomical capability and cannot evaluate cardiac function or anatomy. In addition, pulmonary function testing can be affected by underlying lung disease such as asthma, which is unrelated to the chest wall deformity. In our opinion, pulmonary function testing has a little role in the evaluation of patients with pectus excavatum.
Standard computed tomography (CT) provides excellent anatomical detail for the chest wall deformity and reliably provides objective data about intrathoracic dimensions and displacement of the heart. Intravenous contrast enhancement can demonstrate heart and lung anatomy, but does not provide any functional information about cardiac or pulmonary performance. In addition, it involves significant radiation exposure, which is compounded with serial (i.e. pre- and postoperative) studies, and is of particular concern in this younger patient population. Also, there are certain patients with severe antero-posterior compression on CT who may have little functional impairment; conversely, some individuals are markedly symptomatic, with less-severe findings.
CMR provides the optimal preoperative and postoperative evaluation of pectus excavatum. The resolution is comparable with CT for bony and muscular anatomy and provides objective measurements for intrathoracic anatomy. More importantly, CMR provides accurate and dynamical assessment of cardiovascular function and is highly sensitive for qualitative analysis [11, 12]. An advantage over CT is the lack of radiation exposure in this younger patient population, allowing for safe performance of serial studies. This study suggests that an important, if not sole, underlying cause of exercise intolerance in pectus excavatum is cardiac compression, rather than primarily pulmonary restriction.
The reduced antero-posterior dimension of the chest wall in pectus excavatum produces an actual anatomical limitation on the heart, primarily on the right ventricle and left atrium. Both of these cardiac chambers lie primarily in the midline and are ‘entrapped’ between the posterior wall of the depressed sternum and the vertebral bodies. The right atrium also may be involved. Diastolic filling of these two chambers is essentially fixed due to the bony constraints of the chest wall depression. The left ventricle does not experience compression as the heart is generally rotated laterally into the left chest. Also, apposition of the dynamical anterior RV wall against the inner chest wall may give rise to complaints of chest discomfort. The right atrium and superior vena cava are less likely to be affected, but can be compressed from an asymmetric sternum with more right-sided depression. Systolic function of both ventricles is generally preserved. It is unclear from this series of patients why there is variability in the recovery of RV function. One possible explanation is that there was insufficient time between surgical correction and postoperative imaging for RV recovery. Improvement in ejection fraction after cardiac surgery for ischaemic or valvular heart disease may occur over several months, particularly if the principal impairment has been diastolic dysfunction.
When additional cardiac output is required during exercise, the right ventricle is unable to generate a larger stroke volume due to the restriction on diastolic filling. This fixed stroke volume limits the amount of blood delivered to the pulmonary circulation. Restricted left atrial diastolic (to clarify that this is not during ventricular diastole) further limits LV stroke volume. The patient cannot increase stroke volume and is dependent on increased heart rate to meet exercise demands. Once maximal heart rate is achieved, there is no further increase in cardiac output, and the patient experiences dyspnoea and exercise limitation.
The technique of sternal eversion has been our procedure of choice for adult patients with pectus excavatum. Although minimally invasive techniques have become well-established for the treatment of pectus excavatum in children and selected adults, it is our opinion that the sternal eversion method provides a more complete anatomical relief of the underlying cardiac compression. There is no destruction of bone or cartilage with the sternal eversion technique, and the chest wall plate therefore retains its contour without the use of posteriorly placed hardware. Furthermore, there is no risk of impingement or organ perforation by such hardware, and no requirement for future hardware removal and associated complications of a second operation.
After surgical correction of pectus excavatum with the sternal eversion technique, CMR demonstrates improvement in both anatomical chest wall contour and cardiovascular anatomy. All patients in this series had CMR-documented relief of RV and left atrial compression. Chest wall anatomy is markedly improved and symptoms are relieved in nearly all patients. We recommend CMR as the definitive modality for evaluation of patients with pectus excavatum. Importantly, this study shows that the primary underlying physiological abnormality in pectus excavatum is cardiac compression rather than respiratory restriction.
Conflict of interest: none declared.




