-
PDF
- Split View
-
Views
-
Cite
Cite
Inderpal S. Sarkaria, Nabil P. Rizk, David J. Finley, Manjit S. Bains, Prasad S. Adusumilli, James Huang, Valerie W. Rusch, Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 5, May 2013, Pages e107–e115, https://doi.org/10.1093/ejcts/ezt013
Close - Share Icon Share
Abstract
This study reports an early, single-institution experience of combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy (RAMIE) using a four-arm robotic platform, with special attention given to the pitfalls and complications during procedure development.
We conducted a prospective, single-cohort, observational study of patients undergoing RAMIE at a single institution.
A total of 21 patients (median age, 62 years [range, 37–83 years]) underwent RAMIE with a four-arm robotic platform (17 by Ivor Lewis and 4 by McKeown). Of the patients, 17 (81%) had a complete (R0) resection, and 16 (76%) received induction treatment, the majority (14/21 [67%]) with combined chemoradiation. The median operative time was 556 min (range, 395–807 min), which decreased to 414 min (range, 405–543 min) for the last 5 cases in the series. The median estimated blood loss was 307 cm3 (range, 200–500 cm3), and the median length of hospital stay was 10 days (range, 7–70 days). The median number of lymph nodes resected was 20 (range, 10–49). Five (24%) patients were converted to open procedures. Five patients (24%) had major complications. One (5%) died of complications on postoperative Day 70, and 3 (14%) had clinically significant anastomotic leaks (Grade II or greater, by Common Terminology Criteria for Adverse Events version 3.0). Three patients (14%) in this early experience developed airway fistulas.
While four-arm RAMIE may offer advantages over standard minimally invasive esophagectomy approaches, its adoption in a structured program, with critical evaluation of adverse events and subsequent adjustment of technique, is paramount to maximize patient safety, minimize complications and improve the conduct of operation early in the learning curve. Particular technical consideration should be given to prevention of airway complications.
INTRODUCTION
Minimally invasive esophagectomy (MIE) has become increasingly used and accepted, with reported outcomes compared with those of open approaches [1, 2]. The purported benefits include decreased postoperative pulmonary complications and pain and decreased length of hospital stay.
Robotic approaches to a number of surgical procedures have been increasingly investigated. Possible benefits of laparoscopic and robotic thoracoscopic procedures include steady and improved high-resolution optics under the direct control of the surgeon, articulating (wristed) instruments that allow for fine control during dissection and suturing, and the ability of the surgeon to self-assist with the additional fourth arm on currently available robotic platforms.
Robotic approaches for patients with benign oesophageal pathology, such as achalasia and paraesophageal hernias, have also increased [3, 4]. However, experience with robotic-assisted esophagectomy remains relatively limited, with a small number of reported series using variable hybrid approaches and with inconsistent reporting of the challenges and complications encountered during programmatic development [5–11].
In this study, we report the first experience of complete laparoscopic and thoracoscopic robotic-assisted esophagectomy using a four-arm robotic platform. We describe technical modifications made in response to specific challenges and complications encountered in both the intraoperative and the postoperative management of patients.
PATIENTS AND METHODS
Patient selection
Consecutive patients with oesophageal cancer who presented during the study period and were deemed to be surgical candidates were scheduled for robotic-assisted minimally invasive esophagectomy (RAMIE) using a combined thoracoscopic and laparoscopic approach. The first 6 cases represented consecutive patients presenting to 4 of 7 partners performing oesophageal resections. After patient 6, consecutive patients presenting to only 2 partners (I.S.S. and N.P.R.) were considered for robotic resections. All patients underwent preoperative staging and evaluation, including history and physical exam; upper-gastrointestinal endoscopy and biopsy; fluoroxyglucose-18 positron emission tomography; computed tomography of the chest, abdomen and pelvis and endoscopic ultrasound evaluation. For patients with gastroesophageal junction (GEJ) tumours suspected of significant gastric cardiac involvement (Siewert Type III tumour), laparoscopic staging was performed before the separately scheduled oesophagectomy. Patients deemed to require colon interposition were excluded. Patients with T3 tumours or greater and/or nodal involvement were referred for induction chemoradiation therapy. A waiver of informed consent for retrospective studies was granted by our institutional review board.
The initial 6 cases were performed primarily by an attending thoracic surgeon with advanced experience in MIE (I.S.S.), as well as by various assistants or cosurgeons (resident, fellow or attending), as available. With the intertwined goals of (i) maintaining a focussed experience with a uniform surgical team, (ii) optimizing conduct of the operation and (iii) thus maximizing patient safety during development of this procedure, procedures for all cases after Patient 6 were assisted or coperformed by a dedicated second thoracic attending surgeon with advanced experience in thoracoscopic surgery (N.P.R.) but not MIE. The majority of operations were assisted by nursing and anaesthesiology staff experienced in non-robotic minimally invasive oesophageal resections at our institution.
Data collection
Patient demographic characteristics and outcomes were prospectively collected as part of our institutional oesophageal database. Intraoperative data were obtained from the operative record. Data on complications were both prospectively and retrospectively collected as they occurred, or they were collected by chart review. Complications were graded in accordance with the Common Terminology Criteria for Adverse Events version 3.0 [12].
Operative technique: abdominal phase
Our technique for both Ivor Lewis and McKeown RAMIE was adapted from previously well described and standardized non-robotic MIE techniques [13, 14]. Only specific differences or modifications from these techniques are outlined below. We used a team approach, developed during the course of the series, that comprised 2 attending surgeons, with 1 attending surgeon at the robotic console (I.S.S.) and 1 at the bedside (N.P.R.). An additional assistant (fellow or resident) was seated at the second robotic console. All patients received epidural anaesthesia, in accordance with our standard service procedure.
Patient positioning and port placement
Patients are placed supine on the operating table, with their arms placed at 45° and with a footboard in place. Upper-gastrointestinal endoscopy and bronchoscopy are performed, as necessary, to evaluate the extent of the tumour. The operating table is turned 90°, to situate the robotic cart and arms (Da Vinci, Intuitive Surgical, Sunnyvale, CA, USA) directly over the patient. A reference point 1–2 cm above the xiphoid is marked in the midline, as a marker of the oesophageal hiatus, which all instrumentation must be able to reach. A midline 12-mm camera port is placed above the umbilicus, but no more than 23 cm from the reference point, which is the maximum reach of the current robotic camera. A distance of 9–10 cm is maintained between robotic ports, to minimize arm collisions. For patients with smaller body habitus that do not allow for adequate distance between ports, the camera port may be placed to the right of the midline as an alternative site. Peritoneal distention is accomplished with standard CO2 insufflation. A standard 10-mm, 30-degree laparoscope is used for the initial inspection of the peritoneal cavity and port placement. A left-lateral, subcostal 5-mm port is placed for use with the robotic atraumatic grasper, and a midclavicular 8-mm port is placed 13–15 cm from the reference point, in the left midabdomen. This port is for use with the ultrasonic shears (Harmonic scalpel, Ethicon, Cincinnati, OH, USA), which have a shorter length than other instruments used with the current robotic system. An additional right-lateral, subcostal 5-mm port, for placement of the liver retractor (MediFlex retractor), is placed, as well as a midclavicular, right-midabdominal 8-mm port, for use with the bipolar atraumatic grasper. A 12-mm port is placed between the camera and the right midclavicular ports and is used by the assistant for both suctioning and additional retraction. During later phases of the operation, this port is increased in size to 15 mm, allowing entry of endoscopic staplers during gastric tubularization. This port may also be used as an alternative robotic-camera entry site, to improve gastric visualization during mobilization of the greater curve and gastroepiploic vasculature.
The centre column of the robotic cart is brought over the midline of the patient, the patient is placed in the reverse Trendelenburg position, the robotic arms are docked to the ports and the robotic instruments are inserted. The port placement and the general configuration of the operating suite are shown in Fig. 1.
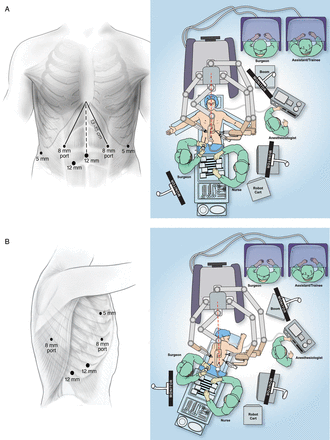
Abdominal phase room setup and port placement (A) and thoracic phase room setup and port placement (B).
Hiatal and retrogastric dissection
After initial hiatal dissection, the left gastric vascular pedicle is exposed from the lesser gastric curve by use of the robotic assistant arm to gently retract the stomach anteriorly. Additional retraction by the bedside assistant is useful to completely expose this area (Fig. 2A). After completion of nodal dissection and ligation of the vascular pedicle, gentle retraction of the stomach by use of the assistant arm allows for exposure and additional dissection of the left crus from the lesser gastric curve.
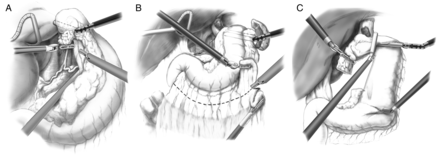
Retrogastric and left gastric vascular pedicle dissection (A), greater gastric curve dissection (B) and gastric conduit formation (C).
Gastric mobilization and tubularization
During short gastric and greater omental mobilization of the stomach, clear visualization of the gastroepiploic vessels is aided by gentle superior and medial retraction of the greater gastric curve, by use of the robotic assistant arm, from a retrogastric position (Fig. 2B). This also aids in visualizing and releasing retrogastric adhesions.
In preparation for gastric tubularization, the left-lateral robotic assistant arm retracts the gastric fundus towards the left upper quadrant with an additional robotic grasper (Cadierre forceps) providing gentle inferior traction on the antrum (Fig. 2C). During Ivor Lewis resection, the conduit and specimen are divided and reapproximated with a heavy suture to allow for properly oriented entry into the chest during the thoracoscopic phase of the operation. During McKeown resection, a standard chest tube is fixed to the divided specimen, which is delivered through the neck incision. The conduit is fixed to the distal end of the chest tube, which is used to gently bring the conduit to the neck, while under direct laparoscopic vision from the abdomen. Suturing is greatly aided by the articulating, wristed robotic instrumentation.
Pyloroplasty
If pyloroplasty is performed, the left-lateral assistant robotic arm is used to gently grasp the antrum of the stomach, with leftward retraction, to better visualize the pylorus. Haemostatic sutures are placed across the pyloric muscle and aid in retraction. The pylorus is opened across its width with the ultrasonic shears and closed transversely with robotic-assisted suturing (Fig. 3).
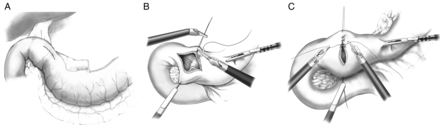
Feeding jejunostomy
Given the field-of-view limitations of the current robotic platform, a standard laparoscopic feeding jejunostomy is placed, using standard non-robotic laparoscopic techniques, at the end of the abdominal phase and after removal of the robotic platform.
Operative technique: thoracoscopic phase
Patient positioning and port placement
After the patient is positioned in the standard left-lateral decubitus position with flexion, a small stab incision is made below the scapular tip. An insufflation needle with a water-filled open syringe is placed into the chest, with water entry confirming safe intrapleural position. CO2 insufflation is instituted at a pressure of 8 mmHg. Port placement, operating layout and robotic cart trajectory over the right shoulder are depicted in Fig. 1. A standard laparoscopic 10-mm camera is placed into the obturator of the 12-mm camera port, which is introduced into the chest, under direct video guidance, in the eighth intercostal space, in the mid- to posterior axillary line. A 5-mm robotic port is placed in the third intercostal space, in the mid- to posterior axillary line, and an 8-mm robotic port is placed in the fifth intercostal space. An additional 8-mm port is placed laterally in approximately the eighth or ninth interspace. A 12-mm assistant port is placed, under direct vision, at the site of the diaphragmatic insertion. This port should lie midway between the camera and the lateral 8-mm robotic ports, to avoid collisions with the bedside assistant's arm. The robot is docked to the ports, and the robotic camera is introduced with a 30° down-facing orientation.
En bloc oesophageal mobilization
The 5-mm robotic assistant arm provides excellent retraction on the lung and oesophagus, largely obviating the need for bedside-assisted retraction, as described for previous techniques [5, 14]. En bloc oesophageal dissection is performed, taking all tissues superiorly along the pericardium from the level of the hiatus and inferior vena cava to the carina and lower trachea, posteriorly along the length of the thoracic aorta, and laterally along the pleural surfaces. The azygous vein is divided. During subcarinal lymph node dissection, to avoid thermal injury, the surgeon must take great care to maintain some distance between the dissection plane and the airway when using the ultrasonic shears. In the latter part of our experience, we routinely replaced the ultrasonic shears with the wristed, bipolar Maryland forceps for the subcarinal airway dissection, to avoid undue thermal contact with the airway. The bedside assistant's use of thoracoscopic suction to maintain a clear surgical field aids in this dissection, as does careful maintenance of haemostasis with the robotic bipolar fenestrated cautery forceps. The use of a Penrose drain around the oesophagus, held by the robotic assistant arm, may aid in retraction and exposure, but often this is not necessary. After mobilization for McKeown resection, the Penrose drain is left in the thoracic inlet and removed during the neck dissection. During posterior dissection, surgical clips placed through the assistant's port or by the robotic clip applier are used liberally, to seal lymphatic perforators.
The specimen and conduit are separated, and the conduit is reattached to the diaphragm, to prevent retraction into the abdomen during the remainder of the dissection, which, again, is simplified with robotic suturing. During deep dissection, the specimen is retracted laterally and superiorly with the atraumatic robotic grasper, allowing clear visualization along the contralateral pleura and left mainstem bronchus.
The oesophagus is divided above the azygos vein by use of the robotic endoshears. The posterior 8-mm robotic port is extended into a miniature access incision 4 cm in length, through which the specimen is removed.
Creation of anastomosis
Intrathoracic anastomotic creation is summarized in Fig. 4. The oesophageal orifice is held open with the aid of the robotic graspers, and the anvil of the end anastomosis stapler is inserted. A robotically sewn ‘baseball stitch’ purse-string suture is placed, as well as a superficial second purse-string for reinforcement.
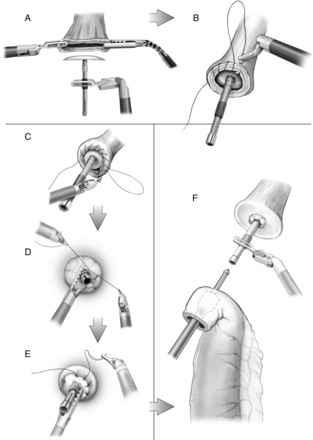
Placement of stapler anvil (A), purse-string sutures (B–E) and placement of end-to-end anastomotic stapler within gastric conduit (F).
The end-to-end anastomosis stapler is introduced through the access incision and placed into the conduit through a proximally created gastrotomy held open by the robotic graspers and the bedside assistant. The stapler spike is brought out just proximal to the vascular arcade, on the greater curve; the spike and anvil are married with the aid of the robotic graspers and the anastomosis is completed with firing of the stapler (Fig. 4). The nasogastric tube is advanced, under direct vision, and the redundant conduit is closed with an endo-gastrointestinal stapler. The procedure is completed with placement of a chest tube, as well as a Jackson-Pratt drain adjacent to the anastomosis posteriorly.
During McKeown resection, an end-to-side, single-layer, hand-sewn anastomosis is created in the neck. A Penrose drain is left adjacent to the anastomosis and brought through a separate skin incision.
RESULTS
Demographic characteristics
Demographic and perioperative data are summarized in Table 1. A total of 21 consecutive patients underwent RAMIE from 25 January 2011 to 1 November 2011: 76% were male (median age, 62 years), 86% had adenocarcinomas and 86% were centred at or above the GEJ (Siewert Type I or II). Seventeen patients (81%) underwent an Ivor Lewis operation, and the remainder underwent a three-hole (McKeown) approach. Sixteen patients underwent induction therapy, with 14 (67%) receiving combined neoadjuvant chemoradiation.
| Variable . | No. (%) . |
|---|---|
| Age, years, median (range) | 62 (37–83) |
| Male | 17 (81) |
| Histological result | |
| Adenocarcinoma | 18 (85) |
| Squamous cell carcinoma | 2 (10) |
| Other | 1 (5) |
| Siewert classification | |
| I | 7 (33) |
| II | 9 (43) |
| III | 2 (10) |
| N/A | 3 (14) |
| Ivor Lewis | 17 (81) |
| Overall stage, TN stage | |
| Stage 0 | 8 (38) |
| Stage I | |
| T1aN0 (IA) | 2 (10) |
| T1bN0 (IA) | 3 (14) |
| Stage IIA | |
| T2N0 | 1 (5) |
| Stage IIB | |
| T3N0 | 2 (10) |
| T1bN1 | 2 (10) |
| Stage IIIA | |
| T3N1 | 2 (10) |
| N/A (GIST) | 1 (5) |
| Induction therapy | 16 (76) |
| Chemotherapy only | 2 (10) |
| Chemotherapy and radiation | 14 (67) |
| Lymph nodes resected, median (range) | 20 (10–49) |
| Extent of resection | |
| R0 | 17 (81) |
| R1 | 4 (19) |
| Length of stay, days, median (range) | 10 (7–70) |
| Variable . | No. (%) . |
|---|---|
| Age, years, median (range) | 62 (37–83) |
| Male | 17 (81) |
| Histological result | |
| Adenocarcinoma | 18 (85) |
| Squamous cell carcinoma | 2 (10) |
| Other | 1 (5) |
| Siewert classification | |
| I | 7 (33) |
| II | 9 (43) |
| III | 2 (10) |
| N/A | 3 (14) |
| Ivor Lewis | 17 (81) |
| Overall stage, TN stage | |
| Stage 0 | 8 (38) |
| Stage I | |
| T1aN0 (IA) | 2 (10) |
| T1bN0 (IA) | 3 (14) |
| Stage IIA | |
| T2N0 | 1 (5) |
| Stage IIB | |
| T3N0 | 2 (10) |
| T1bN1 | 2 (10) |
| Stage IIIA | |
| T3N1 | 2 (10) |
| N/A (GIST) | 1 (5) |
| Induction therapy | 16 (76) |
| Chemotherapy only | 2 (10) |
| Chemotherapy and radiation | 14 (67) |
| Lymph nodes resected, median (range) | 20 (10–49) |
| Extent of resection | |
| R0 | 17 (81) |
| R1 | 4 (19) |
| Length of stay, days, median (range) | 10 (7–70) |
GIST: gastrointestinal stromal tumour; R0: gross and microscopic margins negative; R1: gross margin negative: microscopic margin positive; Siewert I: tumour centred above GEJ; Siewert II: tumour centred at GEJ; Siewert III: tumour centred below GEJ.
| Variable . | No. (%) . |
|---|---|
| Age, years, median (range) | 62 (37–83) |
| Male | 17 (81) |
| Histological result | |
| Adenocarcinoma | 18 (85) |
| Squamous cell carcinoma | 2 (10) |
| Other | 1 (5) |
| Siewert classification | |
| I | 7 (33) |
| II | 9 (43) |
| III | 2 (10) |
| N/A | 3 (14) |
| Ivor Lewis | 17 (81) |
| Overall stage, TN stage | |
| Stage 0 | 8 (38) |
| Stage I | |
| T1aN0 (IA) | 2 (10) |
| T1bN0 (IA) | 3 (14) |
| Stage IIA | |
| T2N0 | 1 (5) |
| Stage IIB | |
| T3N0 | 2 (10) |
| T1bN1 | 2 (10) |
| Stage IIIA | |
| T3N1 | 2 (10) |
| N/A (GIST) | 1 (5) |
| Induction therapy | 16 (76) |
| Chemotherapy only | 2 (10) |
| Chemotherapy and radiation | 14 (67) |
| Lymph nodes resected, median (range) | 20 (10–49) |
| Extent of resection | |
| R0 | 17 (81) |
| R1 | 4 (19) |
| Length of stay, days, median (range) | 10 (7–70) |
| Variable . | No. (%) . |
|---|---|
| Age, years, median (range) | 62 (37–83) |
| Male | 17 (81) |
| Histological result | |
| Adenocarcinoma | 18 (85) |
| Squamous cell carcinoma | 2 (10) |
| Other | 1 (5) |
| Siewert classification | |
| I | 7 (33) |
| II | 9 (43) |
| III | 2 (10) |
| N/A | 3 (14) |
| Ivor Lewis | 17 (81) |
| Overall stage, TN stage | |
| Stage 0 | 8 (38) |
| Stage I | |
| T1aN0 (IA) | 2 (10) |
| T1bN0 (IA) | 3 (14) |
| Stage IIA | |
| T2N0 | 1 (5) |
| Stage IIB | |
| T3N0 | 2 (10) |
| T1bN1 | 2 (10) |
| Stage IIIA | |
| T3N1 | 2 (10) |
| N/A (GIST) | 1 (5) |
| Induction therapy | 16 (76) |
| Chemotherapy only | 2 (10) |
| Chemotherapy and radiation | 14 (67) |
| Lymph nodes resected, median (range) | 20 (10–49) |
| Extent of resection | |
| R0 | 17 (81) |
| R1 | 4 (19) |
| Length of stay, days, median (range) | 10 (7–70) |
GIST: gastrointestinal stromal tumour; R0: gross and microscopic margins negative; R1: gross margin negative: microscopic margin positive; Siewert I: tumour centred above GEJ; Siewert II: tumour centred at GEJ; Siewert III: tumour centred below GEJ.
Perioperative outcomes
The median operative time (from incision to closure of wounds, inclusive of docking and repositioning time) for the entire cohort was 556 min (range, 395–626 min), which decreased to a median of 414 min (range, 405–543 min) for the last 5 cases. Seventeen patients (81%) had complete macroscopic and microscopic (R0) resection. The median blood loss was 300 cm3, and the median length of hospital stay was 10 days (range, 7–70 days).
Conversions
Ten of 21 patients (48%) were converted to either open or non-robotic laparoscopic and thoracoscopic approaches. Conversion to open surgery from minimally invasive approaches occurred for 5 of 21 patients (24%), with no conversions to open surgery among the last 5 patients. Reasons for conversion, either to open surgery or from RAMIE to MIE, included excessive operative time, unclear visualization of the greater gastric curve and/or gastroepiploic arcade, questionable anastomotic integrity, dense adhesions, positive proximal cancer margin by frozen-section analysis and robotic console failure.
Complications
Data on complications are reported in Table 2. Five of 21 patients (24%) had Grade III or greater complications. There was 1 postoperative death, on postoperative Day 70 (a patient who died of respiratory failure secondary to anastomotic leak and tracheobronchial fistula). The overall rate of Grade II or greater anastomotic leak was 14% (3 of 21).
Patient complications by Common Terminology Criteria for Adverse Events version 3.0
| Grade, complication . | No. . |
|---|---|
| Grade I | |
| Anastomotic leak | 4 |
| UTI | 1 |
| Delayed gastric emptying | 1 |
| Grade II | |
| Anastomotic leak | 1 |
| Atrial fibrillation | 1 |
| Pneumonitis | 1 |
| Anastomotic stricture | 1 |
| Wound infection | 1 |
| Empyema | 1 |
| Grade III | |
| TE fistula | 1 |
| Anastomotic leak/TE fistula | 1 |
| Respiratory failure | 1 |
| Anastomotic leak | 1 |
| Recurrent laryngeal nerve palsy | 1 |
| Grade IV | |
| Pulmonary embolus | 2 |
| Grade V | |
| Respiratory failure | 1 |
| Grade, complication . | No. . |
|---|---|
| Grade I | |
| Anastomotic leak | 4 |
| UTI | 1 |
| Delayed gastric emptying | 1 |
| Grade II | |
| Anastomotic leak | 1 |
| Atrial fibrillation | 1 |
| Pneumonitis | 1 |
| Anastomotic stricture | 1 |
| Wound infection | 1 |
| Empyema | 1 |
| Grade III | |
| TE fistula | 1 |
| Anastomotic leak/TE fistula | 1 |
| Respiratory failure | 1 |
| Anastomotic leak | 1 |
| Recurrent laryngeal nerve palsy | 1 |
| Grade IV | |
| Pulmonary embolus | 2 |
| Grade V | |
| Respiratory failure | 1 |
TE: tracheo-oesophageal; UTI: urinary tract infection.
Patient complications by Common Terminology Criteria for Adverse Events version 3.0
| Grade, complication . | No. . |
|---|---|
| Grade I | |
| Anastomotic leak | 4 |
| UTI | 1 |
| Delayed gastric emptying | 1 |
| Grade II | |
| Anastomotic leak | 1 |
| Atrial fibrillation | 1 |
| Pneumonitis | 1 |
| Anastomotic stricture | 1 |
| Wound infection | 1 |
| Empyema | 1 |
| Grade III | |
| TE fistula | 1 |
| Anastomotic leak/TE fistula | 1 |
| Respiratory failure | 1 |
| Anastomotic leak | 1 |
| Recurrent laryngeal nerve palsy | 1 |
| Grade IV | |
| Pulmonary embolus | 2 |
| Grade V | |
| Respiratory failure | 1 |
| Grade, complication . | No. . |
|---|---|
| Grade I | |
| Anastomotic leak | 4 |
| UTI | 1 |
| Delayed gastric emptying | 1 |
| Grade II | |
| Anastomotic leak | 1 |
| Atrial fibrillation | 1 |
| Pneumonitis | 1 |
| Anastomotic stricture | 1 |
| Wound infection | 1 |
| Empyema | 1 |
| Grade III | |
| TE fistula | 1 |
| Anastomotic leak/TE fistula | 1 |
| Respiratory failure | 1 |
| Anastomotic leak | 1 |
| Recurrent laryngeal nerve palsy | 1 |
| Grade IV | |
| Pulmonary embolus | 2 |
| Grade V | |
| Respiratory failure | 1 |
TE: tracheo-oesophageal; UTI: urinary tract infection.
DISCUSSION
MIE has become increasingly performed and reported [1, 2]. To a lesser extent, reported series of hybrid RAMIE approaches using three-arm robotic platforms, generally thoracoscopic in nature, have also increased. These studies have cited advantages over standard thoracoscopy, including improved optics, camera stability and operator-dependent control, as well as greatly improved articulated instrumentation [5, 6, 8, 11].
To our knowledge, only one other published report of complete RAMIE using a combined thoracoscopic and laparoscopic approach exists. Kernstine et al. [5] reported on 6 patients undergoing hybrid procedures and 8 undergoing complete robotic resection with a three-hole approach, by use of a three-arm robotic platform. The thoracic portions were performed with the patients in near-prone position. Among the patients undergoing complete robotic procedures, there was one nonemergent conversion to thoracotomy. Intraoperative airway injury during the chest dissection, related to the ultrasonic dissecting device, occurred in 1 patient and was repaired robotically. The median operating-room time was 11.2 h (range, 9.5–13.0 h). The incidence of major morbidity was 29%, including anastomotic leak (14%), thoracic duct leak (7%) and unilateral and bilateral vocal cord paralyses (14%). There was 1 death at 90 days postoperation (7%; a patient with persistent aspiration pneumonia).
The present study represents the first reported series of combined thoracoscopic and laparoscopic RAMIE using a four-arm robotic platform. The procedure was adapted and developed from a previously described method of transthoracic MIE without prone positioning [13, 14]. Although prone positioning may offer some advantages in lung retraction and dependent clearance of blood from the surgical field, we have not found it to be necessary. Lateral decubitus positioning of the patient is more ‘natural’ and readily adaptable to the majority of thoracic surgeons and anaesthesiologists, and it may minimize difficulties in converting to open procedures when necessary. Furthermore, we have found that the addition of the fourth robotic arm aids in lung retraction, and we have not had significant issues with visualization and exposure secondary to pooling of blood.
The extent of lymphadenectomy and the rate of complete resection are compared with those in other series, for both open surgery and MIE, including our own previous experience [15–18]. Positive microscopic margins (R1) were identified in 4 patients. This included a radial margin in 1 patient who did not undergo preoperative radiation. One patient undergoing Ivor Lewis resection had a positive proximal margin despite an anastomosis just below the thoracic inlet. The final margin from subsequent colon interposition identified no residual tumour in the residual oesophagus. One patient with diffuse proximal squamous carcinoma, after lye ingestion as a child, had a possible viable tumour in the proximal margin after induction chemoradiation. A close endoscopic and radiographic follow-up identified no recurrent tumour at the last follow-up. One patient had a positive gastric margin, although no gross tumour was identified in the stomach preoperatively.
We report a similar overall incidence of major morbidity and mortality, including rates of anastomotic leak, as those in other series, for both open and minimally invasive surgery [15]. Of concern, three upper-gastrointestinal airway fistulas were identified in this early experience. There were no deaths at 30 days postoperation. One patient died of respiratory failure on postoperative Day 70, after developing an anastomotic leak and tracheo-oesophageal fistula on postoperative Day 8. While the patient's fistula was successfully repaired, the patient was unable to recover from the respiratory complications. Operative times decreased significantly during the series, although it is too early to determine whether a plateau was reached.
The presence of a fourth arm allowed additional options for directly controlled self-assistance, retraction and exposure, while simultaneously maintaining use of the primary ‘working’ arms, obviating the need to instruct a bedside assistant and improving the pace and conduct of the operation. In both the abdomen and the chest, retraction provided by the fourth arm eliminated the need for an additional (second) bedside assistant, which is required for lung, oesophageal, pyloric and gastric retraction during standard non-robotic MIE, as described elsewhere [13]. The addition of the dual console allowed for active participation by a second surgeon assistant and facilitated safe and structured teaching of surgical trainees, who performed discrete portions of the operations under direct and closely monitored supervision.
Specific complications, pitfalls and technical considerations
Tracheobronchial fistula
Two patients developed tracheobronchial fistulae secondary to anastomotic leak (1 on Day 7 and 1 at 3 months postoperation), and 1 developed gastrobronchial fistula from the conduit to the left mainstem bronchus, complications not seen in our non-robotic MIE experience. It is possible that thermal injury to the conduit during mobilization in the abdomen and/or to the airway during oesophageal mobilization in the chest, potentially exacerbated by the highly magnified surgical field and lack of tactile feedback to assess pressure on these structures, may contribute to this complication. This must be considered and actively corrected for by the surgeon early in the learning curve, when using devices with lateral heat spread and residual heat retention, such as ultrasonic shears. Great attention must be paid, in particular during dissection around the airway, where even avoiding undue contact (either direct or peripheral) with the instrument, as well as routine ‘dampening’ of the device on neutral tissues to disperse heat energy, may still carry significant risk of injury due to thermal damage not immediately apparent at the time of surgery. We caution against the use of such devices during these portions of the dissection and suggest the use of alternative, ‘wristed’ energy sources with minimal thermal spread, such as the robotic bipolar Maryland dissector, to attenuate the risk of injury during dissection along the mainstem bronchi and carina.
Questionable anastomotic integrity
Two patients were converted to open thoracotomy, to better evaluate and reinforce the anastomosis, after observation of muscular tears in the oesophageal rings following stapler fire. We believe this likely resulted from the placement of the purse-string sutures too far proximal on the distal oesophagus, resulting in excessive ‘bunching’ of tissue around the spike of the anvil. Undue bulk may not allow satisfactory tissue entry into the stapler housing of standard end-to-end anastomosis staplers. We have corrected our technique to place all stitches within 2–3 mm of the oesophageal edge, to avoid an excess of tissue around the anvil spike.
Greater gastric curve visualization
The most common cause of conversion in our series was inadequate visualization of the greater curve anatomy, specifically the gastroepiploic arcade. Visualization may be increased by gentle retraction on the posterior aspect of the stomach by use of the assistant robotic arm to lift the vascular arcade further into view (Fig. 2). Additionally, the robotic-camera port may be moved to the more inferior lateral assistant port, thus allowing for a broader operative view.
In summary, complete thoracoscopic and laparoscopic RAMIE is feasible, and we have found that the improved optics, camera stability and sophisticated instrument articulation provide the greatest advantages between the robotic and the standard minimally invasive approaches. The addition of a fourth arm to current robotic platforms has significantly improved the conduct of operation, and the presence of an additional console aids in surgical assistance, as well as in supervised, mentored instruction. Conversely, limitations of the robotic platform include limited arm manoeuverability, collisions and a more limited surgical field of view, compared with those in standard thoracoscopy and laparoscopy. While these limitations can be attenuated, to some degree, by experience and familiarity with the robotic platform, including systematic placement of the robotic arms, there is significant room for improvement with future iterations of robotic-assisted platforms, including development of safer energy-based dissection technologies. Although the lack of haptic feedback has been cited as a limitation of robotic approaches, we have not found this to be a significant drawback for this operation.
Further study is necessary to better evaluate patient outcomes, with specific attention given to airway complications. While it is unclear whether the airway complications reported here are specific to the robotic approach, we have not witnessed these particular events in our standard MIE. Although experience with robotic esophagectomy is increasing, most studies contain insufficient reporting of complications and of the technical aspects of procedure development. Although this new technology offers possible advantages, its introduction into thoracic procedures should be vetted in careful and controlled environments, as is the case with any new technology. Careful and critical routine evaluation of surgical complications and pitfalls has proven valuable during the development of this procedure and the refinement of the technique. This algorithm may be particularly important in the development of new and emerging technologies applied to conventional operations with established standards of care and outcomes. While innovation is essential to the progress of modern surgery, especially in the age of rapidly increasing proliferation of sophisticated technologies, it is the burden and responsibility of the surgeon to introduce and evaluate these technologies in a structured and rigorous manner, to maintain equipoise and to minimize the risk to patients [19].
Conflict of interest: none declared.
REFERENCES
Author notes
Presented at the Society of Thoracic Surgeons 48th Annual Meeting, Ft. Lauderdale, FL, USA, 28 January–1 February 2012.




