-
PDF
- Split View
-
Views
-
Cite
Cite
Song-Wei Yue, Hua Guo, Yong-Gao Zhang, Jian-Bo Gao, Xiu-Xian Ma, Peng-Xu Ding, The clinical value of computer tomographic angiography for the diagnosis and therapeutic planning of patients with pulmonary sequestration, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 5, May 2013, Pages 946–951, https://doi.org/10.1093/ejcts/ezs484
Close - Share Icon Share
Abstract
This study was conducted to evaluate the clinical value of computed tomographic (CT) angiography for diagnosis and therapeutic planning in patients with pulmonary sequestration.
Forty-three patients with suspected pulmonary sequestration underwent CT angiography before undergoing digital subtraction angiography or surgery. For each patient, CT angiography was used to determine whether the pulmonary sequestration was suitable for coil embolization, surgical resection or conservative treatment. The treatments planned using CT angiography were compared with actual treatment decisions made or treatments administered using digital subtraction angiography or surgery.
Digital subtraction angiography and/or surgery confirmed pulmonary sequestration in 37 patients; six patients had no pulmonary sequestration. The diagnostic performance of CT angiography for pulmonary sequestration in the patient-based evaluation yielded an accuracy of 97.7%, sensitivity of 97.3%, specificity of 100%, positive predictive value (PPV) of 100% and negative predictive value (NPV) of 85.7%. The aberrant systemic artery-based evaluation yielded an accuracy of 98.0%, sensitivity of 97.8%, specificity of 100%, PPV of 100% and NPV of 85.7%. Treatments could be correctly planned using CT angiography with 100% accuracy, sensitivity, specificity, PPV and NPV according to the aneurysm-based evaluation.
We have obtained promising results with a CT angiography-based protocol, rather than a digital subtraction angiography-based protocol, as the only diagnostic and pretreatment planning tool in patients with pulmonary sequestration. The CT angiography-based selection of treatment strategies seems to be safe and effective in the majority of patients with pulmonary sequestration.
INTRODUCTION
Pulmonary sequestration is a rare congenital disorder that is characterized by an area of dysplastic and non-functioning pulmonary tissue that lacks a normal communication with the tracheobronchial tree and has an anomalous systemic blood supply [1]. Digital subtraction angiography is considered to be the gold standard for the diagnosis of pulmonary sequestration [2]. Digital subtraction angiography can clearly identify the aberrant arterial supply of the sequestered lung tissue, but is an invasive, radiation-associated, time-consuming and relatively expensive procedure that requires hospitalization. In recent years, less-invasive imaging techniques have proved to be equally effective and safer alternatives to digital subtraction angiography, including computed tomographic (CT) angiography and magnetic resonance imaging (MRI) [3–7].
Although MRI can reveal the cystic nature of many intralobar sequestrations as well as their variable solid, fluid, hemorrhagic and mucus-containing components, it cannot accurately evaluate lung abnormalities, mainly because of artefacts caused by respiratory motion [4, 5, 8, 9]. In the current clinical practice, CT angiography is the most frequently used noninvasive diagnostic tool for the detection of pulmonary sequestration, because it is faster, has a superior spatial resolution and can yield the most information about the bronchial anatomy and the pulmonary parenchymal lesion [3, 9–14]. Nevertheless, few studies have confirmed whether CT angiography can be used as the sole diagnostic and preoperative planning procedure for the endovascular or surgical treatment of pulmonary sequestration. Therefore, the purpose of the current study was to evaluate the clinical value of spiral CT angiography for the diagnosis and therapeutic planning of pulmonary sequestration.
MATERIALS AND METHODS
Study design
The institutional review board approved the study protocol, and patients or qualifying family members provided informed consent before participation. From May 2008 to December 2011, 43 consecutive patients with suspected pulmonary sequestration underwent CT angiography before undergoing coil embolization, surgical resection or conservative treatment. Digital subtraction angiography or surgery after CT angiography was used to confirm the diagnosis of pulmonary sequestration.
Image acquisition
Computed tomographic angiography
All CT angiography examinations were performed on a 64-row CT scanner (LightSpeed VCT or Discovery CT750 HD, GE Healthcare). The smart preset scan technique was used for enhanced CT angiography with the following parameters: 0.625 mm × 64 slice, 120 kVp, 100–600 mA, 1.375:1 helical pitch and 25.6 s acquisition time. For enhanced CT angiography, a standard dose of 0.7 ml/kg body weight of contrast material in 40 ml of normal saline was administered using a power injector at a rate of 4 ml/s through a 22-gauge needle into the antecubital vein. When the concentration of a contrast medium in the ascending aorta reached 100 HU, the CT device automatically scanned the area from the apex of the lung to the level of the upper abdomen. The acquired image data sets were then transferred to a workstation (GE AW4.3 or GE AW4.4, GE Medical), where three-dimensional (3D) image reconstruction was performed with a 732 × 732 matrix. The reconstruction included the development of oblique, coronal and sagittal maximum intensity projections, multiplanar reconstruction and 3D volume-rendered images of the airway and thoracic vascular structures.
Each patient was assessed for pulmonary sequestration after a collateral artery originating from the aorta was identified in the CT angiography scans. The site of origin, distribution and course of the sequestered tissue were evaluated using multiplanar reconstructions, maximum intensity projections and volume-rendered images by adjusting the value of translucency or slab thickness. The diameter of the sequestered tissue was measured on multiplanar reconstructions. The pulmonary arteries and veins of the affected lung were also evaluated. Each segmental bronchus of the involved lung was reconstructed using multiplanar reconstructions and maximum intensity projections. The number and the course of vascular branches and the lung volume were also evaluated.
After diagnostic CT angiography, an appropriate treatment strategy was selected from coil embolization, surgical resection and conservative treatment, by the attending interventional radiologists and surgeon in consensus. In this study, there were four categories of treatment options: (i) coil embolization; (ii) surgery; (iii) unsuccessful attempt to place coils or perform surgical clipping, with no further therapy and (iv) conservative treatment.
Digital subtraction angiography
Digital subtraction angiography was performed by interventional radiologists after CT angiography examinations. Conventional 2D digital subtraction angiography was performed on a monoplanar unit (Axiom Artis VB22N, Siemens) with a 1024 × 1024 matrix and a 17 cm × 20 cm field of view. The access routes for angiography were the umbilical artery (neonates) and the femoral artery. After doing a complete haemodynamic evaluation, an initial aortogram was obtained to show the arterial map of the aberrant systemic arteries supplying the sequestered region. The abnormal vessels were then selectively catheterized with a 4- or 5-Fr catheter. Angiography was performed to view the size, location and venous drainage of the sequestration. The feeding artery was embolized with a stainless steel coil (Tornado Embolization Microcoil, Cook Medical). Angiography was repeated at the end of the procedure to check whether the embolization was successful. The size of the coil depended on the size of the aberrant artery. Two observers, who were blinded to all clinical and previous imaging results, identified and analyzed all sequestrations together.
Image review
Three observers were blinded to all clinical, digital subtraction angiography and surgery results. They independently analyzed all CT angiography data sets on an offline workstation from multiple, on-screen viewing angles. The source images, multiplanar reconstructions, maximum intensity projections and volume-rendered images were presented on-screen, thus allowing for adjustment of the appropriate threshold of the window width and level. In the presence of interobserver discrepancies in the detection of pulmonary sequestration, a consensus or a majority decision was obtained.
Treatment planning with computed tomographic angiography
The three observers were asked to use the CT angiography data sets to recommend endovascular treatment with coil embolization, surgical resection or conservative treatment. The CT angiography-based selection criteria for the treatment methods are shown in Table 1.
In our institution, endovascular therapy with coils is considered the first-line treatment for pulmonary sequestration. This treatment is considered feasible only when the aberrant systemic artery can be clearly seen on CT angiography and the age of the patient is <1 week or >3 months [15].
Surgical resection is considered when pulmonary sequestration has been confirmed on CT angiography and the age of the patients is between 1 week and 3 months or the patient has associated congenital heart disease, such as severe mitral valve regurgitation and valve prolapse, partial anomaly of pulmonary venous return and atrial septal defect.
Conservative treatment is considered when the patient has no pulmonary sequestration on CT angiography, the pulmonary sequestration is difficult to treat with endovascular therapy or surgical clipping, or when such treatment is unsuccessful.
CTA-based selection criteria for treatment methods in patients with pulmonary sequestration
| Treatment methods . | Selection criteria . |
|---|---|
| Endovascular treatment | Pulmonary sequestration and patient age <1 week or >3 months |
| Patients' wish to be treated with endovascular treatment | |
| Clear depiction of an aberrant systemic artery on CTA | |
| Refusal to undergo surgery | |
| Surgical clipping | Pulmonary sequestration and patient age between 1 week and 3 months |
| Associated congenital heart disease | |
| Refusal to undergo endovascular treatment | |
| Conservative treatment | No pulmonary sequestration |
| Difficult or unsuccessful endovascular treatment and/or surgical resection |
| Treatment methods . | Selection criteria . |
|---|---|
| Endovascular treatment | Pulmonary sequestration and patient age <1 week or >3 months |
| Patients' wish to be treated with endovascular treatment | |
| Clear depiction of an aberrant systemic artery on CTA | |
| Refusal to undergo surgery | |
| Surgical clipping | Pulmonary sequestration and patient age between 1 week and 3 months |
| Associated congenital heart disease | |
| Refusal to undergo endovascular treatment | |
| Conservative treatment | No pulmonary sequestration |
| Difficult or unsuccessful endovascular treatment and/or surgical resection |
CTA: computed tomographic angiography.
CTA-based selection criteria for treatment methods in patients with pulmonary sequestration
| Treatment methods . | Selection criteria . |
|---|---|
| Endovascular treatment | Pulmonary sequestration and patient age <1 week or >3 months |
| Patients' wish to be treated with endovascular treatment | |
| Clear depiction of an aberrant systemic artery on CTA | |
| Refusal to undergo surgery | |
| Surgical clipping | Pulmonary sequestration and patient age between 1 week and 3 months |
| Associated congenital heart disease | |
| Refusal to undergo endovascular treatment | |
| Conservative treatment | No pulmonary sequestration |
| Difficult or unsuccessful endovascular treatment and/or surgical resection |
| Treatment methods . | Selection criteria . |
|---|---|
| Endovascular treatment | Pulmonary sequestration and patient age <1 week or >3 months |
| Patients' wish to be treated with endovascular treatment | |
| Clear depiction of an aberrant systemic artery on CTA | |
| Refusal to undergo surgery | |
| Surgical clipping | Pulmonary sequestration and patient age between 1 week and 3 months |
| Associated congenital heart disease | |
| Refusal to undergo endovascular treatment | |
| Conservative treatment | No pulmonary sequestration |
| Difficult or unsuccessful endovascular treatment and/or surgical resection |
CTA: computed tomographic angiography.
Statistical analyses
Categorical variables (demographic and basic characteristics) were expressed as numbers and percentages and were compared using the χ2 test. Continuous variables were expressed as mean ± standard deviation and compared using an unpaired t-test, if normally distributed. Descriptive statistics were assessed on three levels: patient by patient (no pulmonary sequestration or pulmonary sequestration per patient) and artery by artery. The diagnostic performance parameters of CT angiography compared with digital subtraction angiography in the detection of pulmonary sequestration [i.e. accuracy, sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV)] were expressed as percentages.
Good correlation with the proposed treatment protocol was defined as agreement between the treatment recommended by the radiologists or surgeons on the basis of CT angiography findings and the treatment actually administered by the attending radiologists or surgeon on the basis of CT angiography findings. Deviation from the treatment protocol was defined as a difference between the treatment recommended on the basis of CT angiography examination and the treatment actually administered on the basis of digital subtraction angiography findings. On the basis of this dichotomization, accuracy, sensitivity, specificity, PPV and NPV were calculated. Interobserver reliability with percentages of agreement between the observer evaluations of the CT angiography images was calculated with kappa (κ) statistics. Statistical analyses were performed using SPSS (version 13.0; SPSS Inc., Chicago, IL, USA).
RESULTS
Patient population
A total of 43 consecutive patients were enrolled in the study, including 26 male patients and 17 female patients, with a mean age of 4.73 ± 9.15 years (range: 1 day to 45 years). The basic characteristics of the patients are summarized in Table 2.
| Characteristic . | All patients (n = 43) . | PS (n = 37) . | Non-PS (n = 6) . | P-value . |
|---|---|---|---|---|
| Age (years) | 4.73 ± 9.15 | 3.69 ± 8.74 | 11.16 ± 9.95 | 0.063 |
| Male sex [n (%)] | 26 (48.2) | 23 (41.5) | 3 (1.8) | 0.001 |
| Laterality [n (%)] | ||||
| Left lower lobe | 27 (31.2) | 24 (39.0) | 3 (15.5) | <0.001 |
| Right lower lobe | 16 (13.0) | 13 (39.0) | 3 (39.0) | — |
| Classification [n (%)] | — | — | — | — |
| Intralobar | — | 22 (4.2) | — | — |
| Extralobar | — | 15 (4.2) | — | — |
| Aberrant systemic artery [n (%)] | — | — | — | — |
| Descending aorta | — | 27 | — | — |
| Abdominal aorta | — | 10 | — | — |
| Single artery | — | 31 | — | — |
| Multiple artery | — | 6 | — | — |
| Draining vein [n (%)] | — | — | — | |
| Pulmonary vein | — | 25 | — | — |
| Azygos vein | — | 9 | — | — |
| Hemiazygos vein | — | 1 | — | — |
| Portal vein | — | 2 | — | — |
| Characteristic . | All patients (n = 43) . | PS (n = 37) . | Non-PS (n = 6) . | P-value . |
|---|---|---|---|---|
| Age (years) | 4.73 ± 9.15 | 3.69 ± 8.74 | 11.16 ± 9.95 | 0.063 |
| Male sex [n (%)] | 26 (48.2) | 23 (41.5) | 3 (1.8) | 0.001 |
| Laterality [n (%)] | ||||
| Left lower lobe | 27 (31.2) | 24 (39.0) | 3 (15.5) | <0.001 |
| Right lower lobe | 16 (13.0) | 13 (39.0) | 3 (39.0) | — |
| Classification [n (%)] | — | — | — | — |
| Intralobar | — | 22 (4.2) | — | — |
| Extralobar | — | 15 (4.2) | — | — |
| Aberrant systemic artery [n (%)] | — | — | — | — |
| Descending aorta | — | 27 | — | — |
| Abdominal aorta | — | 10 | — | — |
| Single artery | — | 31 | — | — |
| Multiple artery | — | 6 | — | — |
| Draining vein [n (%)] | — | — | — | |
| Pulmonary vein | — | 25 | — | — |
| Azygos vein | — | 9 | — | — |
| Hemiazygos vein | — | 1 | — | — |
| Portal vein | — | 2 | — | — |
PS: pulmonary sequestration.
| Characteristic . | All patients (n = 43) . | PS (n = 37) . | Non-PS (n = 6) . | P-value . |
|---|---|---|---|---|
| Age (years) | 4.73 ± 9.15 | 3.69 ± 8.74 | 11.16 ± 9.95 | 0.063 |
| Male sex [n (%)] | 26 (48.2) | 23 (41.5) | 3 (1.8) | 0.001 |
| Laterality [n (%)] | ||||
| Left lower lobe | 27 (31.2) | 24 (39.0) | 3 (15.5) | <0.001 |
| Right lower lobe | 16 (13.0) | 13 (39.0) | 3 (39.0) | — |
| Classification [n (%)] | — | — | — | — |
| Intralobar | — | 22 (4.2) | — | — |
| Extralobar | — | 15 (4.2) | — | — |
| Aberrant systemic artery [n (%)] | — | — | — | — |
| Descending aorta | — | 27 | — | — |
| Abdominal aorta | — | 10 | — | — |
| Single artery | — | 31 | — | — |
| Multiple artery | — | 6 | — | — |
| Draining vein [n (%)] | — | — | — | |
| Pulmonary vein | — | 25 | — | — |
| Azygos vein | — | 9 | — | — |
| Hemiazygos vein | — | 1 | — | — |
| Portal vein | — | 2 | — | — |
| Characteristic . | All patients (n = 43) . | PS (n = 37) . | Non-PS (n = 6) . | P-value . |
|---|---|---|---|---|
| Age (years) | 4.73 ± 9.15 | 3.69 ± 8.74 | 11.16 ± 9.95 | 0.063 |
| Male sex [n (%)] | 26 (48.2) | 23 (41.5) | 3 (1.8) | 0.001 |
| Laterality [n (%)] | ||||
| Left lower lobe | 27 (31.2) | 24 (39.0) | 3 (15.5) | <0.001 |
| Right lower lobe | 16 (13.0) | 13 (39.0) | 3 (39.0) | — |
| Classification [n (%)] | — | — | — | — |
| Intralobar | — | 22 (4.2) | — | — |
| Extralobar | — | 15 (4.2) | — | — |
| Aberrant systemic artery [n (%)] | — | — | — | — |
| Descending aorta | — | 27 | — | — |
| Abdominal aorta | — | 10 | — | — |
| Single artery | — | 31 | — | — |
| Multiple artery | — | 6 | — | — |
| Draining vein [n (%)] | — | — | — | |
| Pulmonary vein | — | 25 | — | — |
| Azygos vein | — | 9 | — | — |
| Hemiazygos vein | — | 1 | — | — |
| Portal vein | — | 2 | — | — |
PS: pulmonary sequestration.
Results of digital subtraction angiography and surgery
Suspected pulmonary sequestration observed on CT angiography was confirmed using digital subtraction angiography or surgery in 37 patients; the remaining six patients had no demonstrable pulmonary sequestration. Digital subtraction angiography or surgery revealed 45 aberrant systemic arteries in the 37 confirmed pulmonary sequestration patients. Two patients had three aberrant systemic arteries each, whereas four patients had two aberrant arteries each; a single aberrant artery was detected in the remaining 31 patients.
Diagnostic performance of computed tomographic angiography
The diagnostic accuracy, sensitivity, specificity, PPV and NPV of CT angiography for patient- and artery-based evaluations are detailed in Table 3. Sample CT angiography and digital subtraction angiography images from a single patient are shown in Fig. 1. Of the 43 sequestrations observed on CT angiography, 37 were confirmed on digital subtraction angiography or surgery; 6 patients had no demonstrable pulmonary sequestration. CT angiography showed a total of 44 aberrant systemic arteries in 37 patients. One patient was found to have three aberrant arteries on CT angiography; 5 patients had two aberrant arteries each and a single aberrant artery was detected in 31 patients. In one patient, three aberrant systemic arteries were detected on digital subtraction angiography, but only two of these arteries were observed on CT angiography, i.e. there was one false-negative result.
Diagnostic performance of CTA vs DSA for pulmonary sequestration in patient- and artery-based evaluations
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 36 | 6 | 0 | 1 | 0.89–1.0 | 97.3 (36/37) | 100 | 100 | 85.7 (6/7) | 97.7 (42/43) |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
| Single artery | 37 | 31 | 6 | 0 | 0 | 0.87–1.0 | 100 | 100 | 100 | 100 | 100 |
| Multiple arteries | 20 | 13 | 6 | 0 | 1 | 1 | 92.9 (13/14) | 100 | 100 | 85.7 (6/7) | 95.0 (19/20) |
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 36 | 6 | 0 | 1 | 0.89–1.0 | 97.3 (36/37) | 100 | 100 | 85.7 (6/7) | 97.7 (42/43) |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
| Single artery | 37 | 31 | 6 | 0 | 0 | 0.87–1.0 | 100 | 100 | 100 | 100 | 100 |
| Multiple arteries | 20 | 13 | 6 | 0 | 1 | 1 | 92.9 (13/14) | 100 | 100 | 85.7 (6/7) | 95.0 (19/20) |
CTA: computed tomographic angiography; DSA: digital subtraction angiography; TP: true positive; TN: true negative; FP: false positive; FN: false negative; PPV: positive predictive value; NPV: negative predictive value.
Diagnostic performance of CTA vs DSA for pulmonary sequestration in patient- and artery-based evaluations
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 36 | 6 | 0 | 1 | 0.89–1.0 | 97.3 (36/37) | 100 | 100 | 85.7 (6/7) | 97.7 (42/43) |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
| Single artery | 37 | 31 | 6 | 0 | 0 | 0.87–1.0 | 100 | 100 | 100 | 100 | 100 |
| Multiple arteries | 20 | 13 | 6 | 0 | 1 | 1 | 92.9 (13/14) | 100 | 100 | 85.7 (6/7) | 95.0 (19/20) |
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 36 | 6 | 0 | 1 | 0.89–1.0 | 97.3 (36/37) | 100 | 100 | 85.7 (6/7) | 97.7 (42/43) |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
| Single artery | 37 | 31 | 6 | 0 | 0 | 0.87–1.0 | 100 | 100 | 100 | 100 | 100 |
| Multiple arteries | 20 | 13 | 6 | 0 | 1 | 1 | 92.9 (13/14) | 100 | 100 | 85.7 (6/7) | 95.0 (19/20) |
CTA: computed tomographic angiography; DSA: digital subtraction angiography; TP: true positive; TN: true negative; FP: false positive; FN: false negative; PPV: positive predictive value; NPV: negative predictive value.
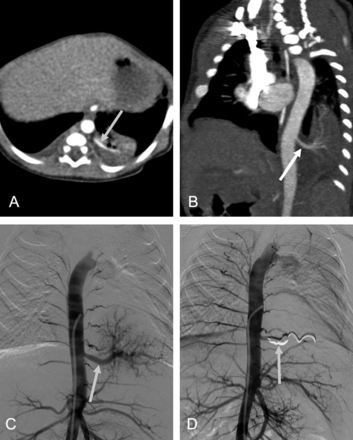
A 4-day-old girl with pulmonary sequestration in the left lower lobe. (A) Axial multiplanar reconstruction image demonstrates a homogenous, solid mass in the posterobasal segment of the left lower lobe supplied by an aberrant systemic artery (arrow) arising from the aorta. (B) Coronal maximum intensity projection exhibits an aberrant systemic artery (arrow) originating from the descending aorta and supplying the mass in the left lower lobe. (C) Pre-embolization aortography shows the aberrant artery (arrow) branching from the descending aorta. (D) Postembolization angiography confirms the complete occlusion of the aberrant artery with coils (arrow).
Treatment planning of computed tomographic angiography
A good correlation was found between with the treatment protocol proposed using CT angiography results and the actual treatment administered according to digital subtraction angiography or surgical findings (Table 4, Fig. 1). Of the 37 patients with confirmed pulmonary sequestration, 35 were treated with embolization coils, and 2 underwent surgical resection; the remaining 6 patients without pulmonary sequestration received conservative treatment. The patient with one false-negative aberrant systemic artery on CT angiography was successfully treated with coil embolization. No deviations were found between the proposed CT angiography-based treatment protocol and the actual treatment administered (Table 5).
Correlation between treatments planned using CT angiography and actual treatments administered in patients with pulmonary sequestration
| . | Treatment methods . | Treatment planned using CTA . | Actual treatment administered . |
|---|---|---|---|
| Radical treatment | Endovascular treatment (coil embolization) | 35 | 35 |
| Surgical resection | 2 | 2 | |
| Nonradical treatment | Conservative treatment | ||
| No pulmonary sequestration | 6 | 6 | |
| Difficult or unsuccessful treatment by coils and/or surgical resection | 0 | 0 |
| . | Treatment methods . | Treatment planned using CTA . | Actual treatment administered . |
|---|---|---|---|
| Radical treatment | Endovascular treatment (coil embolization) | 35 | 35 |
| Surgical resection | 2 | 2 | |
| Nonradical treatment | Conservative treatment | ||
| No pulmonary sequestration | 6 | 6 | |
| Difficult or unsuccessful treatment by coils and/or surgical resection | 0 | 0 |
CTA: computed tomographic angiography.
Correlation between treatments planned using CT angiography and actual treatments administered in patients with pulmonary sequestration
| . | Treatment methods . | Treatment planned using CTA . | Actual treatment administered . |
|---|---|---|---|
| Radical treatment | Endovascular treatment (coil embolization) | 35 | 35 |
| Surgical resection | 2 | 2 | |
| Nonradical treatment | Conservative treatment | ||
| No pulmonary sequestration | 6 | 6 | |
| Difficult or unsuccessful treatment by coils and/or surgical resection | 0 | 0 |
| . | Treatment methods . | Treatment planned using CTA . | Actual treatment administered . |
|---|---|---|---|
| Radical treatment | Endovascular treatment (coil embolization) | 35 | 35 |
| Surgical resection | 2 | 2 | |
| Nonradical treatment | Conservative treatment | ||
| No pulmonary sequestration | 6 | 6 | |
| Difficult or unsuccessful treatment by coils and/or surgical resection | 0 | 0 |
CTA: computed tomographic angiography.
Treatments planned using CT angiography with regard to patient- and artery-based evaluations
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 37 | 6 | 0 | 0 | 1.0 | 100 | 100 | 100 | 100 | 100 |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 37 | 6 | 0 | 0 | 1.0 | 100 | 100 | 100 | 100 | 100 |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
TP: true positive; TN: true negative; FP: false positive; FN: false negative; PPV: positive predictive value; NPV: negative predictive value.
Treatments planned using CT angiography with regard to patient- and artery-based evaluations
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 37 | 6 | 0 | 0 | 1.0 | 100 | 100 | 100 | 100 | 100 |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
| . | N . | TP . | TN . | FP . | FN . | κ . | Sensitivity (%) . | Specificity (%) . | PPV (%) . | NPV (%) . | Accuracy (%) . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient-based evaluation | |||||||||||
| All patients | 43 | 37 | 6 | 0 | 0 | 1.0 | 100 | 100 | 100 | 100 | 100 |
| Aneurysm-based evaluation | |||||||||||
| All arteries | 51 | 44 | 6 | 0 | 1 | 0.89–1.0 | 97.8 (44/45) | 100 | 100 | 85.7 (6/7) | 98.0 (50/51) |
TP: true positive; TN: true negative; FP: false positive; FN: false negative; PPV: positive predictive value; NPV: negative predictive value.
DISCUSSION
Pulmonary sequestration is a rare congenital malformation that accounts for 0.15–6.4% of all pulmonary malformations [16, 17]. Pulmonary sequestration is an area of non-functioning bronchopulmonary tissue that is separate from the tracheobronchial tree and receives arterial blood from the systemic circulation. The aberrant systemic arterial supply may involve a single or multiple vessels, and the latter accounts for 15–20% of pulmonary sequestration cases [18]. Pulmonary sequestration often involves the lung parenchyma and its vascularization and is classified as intra- or extralobar [1]. This condition may present in childhood or adulthood. Although patients may be clinically asymptomatic, they may develop complications such as recurrent pneumonia in a persistent location.
Imaging studies for the investigation of a suspected case of pulmonary sequestration have two principal objectives: to rule out other pathologies and to confirm the presence of an anomalous arterial supply [9, 19]. The most common presentation on plain radiographs is a homogeneous opacity in the posterior basal segment of the left lower lobe. For a definitive diagnosis of pulmonary sequestration, the systemic arterial supply to the sequestered portion must be identified, as this helps to distinguish sequestration from other causes of the lung opacity, such as bronchiectasis, atelectasis and bronchial atresia. Because accessory arteries and venous drainage are adequately visualized intraoperatively, some institutions consider the localization of the aberrant systemic artery alone to be essential for the preoperative assessment of symptomatic pulmonary sequestration [20].
Conventionally, the diagnosis of pulmonary sequestration requires arteriography to identify abnormal systemic vessels supplying the abnormal portion of the lung and to provide valuable information for preoperative planning [2, 19]. In recent years, with the development of imaging techniques, less-invasive techniques have proven to be equally effective and safer alternatives to angiography, including CT angiography and MRI [3–7]. CT angiography is the most widely used, noninvasive diagnostic tool for the detection of pulmonary sequestration in the clinical setting. CT and CT angiography cannot only delineate the origin and course of the anomalous systemic artery and venous drainage accurately, but also evaluate the abnormal lung parenchyma and the airways.
Spiral CT angiography has several advantages over Doppler sonography and MRI in the evaluation of pulmonary sequestration [2]. These advantages arise from the volume acquisition (slice reconstruction, multiplanar reconstruction and 3D reformatting) and faster scanning (reduced need for sedation and intravenous contrast media) associated with CT angiography [20]. This technique is less expensive than multiplanar reconstructions and can be used in claustrophobic patients and those with metallic implants. Compared with other techniques for the evaluation of pulmonary sequestration, CT angiography yields the maximum information about the lung parenchyma, including the airways. Conditions such as bronchiectasis, atelectasis (including that due to an endobronchial lesion), other bronchopulmonary foregut malformations and bronchial atresia can clinically and radiographically resemble pulmonary sequestration. These are not amenable to diagnosis by colour Doppler sonography and MRI, but can be detected with a high accuracy by CT angiography [20]. Therefore, CT angiography is the sole, minimally invasive diagnostic method for pulmonary sequestration.
Surgical resection is the conventional standard treatment for symptomatic pulmonary sequestration in order to prevent possible infection [21, 22], but in asymptomatic patients, the value of surgical treatment is debatable [23]. Recently, endovascular treatment with coil embolization has emerged as a less-invasive alternative to surgery [15, 24, 25], especially in neonates and children. This method can be used to protect the lungs from excessive intraoperative bronchial haemorrhage. In our study, all patients were successfully treated with coil embolization without the occurrence of complications. We used coil embolization to treat pulmonary sequestration in order to prevent infection and shortness of breath and to determine whether asymptomatic patients should be treated by this method. In this study, two patients who were asymptomatic refused surgery.
This prospective study was based on our hypothesis that CT angiography could replace digital subtraction angiography as a reliable diagnostic and pretreatment planning tool for patients with pulmonary sequestration. We found that CT angiography cannot only accurately identify the presence of pulmonary sequestration, but also demonstrate a good correlation with digital subtraction angiography as a treatment planning tool for pulmonary sequestration. The use of CT angiography to plan treatment strategies before digital subtraction angiography is a feasible and effective option in patients with pulmonary sequestration.
Although many studies have assessed the validity of CT angiography as a diagnostic technique for pulmonary sequestration, few have evaluated the clinical implications of using CT angiography instead of digital subtraction angiography as a diagnostic and pretreatment planning tool. If CT angiography is to serve as a noninvasive replacement for digital subtraction angiography in the selection of an appropriate treatment, it must provide precise information about the site and number of the aberrant systemic arteries and their relationships with the aorta. In addition, the shape and size of the aberrant artery must be determined before endovascular treatment with coil embolization is undertaken.
In this study, the high accuracy (>95%) and sensitivity (>95%) of CT angiography for pulmonary sequestration showed that this technique is equivalent to the gold standard, digital subtraction angiography and thus can replace the latter as a diagnostic tool for pulmonary sequestration. Moreover, our results suggest that pulmonary sequestration can be reliably detected using the maximum intensity projections or volume-rendered algorithm, which means that the size, shape and location of a pulmonary sequestration as well as its relationship with the aorta and adjacent vascular structures can be clearly visualized with CT angiography. Compared with the actual treatment decisions made using digital subtraction angiography, the treatments planned with CT angiography had high accuracy, sensitivity, PPV and NPV in both patient- and artery-based evaluations. The high sensitivity and accuracy rates (both >90%) suggest the potential of CT angiography as an effective primary tool in pretreatment planning for most patients with pulmonary sequestration.
The study had some limitations. First, this is a single-centre study and the patient population was small. The small sample size prevents us from generalizing our results. Second, pulmonary sequestration can present as a paediatric condition, and CT angiography scanning involves the exposure of the patients to ionizing radiation and the administration of intravenous contrast material.
In summary, we have demonstrated promising results of a CT angiography-based protocol, rather than a digital subtraction angiography-based protocol, as the sole diagnostic and pretreatment planning tool for patients with pulmonary sequestration. It seems safe and effective to make decisions regarding treatment on the basis of CT angiography, without performing digital subtraction angiography, in the majority of patients with pulmonary sequestration.
Conflict of interest: none declared.




