-
PDF
- Split View
-
Views
-
Cite
Cite
Hunaid A. Vohra, Robert N. Whistance, Laurent De Kerchove, Prakash Punjabi, Gebrine El Khoury, Valve-preserving surgery on the bicuspid aortic valve, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 5, May 2013, Pages 888–898, https://doi.org/10.1093/ejcts/ezs664
Close - Share Icon Share
Abstract
Valve repair has emerged as an important intervention for the management of bicuspid aortic valve disease. This systematic review aims to assess the safety, efficacy and durability of bicuspid aortic valve repair. Initial searches yielded 682 abstracts, reduced by de-duplication to 370, of which 56 full papers were accessed and 30 met the inclusion criteria. Overall, 163 unique outcomes for bicuspid aortic valve-preserving surgery were reported on 280 occasions. Bicuspid aortic valve-preserving surgery exhibited low operative mortality (0.0–5.2%), excellent 5-year survival (82–100%) and 43–100% 5-year freedom from reoperation. Bicuspid aortic valve repair is safe and efficacious, but concerns regarding its durability necessitate further standardized outcome assessments.
INTRODUCTION
The bicuspid aortic valve (BAV) is the most common congenital cardiovascular abnormality, with a prevalence of 0.9–2.5% in the general population [1, 2]. Aortic insufficiency (AI) (due to prolapse or restriction) and stenosis (AS) are frequent complications, with the peak incidence of AI occurring at 30 years and AS typically developing in the seventh decade [2, 3] (Fig. 1). The proximal aorta is prone to dilatation and ∼50–60% of patients with BAV will be affected [4]. The reasons for this are unclear, but it is likely that underlying abnormalities in the aortic wall contribute [5, 6]. Progressive aortic dilatation can lead to catastrophic rupture or dissection, which is often fatal. The goals of surgical intervention in patients with BAV are therefore aimed at correcting aortic valve haemodynamics and managing the presence of aortic aneurysm formation.
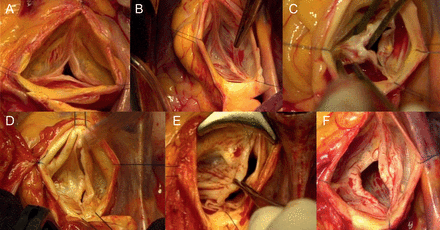
Showing different aortic valve phenotypes: (A) normal tricuspid, (B) normal bicuspid, (C) stenosed bicuspid, (D and E) regurgitant bicuspid with prolapsing cusp and (F) regurgitant bicuspid with restrictive cusp.
Treatment options for complicated BAV are diverse (Fig. 2). Aortic valve replacement (AVR), with or without replacement of the proximal aorta, is an effective intervention in patients with AI or AS. However, mechanical valves require life-long anticoagulation, and bio-prosthetic implants are associated with high rates of degeneration and the need for revision surgery [7, 8]. The Ross procedure is an efficacious option for young patients with stenotic BAV because of proven durability, a low risk of thromboembolism and beneficial effects on long-term survival [9]. The presence of AI and aortic dilatation, which are frequently seen in BAV, are however important risk factors for pulmonary autograft failure [10, 11]. Novel valve-sparing techniques have emerged as viable management options in patients with BAV. These can be categorized broadly into interventions on the AV itself and those aimed at repairing the proximal aorta, although both procedures are often performed concurrently. Procedures to repair the BAV include AV tricuspidisation, leaflet resuspension, triangular resection, commissural plication, circumferential annuloplasty, pericardial patch repair for leaflet perforation and pericardial extension valvuloplasty. When aortic dilatation is also present, aortic root remodelling (Yacoub procedure), reimplantation (David procedure) or replacement is also effective.
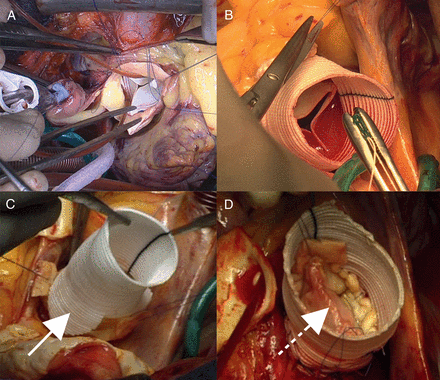
(A) Bioprosthetic aortic valve replacement, (B) modified Bentall procedure (with a Dacron graft sutured to a bioprosthesis; Bio-Bentall), (C) remodelling procedure (white arrow showing tongue of graft to fit scalloped sinus) and (D) reimplantation procedure (note dotted white arrow showing resuspension of cusp leaflets in this specimen).
Studies of patients with tricuspid aortic valve (TAV) have highlighted a number of benefits for preserving the native AV. These include the avoidance of the haemorrhagic complications of long-term anticoagulation and reduced rates of thromboembolism, infective endocarditis (IE) and valvular degeneration compared with AVR [12–14]. However, the longer duration of ischaemic time may not be suitable for high-risk patients, and a proportion of patients undergoing AV repair will require surgical reintervention for progressive disease. At present, little work has been carried out to assess the outcomes of AV repair and aortic root interventions in patients with BAV. This systematic review summarizes the perioperative, long-term and functional outcomes of patients undergoing valve-sparing surgery on the BAV.
METHODS
Search strategy
The OVID SP versions of MEDLINE and EMBASE were searched using terms for ‘aortic valve’, ‘bicuspid’ and ‘repair’ separated by the Boolean operator ‘AND’ (Table 1). Additional search terms captured articles pertaining to recognized AV-sparing procedures (‘David’ and ‘Yacoub’ procedures). The study was limited to publications reporting outcomes for humans in English language, while no time limit was imposed. Reference lists and leading journals were hand-searched for additional articles. All relevant citations were collated using Reference Manager 12 (Thomson Reuters, New York, NY, USA) and duplicates removed.
| Search criteria . | Search terms . |
|---|---|
| Medline | 1. exp Aortic Valve/ |
| 2. exp Aortic Valve Insufficiency/ | |
| 3. exp Aortic Valve Stenosis/ | |
| 4. exp Aortic Aneurysm/ | |
| 5. exp Aneurysm, Dissecting/ | |
| 6. exp Cardiac Surgical Procedures/ | |
| 7. exp Heart Valve Diseases/ | |
| 8. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 9. (aortic adj3 valve adj3 repair).tw | |
| 10. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 11. or/1–10 | |
| 12. bicuspid.tw | |
| 13. repair.tw | |
| 14. remodeling.tw | |
| 15. or/13–14 | |
| 16. 11 and 12 and 15 | |
| 17. (david adj3 procedure).tw | |
| 18. (yacoub adj3 procedure).tw | |
| 19. or/16–18 | |
| Embase | 1. exp aortic valve repair/ |
| 2. exp aortic valve regurgitation/ | |
| 3. exp aortic valve disease/ | |
| 4. exp aorta valve/ | |
| 5. exp heart valve surgery/ | |
| 6. exp valvuloplasty/ | |
| 7. exp aortic dissection/ | |
| 8. exp aortic aneurysm/ | |
| 9. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 10. (aortic adj3 valve adj3 repair).tw | |
| 11. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 12. or/1–11 | |
| 13. bicuspid.tw | |
| 14. repair.tw | |
| 15. remodeling.tw | |
| 16. or/14–15 | |
| 17. 12 and 13 and 16 | |
| 18. (david adj3 procedure).tw | |
| 19. (yacoub adj3 procedure).tw | |
| 20. or/17–19 |
| Search criteria . | Search terms . |
|---|---|
| Medline | 1. exp Aortic Valve/ |
| 2. exp Aortic Valve Insufficiency/ | |
| 3. exp Aortic Valve Stenosis/ | |
| 4. exp Aortic Aneurysm/ | |
| 5. exp Aneurysm, Dissecting/ | |
| 6. exp Cardiac Surgical Procedures/ | |
| 7. exp Heart Valve Diseases/ | |
| 8. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 9. (aortic adj3 valve adj3 repair).tw | |
| 10. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 11. or/1–10 | |
| 12. bicuspid.tw | |
| 13. repair.tw | |
| 14. remodeling.tw | |
| 15. or/13–14 | |
| 16. 11 and 12 and 15 | |
| 17. (david adj3 procedure).tw | |
| 18. (yacoub adj3 procedure).tw | |
| 19. or/16–18 | |
| Embase | 1. exp aortic valve repair/ |
| 2. exp aortic valve regurgitation/ | |
| 3. exp aortic valve disease/ | |
| 4. exp aorta valve/ | |
| 5. exp heart valve surgery/ | |
| 6. exp valvuloplasty/ | |
| 7. exp aortic dissection/ | |
| 8. exp aortic aneurysm/ | |
| 9. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 10. (aortic adj3 valve adj3 repair).tw | |
| 11. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 12. or/1–11 | |
| 13. bicuspid.tw | |
| 14. repair.tw | |
| 15. remodeling.tw | |
| 16. or/14–15 | |
| 17. 12 and 13 and 16 | |
| 18. (david adj3 procedure).tw | |
| 19. (yacoub adj3 procedure).tw | |
| 20. or/17–19 |
| Search criteria . | Search terms . |
|---|---|
| Medline | 1. exp Aortic Valve/ |
| 2. exp Aortic Valve Insufficiency/ | |
| 3. exp Aortic Valve Stenosis/ | |
| 4. exp Aortic Aneurysm/ | |
| 5. exp Aneurysm, Dissecting/ | |
| 6. exp Cardiac Surgical Procedures/ | |
| 7. exp Heart Valve Diseases/ | |
| 8. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 9. (aortic adj3 valve adj3 repair).tw | |
| 10. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 11. or/1–10 | |
| 12. bicuspid.tw | |
| 13. repair.tw | |
| 14. remodeling.tw | |
| 15. or/13–14 | |
| 16. 11 and 12 and 15 | |
| 17. (david adj3 procedure).tw | |
| 18. (yacoub adj3 procedure).tw | |
| 19. or/16–18 | |
| Embase | 1. exp aortic valve repair/ |
| 2. exp aortic valve regurgitation/ | |
| 3. exp aortic valve disease/ | |
| 4. exp aorta valve/ | |
| 5. exp heart valve surgery/ | |
| 6. exp valvuloplasty/ | |
| 7. exp aortic dissection/ | |
| 8. exp aortic aneurysm/ | |
| 9. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 10. (aortic adj3 valve adj3 repair).tw | |
| 11. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 12. or/1–11 | |
| 13. bicuspid.tw | |
| 14. repair.tw | |
| 15. remodeling.tw | |
| 16. or/14–15 | |
| 17. 12 and 13 and 16 | |
| 18. (david adj3 procedure).tw | |
| 19. (yacoub adj3 procedure).tw | |
| 20. or/17–19 |
| Search criteria . | Search terms . |
|---|---|
| Medline | 1. exp Aortic Valve/ |
| 2. exp Aortic Valve Insufficiency/ | |
| 3. exp Aortic Valve Stenosis/ | |
| 4. exp Aortic Aneurysm/ | |
| 5. exp Aneurysm, Dissecting/ | |
| 6. exp Cardiac Surgical Procedures/ | |
| 7. exp Heart Valve Diseases/ | |
| 8. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 9. (aortic adj3 valve adj3 repair).tw | |
| 10. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 11. or/1–10 | |
| 12. bicuspid.tw | |
| 13. repair.tw | |
| 14. remodeling.tw | |
| 15. or/13–14 | |
| 16. 11 and 12 and 15 | |
| 17. (david adj3 procedure).tw | |
| 18. (yacoub adj3 procedure).tw | |
| 19. or/16–18 | |
| Embase | 1. exp aortic valve repair/ |
| 2. exp aortic valve regurgitation/ | |
| 3. exp aortic valve disease/ | |
| 4. exp aorta valve/ | |
| 5. exp heart valve surgery/ | |
| 6. exp valvuloplasty/ | |
| 7. exp aortic dissection/ | |
| 8. exp aortic aneurysm/ | |
| 9. aort$.tw adj3 (valv$.tw or steno$.tw or regurg$.tw or insufficien$.tw or aneurysm.tw or dissect$.tw) | |
| 10. (aortic adj3 valve adj3 repair).tw | |
| 11. (aortic adj3 sparing adj3 root adj3 replacement).tw | |
| 12. or/1–11 | |
| 13. bicuspid.tw | |
| 14. repair.tw | |
| 15. remodeling.tw | |
| 16. or/14–15 | |
| 17. 12 and 13 and 16 | |
| 18. (david adj3 procedure).tw | |
| 19. (yacoub adj3 procedure).tw | |
| 20. or/17–19 |
Study selection
The titles and abstracts of identified publications were screened by one reviewer (R.N.W.) and checked by a second researcher (H.A.V.). Included were publications reporting outcomes of BAV repair. Thoracic aneurysm repair or procedures involving the aortic root or ascending aorta were also included, provided the native BAV was preserved. Excluded were studies solely of AVR, articles where the outcomes of bicuspid and TAV surgery were not presented independently and abstracts of conference proceedings (due to the high probability of missing data). Case series were defined as studies comprising a single group of participants and cohort studies were those that compared two or more different groups of participants. Full papers of potentially relevant studies were obtained and articles not meeting the eligibility criteria were excluded.
Data extraction
The following data were extracted from included articles: (i) year of publication; (ii) country in which the study was undertaken; (iii) demographics of participants; (iv) study design; (v) the nature of the interventions and comparators; and (vi) the impact of the interventions on outcome measures (see Supplementary Material). Where studies reported outcomes for both tricuspid and bicuspid aortic valve surgery, only the endpoints for patients with bicuspid aortic valves were extracted. All data were recorded on a dedicated data extraction form and entered onto a Microsoft Excel (Microsoft, Washington, DC, USA) spreadsheet to facilitate data management and analysis. A second reviewer (H.A.V.) checked the data extraction and any disagreements were discussed with the senior author.
Data synthesis
The standard of outcome reporting was considered by calculating the proportion of endpoints that specified precisely when they were measured. Outcome reporting was deemed to be of a high standard when the time over which the endpoint was measured was reported (e.g. 30-day mortality, 5-year overall survival). Poor outcome reporting was defined as failure to specify the time period during which the outcome occurred (e.g. operative mortality, late death). Outcomes were divided into (i) perioperative outcomes, (ii) long-term morbidity and mortality and (iii) functional and echocardiographic endpoints.
RESULTS
The search strategy (Table 1) yielded 682 abstracts, of which 370 remained after removal of duplicates (Fig. 3). A total of 56 full papers were accessed and 29 of these fulfilled the eligibility criteria. One further publication was identified through hand searching of reference lists. Thirty articles were consequently included in the review (Table 2) [4, 15–41]. These were published between 1991 and 2011 in eight countries, including Germany (n = 11), USA (n = 9) and Canada (n = 6). The majority of studies were retrospective (n = 29), and there were 18 case series and 12 cohort studies. Twenty-five studies were of combined AV repair and procedures on the aortic root or ascending aorta (replacement, remodelling or reimplantation) [4, 14–22, 24–27, 29–31, 34, 35, 37–41], 5 reported outcomes of AV repair alone [23, 28, 32, 33, 36], 19 included only patients with BAV [4, 18, 19, 21–24, 27–29, 31–34, 36, 38–41] and a further 11 included patients with both BAV and TAV [12, 14–17, 20, 25, 26, 30, 35, 37]. +
| Author, year and country . | Demographicsa . | Study design . | Outcomesa . | Resultsa . |
|---|---|---|---|---|
| Aicher et al. (2004), Germany [15] | n = 60 Male 83.3% Mean age 53 ± 12 years Aortic dissection 6.7% | Retrospective cohort study comparing valve-sparing aortic root replacement in patients with TAV or BAV | In-hospital mortality Postoperative AI grade 5-year overall survival TE events/endocarditis during follow-up Reoperation during follow-up 5-year mean AV gradient 5-year freedom from AI > II 5-year freedom from reoperation | 0% 0.8 ± 0.7 82% 0% 1.7% 4.5 ± 2.3 mmHg 96% 98% |
| Aicher et al. (2007), Germany [16] | n = 81 Male 85.2% Aortic dissection 7.4% AI > II 55.6% Marfan 0% | Retrospective cohort study of aortic root remodelling in patients with BAV, AI and dilatation of the ascending aorta. Comparison made with patients with TAV | Hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0% 94% 87% 97% 99% |
| Aicher et al. (2010), Germany [17] | n = 205 | Retrospective cohort study comparing AV repair in patients with BAV and TAV, limited raw data presented for BAV | 5-year freedom from AI > II 10-year freedom from AI > II | 86% 83% |
| Aicher et al. (2011), Germany [4] | n = 316 Male 84.8% Age range 3–79 years Aortic dissection 8% Severe AI 72.8% | Retrospective case series evaluating the effect of valve configuration on outcomes after repair of BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0.63% 92% 81% 81% 84% |
| Alsoufi et al. (2005), Canada [18] | n = 71 Male 87.3% Mean age 41.5 ± 13.2 years | Retrospective case series of prospectively collected data on AV repair for AI secondary to BAV, aortic remodelling/ replacement included | Hospital mortality Postoperative AI > II 8-year overall survival Freedom from TE/haemorrhage 8-year freedom from reoperation 8-year freedom from endocarditis 8-year freedom from AI > II | 0% 0% 96.7% 100% 82.3% 90% 44.2% |
| Ashikhmina et al. (2010), USA [19] | n = 108 Male 91% Mean age 41 years | Retrospective case series of BAV repair. Patients with valve-sparing aortic root replacements were excluded | In-hospital mortality 10-year overall survival 10-year freedom from reoperation 10-year freedom from AVR | 0% 87% 64% 49% |
| Badiu et al. (2010), Germany [20] | n = 11 Male 100% Mean age 37 ± 15.8 years Aortic dissection 0% Marfan 0% | Retrospective cohort study comparing BAV and TAV repair for AI | Operative mortality 5-year overall survival 5-year freedom from reoperation 5-year freedom from AI 5-year freedom from TE events | 0% 100% 100% 57.1% 95.9% |
| Bakhtiary et al. (2009), Germany [21] | n = 14 Male 92.9% Mean age 58 ± 5 years AI > I 78.6% | Retrospective case series of patients undergoing the modified David procedure for incompetent BAV. | Endocarditis NE Operative death In-hospital death Late death | 0% 0% 0% 0% 0% |
| Boodhwani et al. (2009), Belgium [12] | n = 90 | Retrospective cohort study comparing aortic valve repair for AI in BAV and TAV, limited raw data presented for BAV | Comparison of freedom from AI recurrence between BAV and TAV | P = 0.7 |
| Boodhwani et al. (2010), Belgium [22] | n = 122 Male 92% Mean age 44 ± 11 years AI > II 86.1% | Retrospective case series of patients undergoing surgery for BAV in association with either AI or dilatation of the proximal aorta | In-hospital mortality Discharge AI < II 8-year overall survival 5-year freedom from AI > II 8-year freedom from AV reoperation 8-year freedom from AVR 8-year freedom from TE and bleeding | 0% 93% 97 ± 2% 94 ± 3% 83 ± 5% 90 ± 5% 96 ± 2% |
| Casselman et al. (1999), USA [23] | n = 94 Male 93% Mean age 38 ± 10 years | Retrospective case series of aortic valve repair in patients with BAV and AI | Immediate reoperation Immediate postoperative AI > II 7-year freedom from AV reoperation | 8.5% 2.1% 84% |
| Davierwala et al. (2003), Canada [24] | n = 44 Mean age 39.3 ± 12.1 years Male 93.2% Preoperative CCF 13.6% | Retrospective cohort study of prospectively collected data comparing AV repair to AVR in patients with AI caused by BAV. Procedures on the aorta were included | Operative mortality Late death TE/Haemorrhagic events 5-year freedom from AI > I 5-year freedom from AV reoperation | 0% 0% 0% 79 ± 8% 91 ± 5% |
| de Kerchove et al. (2009), Belgium [25] | n = 54 | Retrospective cohort study comparing the impact of preoperative AI on outcome after aortic valve-sparing surgery, limited raw data presented for BAV | 5-year freedom from AI > II 8-year freedom from AV reoperation | 98 ± 2% 91 ± 9% |
| Delius et al. (1998), USA [26] | n = 16 | Retrospective cohort study of aortic valve repair in patients with either subvalvular or supravalvular aortic stenosis. Comparison made between BAV and TAV | 10-year overall survival 5-year freedom from reoperation 5-year freedom from AVR | 100% 43% 43% |
| Doss et al. (2010), Germany [27] | n = 66 (A) n = 49; (B) n = 17 Mean age 41.2 ± 12 years (A) 58 years; (B) 39 yrs Male 78.8% (A) 82.3%; (B) 77.6% AI > II 95.4% (A) 82.3%; (B) 100% | Retrospective cohort study of (A) patch augmentation plus reduction aortoplasty vs (B) modified David procedure in patients with BAV and AI | 5-year mortality 5-year reoperation 5-year endocarditis 5-year conduction disturbance/thromboembolism/AI > I | (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 0% (B) 0% |
| Fraser et al. (1994), USA [28] | n = 72 Male 94.4% Mean age 39 ± 11 years | Retrospective case series of AV repair for AI secondary to BAV | Operative death Late death 3-year freedom from AV reoperation | 0% 0% 89.5% |
| Kin et al. (2003), Japan [29] | n = 19 Male 98% Mean age 42 ± 17 years | Retrospective case series of AV repair for AI secondary to BAV, some patients underwent concomitant procedures on the aorta | Hospital death Early reoperation Reoperation at follow-up Late death 5-year overall survival 5-year freedom from AV reoperation | 5.2% 5.2% 15.8% 5.2% 90 ± 7% 76 ± 23% |
| Lausberg et al. (2006), Germany [30] | n = 89 n = 34; (B) n = 56 | Retrospective cohort study comparing (A) AV repair alone to (B) AV repair plus aortic root remodelling in patients with AI, limited data for BAV alone | Freedom from significant AI in (A) Freedom from reoperation in (A) | (A) 89.1% (A) 100% |
| Mangini et al. (2010), Italy [31] | n = 31 Mean age 49.9 ± 17.3 years Male 83.9% AI > I 96.8% | Prospective case series of patients undergoing repair of BAV for AI | 30-day operative mortality Discharge AI > I 5-year freedom from reoperation | 3.2% 3.2% 96.6% |
| McMullan et al. (2007), Australia [32] | n = 21 Median age 12.6 years Mean follow-up 36.4 months | Retrospective cohort study of tricuspidisation with cusp extension vs Ross procedure in children with AI or AS associated with BAV | Early reoperation Endocarditis during follow-up AVR during follow-up AI > 2 during follow-up | 9.5% 4.8% 9.5% 19.0% |
| Minakata et al. (2004), USA [14] | n = 54 | Retrospective case series of AV repair for AI, limited raw data presented for BAV | Reoperation during index admission Reoperation during follow-up 5-year reoperation rate | 3.7% 11.1% 9% |
| Moidl et al. (1995), Austria [33] | n = 14 Mean age 30.9 ± 12 years Male 92.9% Preoperative AI grade 3.5 ± 0.1 | Retrospective case series of valve-sparing correction of AI and BAV | Reoperation during index admission | 21.4% |
| Nash et al. (2004), USA [34] | n = 77 Mean age 38 ± 10 years Male 93% | Retrospective case series of the echocardiographic factors that predict successful AV repair in patients with BAV and AI | AVR during index admission Reoperation during index admission Perioperative death Thromboembolism Endocarditis | 2.6% 3.9% 0% 0% 0% |
| Odim et al. (2005), USA [35] | n = 39 | Retrospective case series of AV repair with pericardial leaflet extension | Early mortality 2-year freedom from reoperation | 2.6% 70–90% |
| Pretre et al. (2006), Switzerland [36] | n = 12 Median age 18 years Male 75% | Retrospective case series of AV repair with tricuspidization of the BAV | Postoperative morbidity AR > I | 0% 8.3% |
| Rao et al. (2000), Canada [37] | n = 23 | Retrospective case series of AV repair for multiple pathologies, limited raw data presented for BAV | Composite endpoint of reoperation or AI | No difference between BAV and TAV (P > 0.05) |
| Schafers et al. (2000), Germany [38] | n = 16 Age range 35–73 years Male 75% Aortic dissection 6.3% | Retrospective case series of AV repair and root replacement in patients with BAV, AI and aortic dilatation | In-hospital mortality AV reoperation during follow-up Reoperation during follow-up | 0% 0% 6.3% |
| Schafers et al. (2007), Germany [39] | n = 173 Mean age 48 ± 16 years Male 80.3% | Retrospective cohort study comparing (A) root remodelling, (B) AV repair + supracommissural aortic replacement, (C) AV repair alone | In-hospital mortality TE Endocarditis | (A) 0% (B) 2.6% (C) 1.8% 0% (A) 0% (B) 0% (C) 1.3% |
| Schafers et al. (2010), Germany [40] | n = 153 Mean age 51 ± 12 years Male 86.9% Preoperative AI grade 2.6 ± 0.8 Aortic dissection 3.9% | Retrospective case series of valve-preserving root replacement for AI and BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > I 10-year freedom from reoperation 10-year freedom from AVR TE events Endocarditis 10-year freedom from AV complications | 0.7% 91% 90% 95% 97% 2.6% 0% 91% |
| Veldtman et al. (2006), USA [41] | n = 21 Mean age 45 ± 12 years Male 61.9% Marfan 9.5% AI > I 19.0% | Retrospective case series of aortic root repair or replacement with preservation of the BAV | Perioperative death Late death Reoperation | 0% 4.8% 9.5% |
| Author, year and country . | Demographicsa . | Study design . | Outcomesa . | Resultsa . |
|---|---|---|---|---|
| Aicher et al. (2004), Germany [15] | n = 60 Male 83.3% Mean age 53 ± 12 years Aortic dissection 6.7% | Retrospective cohort study comparing valve-sparing aortic root replacement in patients with TAV or BAV | In-hospital mortality Postoperative AI grade 5-year overall survival TE events/endocarditis during follow-up Reoperation during follow-up 5-year mean AV gradient 5-year freedom from AI > II 5-year freedom from reoperation | 0% 0.8 ± 0.7 82% 0% 1.7% 4.5 ± 2.3 mmHg 96% 98% |
| Aicher et al. (2007), Germany [16] | n = 81 Male 85.2% Aortic dissection 7.4% AI > II 55.6% Marfan 0% | Retrospective cohort study of aortic root remodelling in patients with BAV, AI and dilatation of the ascending aorta. Comparison made with patients with TAV | Hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0% 94% 87% 97% 99% |
| Aicher et al. (2010), Germany [17] | n = 205 | Retrospective cohort study comparing AV repair in patients with BAV and TAV, limited raw data presented for BAV | 5-year freedom from AI > II 10-year freedom from AI > II | 86% 83% |
| Aicher et al. (2011), Germany [4] | n = 316 Male 84.8% Age range 3–79 years Aortic dissection 8% Severe AI 72.8% | Retrospective case series evaluating the effect of valve configuration on outcomes after repair of BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0.63% 92% 81% 81% 84% |
| Alsoufi et al. (2005), Canada [18] | n = 71 Male 87.3% Mean age 41.5 ± 13.2 years | Retrospective case series of prospectively collected data on AV repair for AI secondary to BAV, aortic remodelling/ replacement included | Hospital mortality Postoperative AI > II 8-year overall survival Freedom from TE/haemorrhage 8-year freedom from reoperation 8-year freedom from endocarditis 8-year freedom from AI > II | 0% 0% 96.7% 100% 82.3% 90% 44.2% |
| Ashikhmina et al. (2010), USA [19] | n = 108 Male 91% Mean age 41 years | Retrospective case series of BAV repair. Patients with valve-sparing aortic root replacements were excluded | In-hospital mortality 10-year overall survival 10-year freedom from reoperation 10-year freedom from AVR | 0% 87% 64% 49% |
| Badiu et al. (2010), Germany [20] | n = 11 Male 100% Mean age 37 ± 15.8 years Aortic dissection 0% Marfan 0% | Retrospective cohort study comparing BAV and TAV repair for AI | Operative mortality 5-year overall survival 5-year freedom from reoperation 5-year freedom from AI 5-year freedom from TE events | 0% 100% 100% 57.1% 95.9% |
| Bakhtiary et al. (2009), Germany [21] | n = 14 Male 92.9% Mean age 58 ± 5 years AI > I 78.6% | Retrospective case series of patients undergoing the modified David procedure for incompetent BAV. | Endocarditis NE Operative death In-hospital death Late death | 0% 0% 0% 0% 0% |
| Boodhwani et al. (2009), Belgium [12] | n = 90 | Retrospective cohort study comparing aortic valve repair for AI in BAV and TAV, limited raw data presented for BAV | Comparison of freedom from AI recurrence between BAV and TAV | P = 0.7 |
| Boodhwani et al. (2010), Belgium [22] | n = 122 Male 92% Mean age 44 ± 11 years AI > II 86.1% | Retrospective case series of patients undergoing surgery for BAV in association with either AI or dilatation of the proximal aorta | In-hospital mortality Discharge AI < II 8-year overall survival 5-year freedom from AI > II 8-year freedom from AV reoperation 8-year freedom from AVR 8-year freedom from TE and bleeding | 0% 93% 97 ± 2% 94 ± 3% 83 ± 5% 90 ± 5% 96 ± 2% |
| Casselman et al. (1999), USA [23] | n = 94 Male 93% Mean age 38 ± 10 years | Retrospective case series of aortic valve repair in patients with BAV and AI | Immediate reoperation Immediate postoperative AI > II 7-year freedom from AV reoperation | 8.5% 2.1% 84% |
| Davierwala et al. (2003), Canada [24] | n = 44 Mean age 39.3 ± 12.1 years Male 93.2% Preoperative CCF 13.6% | Retrospective cohort study of prospectively collected data comparing AV repair to AVR in patients with AI caused by BAV. Procedures on the aorta were included | Operative mortality Late death TE/Haemorrhagic events 5-year freedom from AI > I 5-year freedom from AV reoperation | 0% 0% 0% 79 ± 8% 91 ± 5% |
| de Kerchove et al. (2009), Belgium [25] | n = 54 | Retrospective cohort study comparing the impact of preoperative AI on outcome after aortic valve-sparing surgery, limited raw data presented for BAV | 5-year freedom from AI > II 8-year freedom from AV reoperation | 98 ± 2% 91 ± 9% |
| Delius et al. (1998), USA [26] | n = 16 | Retrospective cohort study of aortic valve repair in patients with either subvalvular or supravalvular aortic stenosis. Comparison made between BAV and TAV | 10-year overall survival 5-year freedom from reoperation 5-year freedom from AVR | 100% 43% 43% |
| Doss et al. (2010), Germany [27] | n = 66 (A) n = 49; (B) n = 17 Mean age 41.2 ± 12 years (A) 58 years; (B) 39 yrs Male 78.8% (A) 82.3%; (B) 77.6% AI > II 95.4% (A) 82.3%; (B) 100% | Retrospective cohort study of (A) patch augmentation plus reduction aortoplasty vs (B) modified David procedure in patients with BAV and AI | 5-year mortality 5-year reoperation 5-year endocarditis 5-year conduction disturbance/thromboembolism/AI > I | (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 0% (B) 0% |
| Fraser et al. (1994), USA [28] | n = 72 Male 94.4% Mean age 39 ± 11 years | Retrospective case series of AV repair for AI secondary to BAV | Operative death Late death 3-year freedom from AV reoperation | 0% 0% 89.5% |
| Kin et al. (2003), Japan [29] | n = 19 Male 98% Mean age 42 ± 17 years | Retrospective case series of AV repair for AI secondary to BAV, some patients underwent concomitant procedures on the aorta | Hospital death Early reoperation Reoperation at follow-up Late death 5-year overall survival 5-year freedom from AV reoperation | 5.2% 5.2% 15.8% 5.2% 90 ± 7% 76 ± 23% |
| Lausberg et al. (2006), Germany [30] | n = 89 n = 34; (B) n = 56 | Retrospective cohort study comparing (A) AV repair alone to (B) AV repair plus aortic root remodelling in patients with AI, limited data for BAV alone | Freedom from significant AI in (A) Freedom from reoperation in (A) | (A) 89.1% (A) 100% |
| Mangini et al. (2010), Italy [31] | n = 31 Mean age 49.9 ± 17.3 years Male 83.9% AI > I 96.8% | Prospective case series of patients undergoing repair of BAV for AI | 30-day operative mortality Discharge AI > I 5-year freedom from reoperation | 3.2% 3.2% 96.6% |
| McMullan et al. (2007), Australia [32] | n = 21 Median age 12.6 years Mean follow-up 36.4 months | Retrospective cohort study of tricuspidisation with cusp extension vs Ross procedure in children with AI or AS associated with BAV | Early reoperation Endocarditis during follow-up AVR during follow-up AI > 2 during follow-up | 9.5% 4.8% 9.5% 19.0% |
| Minakata et al. (2004), USA [14] | n = 54 | Retrospective case series of AV repair for AI, limited raw data presented for BAV | Reoperation during index admission Reoperation during follow-up 5-year reoperation rate | 3.7% 11.1% 9% |
| Moidl et al. (1995), Austria [33] | n = 14 Mean age 30.9 ± 12 years Male 92.9% Preoperative AI grade 3.5 ± 0.1 | Retrospective case series of valve-sparing correction of AI and BAV | Reoperation during index admission | 21.4% |
| Nash et al. (2004), USA [34] | n = 77 Mean age 38 ± 10 years Male 93% | Retrospective case series of the echocardiographic factors that predict successful AV repair in patients with BAV and AI | AVR during index admission Reoperation during index admission Perioperative death Thromboembolism Endocarditis | 2.6% 3.9% 0% 0% 0% |
| Odim et al. (2005), USA [35] | n = 39 | Retrospective case series of AV repair with pericardial leaflet extension | Early mortality 2-year freedom from reoperation | 2.6% 70–90% |
| Pretre et al. (2006), Switzerland [36] | n = 12 Median age 18 years Male 75% | Retrospective case series of AV repair with tricuspidization of the BAV | Postoperative morbidity AR > I | 0% 8.3% |
| Rao et al. (2000), Canada [37] | n = 23 | Retrospective case series of AV repair for multiple pathologies, limited raw data presented for BAV | Composite endpoint of reoperation or AI | No difference between BAV and TAV (P > 0.05) |
| Schafers et al. (2000), Germany [38] | n = 16 Age range 35–73 years Male 75% Aortic dissection 6.3% | Retrospective case series of AV repair and root replacement in patients with BAV, AI and aortic dilatation | In-hospital mortality AV reoperation during follow-up Reoperation during follow-up | 0% 0% 6.3% |
| Schafers et al. (2007), Germany [39] | n = 173 Mean age 48 ± 16 years Male 80.3% | Retrospective cohort study comparing (A) root remodelling, (B) AV repair + supracommissural aortic replacement, (C) AV repair alone | In-hospital mortality TE Endocarditis | (A) 0% (B) 2.6% (C) 1.8% 0% (A) 0% (B) 0% (C) 1.3% |
| Schafers et al. (2010), Germany [40] | n = 153 Mean age 51 ± 12 years Male 86.9% Preoperative AI grade 2.6 ± 0.8 Aortic dissection 3.9% | Retrospective case series of valve-preserving root replacement for AI and BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > I 10-year freedom from reoperation 10-year freedom from AVR TE events Endocarditis 10-year freedom from AV complications | 0.7% 91% 90% 95% 97% 2.6% 0% 91% |
| Veldtman et al. (2006), USA [41] | n = 21 Mean age 45 ± 12 years Male 61.9% Marfan 9.5% AI > I 19.0% | Retrospective case series of aortic root repair or replacement with preservation of the BAV | Perioperative death Late death Reoperation | 0% 4.8% 9.5% |
aWhere studies have included both patients with BAV and TAV repair, only demographics, outcomes and results of patients with BAV are reported.
AI: aortic insufficiency; AS: aortic stenosis; AV: aortic valve; AVR: aortic valve replacement; BAV: bicuspid aortic valve; CCF: congestive cardiac failure; TAV: tricuspid aortic valve; TE: thromboembolism.
| Author, year and country . | Demographicsa . | Study design . | Outcomesa . | Resultsa . |
|---|---|---|---|---|
| Aicher et al. (2004), Germany [15] | n = 60 Male 83.3% Mean age 53 ± 12 years Aortic dissection 6.7% | Retrospective cohort study comparing valve-sparing aortic root replacement in patients with TAV or BAV | In-hospital mortality Postoperative AI grade 5-year overall survival TE events/endocarditis during follow-up Reoperation during follow-up 5-year mean AV gradient 5-year freedom from AI > II 5-year freedom from reoperation | 0% 0.8 ± 0.7 82% 0% 1.7% 4.5 ± 2.3 mmHg 96% 98% |
| Aicher et al. (2007), Germany [16] | n = 81 Male 85.2% Aortic dissection 7.4% AI > II 55.6% Marfan 0% | Retrospective cohort study of aortic root remodelling in patients with BAV, AI and dilatation of the ascending aorta. Comparison made with patients with TAV | Hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0% 94% 87% 97% 99% |
| Aicher et al. (2010), Germany [17] | n = 205 | Retrospective cohort study comparing AV repair in patients with BAV and TAV, limited raw data presented for BAV | 5-year freedom from AI > II 10-year freedom from AI > II | 86% 83% |
| Aicher et al. (2011), Germany [4] | n = 316 Male 84.8% Age range 3–79 years Aortic dissection 8% Severe AI 72.8% | Retrospective case series evaluating the effect of valve configuration on outcomes after repair of BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0.63% 92% 81% 81% 84% |
| Alsoufi et al. (2005), Canada [18] | n = 71 Male 87.3% Mean age 41.5 ± 13.2 years | Retrospective case series of prospectively collected data on AV repair for AI secondary to BAV, aortic remodelling/ replacement included | Hospital mortality Postoperative AI > II 8-year overall survival Freedom from TE/haemorrhage 8-year freedom from reoperation 8-year freedom from endocarditis 8-year freedom from AI > II | 0% 0% 96.7% 100% 82.3% 90% 44.2% |
| Ashikhmina et al. (2010), USA [19] | n = 108 Male 91% Mean age 41 years | Retrospective case series of BAV repair. Patients with valve-sparing aortic root replacements were excluded | In-hospital mortality 10-year overall survival 10-year freedom from reoperation 10-year freedom from AVR | 0% 87% 64% 49% |
| Badiu et al. (2010), Germany [20] | n = 11 Male 100% Mean age 37 ± 15.8 years Aortic dissection 0% Marfan 0% | Retrospective cohort study comparing BAV and TAV repair for AI | Operative mortality 5-year overall survival 5-year freedom from reoperation 5-year freedom from AI 5-year freedom from TE events | 0% 100% 100% 57.1% 95.9% |
| Bakhtiary et al. (2009), Germany [21] | n = 14 Male 92.9% Mean age 58 ± 5 years AI > I 78.6% | Retrospective case series of patients undergoing the modified David procedure for incompetent BAV. | Endocarditis NE Operative death In-hospital death Late death | 0% 0% 0% 0% 0% |
| Boodhwani et al. (2009), Belgium [12] | n = 90 | Retrospective cohort study comparing aortic valve repair for AI in BAV and TAV, limited raw data presented for BAV | Comparison of freedom from AI recurrence between BAV and TAV | P = 0.7 |
| Boodhwani et al. (2010), Belgium [22] | n = 122 Male 92% Mean age 44 ± 11 years AI > II 86.1% | Retrospective case series of patients undergoing surgery for BAV in association with either AI or dilatation of the proximal aorta | In-hospital mortality Discharge AI < II 8-year overall survival 5-year freedom from AI > II 8-year freedom from AV reoperation 8-year freedom from AVR 8-year freedom from TE and bleeding | 0% 93% 97 ± 2% 94 ± 3% 83 ± 5% 90 ± 5% 96 ± 2% |
| Casselman et al. (1999), USA [23] | n = 94 Male 93% Mean age 38 ± 10 years | Retrospective case series of aortic valve repair in patients with BAV and AI | Immediate reoperation Immediate postoperative AI > II 7-year freedom from AV reoperation | 8.5% 2.1% 84% |
| Davierwala et al. (2003), Canada [24] | n = 44 Mean age 39.3 ± 12.1 years Male 93.2% Preoperative CCF 13.6% | Retrospective cohort study of prospectively collected data comparing AV repair to AVR in patients with AI caused by BAV. Procedures on the aorta were included | Operative mortality Late death TE/Haemorrhagic events 5-year freedom from AI > I 5-year freedom from AV reoperation | 0% 0% 0% 79 ± 8% 91 ± 5% |
| de Kerchove et al. (2009), Belgium [25] | n = 54 | Retrospective cohort study comparing the impact of preoperative AI on outcome after aortic valve-sparing surgery, limited raw data presented for BAV | 5-year freedom from AI > II 8-year freedom from AV reoperation | 98 ± 2% 91 ± 9% |
| Delius et al. (1998), USA [26] | n = 16 | Retrospective cohort study of aortic valve repair in patients with either subvalvular or supravalvular aortic stenosis. Comparison made between BAV and TAV | 10-year overall survival 5-year freedom from reoperation 5-year freedom from AVR | 100% 43% 43% |
| Doss et al. (2010), Germany [27] | n = 66 (A) n = 49; (B) n = 17 Mean age 41.2 ± 12 years (A) 58 years; (B) 39 yrs Male 78.8% (A) 82.3%; (B) 77.6% AI > II 95.4% (A) 82.3%; (B) 100% | Retrospective cohort study of (A) patch augmentation plus reduction aortoplasty vs (B) modified David procedure in patients with BAV and AI | 5-year mortality 5-year reoperation 5-year endocarditis 5-year conduction disturbance/thromboembolism/AI > I | (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 0% (B) 0% |
| Fraser et al. (1994), USA [28] | n = 72 Male 94.4% Mean age 39 ± 11 years | Retrospective case series of AV repair for AI secondary to BAV | Operative death Late death 3-year freedom from AV reoperation | 0% 0% 89.5% |
| Kin et al. (2003), Japan [29] | n = 19 Male 98% Mean age 42 ± 17 years | Retrospective case series of AV repair for AI secondary to BAV, some patients underwent concomitant procedures on the aorta | Hospital death Early reoperation Reoperation at follow-up Late death 5-year overall survival 5-year freedom from AV reoperation | 5.2% 5.2% 15.8% 5.2% 90 ± 7% 76 ± 23% |
| Lausberg et al. (2006), Germany [30] | n = 89 n = 34; (B) n = 56 | Retrospective cohort study comparing (A) AV repair alone to (B) AV repair plus aortic root remodelling in patients with AI, limited data for BAV alone | Freedom from significant AI in (A) Freedom from reoperation in (A) | (A) 89.1% (A) 100% |
| Mangini et al. (2010), Italy [31] | n = 31 Mean age 49.9 ± 17.3 years Male 83.9% AI > I 96.8% | Prospective case series of patients undergoing repair of BAV for AI | 30-day operative mortality Discharge AI > I 5-year freedom from reoperation | 3.2% 3.2% 96.6% |
| McMullan et al. (2007), Australia [32] | n = 21 Median age 12.6 years Mean follow-up 36.4 months | Retrospective cohort study of tricuspidisation with cusp extension vs Ross procedure in children with AI or AS associated with BAV | Early reoperation Endocarditis during follow-up AVR during follow-up AI > 2 during follow-up | 9.5% 4.8% 9.5% 19.0% |
| Minakata et al. (2004), USA [14] | n = 54 | Retrospective case series of AV repair for AI, limited raw data presented for BAV | Reoperation during index admission Reoperation during follow-up 5-year reoperation rate | 3.7% 11.1% 9% |
| Moidl et al. (1995), Austria [33] | n = 14 Mean age 30.9 ± 12 years Male 92.9% Preoperative AI grade 3.5 ± 0.1 | Retrospective case series of valve-sparing correction of AI and BAV | Reoperation during index admission | 21.4% |
| Nash et al. (2004), USA [34] | n = 77 Mean age 38 ± 10 years Male 93% | Retrospective case series of the echocardiographic factors that predict successful AV repair in patients with BAV and AI | AVR during index admission Reoperation during index admission Perioperative death Thromboembolism Endocarditis | 2.6% 3.9% 0% 0% 0% |
| Odim et al. (2005), USA [35] | n = 39 | Retrospective case series of AV repair with pericardial leaflet extension | Early mortality 2-year freedom from reoperation | 2.6% 70–90% |
| Pretre et al. (2006), Switzerland [36] | n = 12 Median age 18 years Male 75% | Retrospective case series of AV repair with tricuspidization of the BAV | Postoperative morbidity AR > I | 0% 8.3% |
| Rao et al. (2000), Canada [37] | n = 23 | Retrospective case series of AV repair for multiple pathologies, limited raw data presented for BAV | Composite endpoint of reoperation or AI | No difference between BAV and TAV (P > 0.05) |
| Schafers et al. (2000), Germany [38] | n = 16 Age range 35–73 years Male 75% Aortic dissection 6.3% | Retrospective case series of AV repair and root replacement in patients with BAV, AI and aortic dilatation | In-hospital mortality AV reoperation during follow-up Reoperation during follow-up | 0% 0% 6.3% |
| Schafers et al. (2007), Germany [39] | n = 173 Mean age 48 ± 16 years Male 80.3% | Retrospective cohort study comparing (A) root remodelling, (B) AV repair + supracommissural aortic replacement, (C) AV repair alone | In-hospital mortality TE Endocarditis | (A) 0% (B) 2.6% (C) 1.8% 0% (A) 0% (B) 0% (C) 1.3% |
| Schafers et al. (2010), Germany [40] | n = 153 Mean age 51 ± 12 years Male 86.9% Preoperative AI grade 2.6 ± 0.8 Aortic dissection 3.9% | Retrospective case series of valve-preserving root replacement for AI and BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > I 10-year freedom from reoperation 10-year freedom from AVR TE events Endocarditis 10-year freedom from AV complications | 0.7% 91% 90% 95% 97% 2.6% 0% 91% |
| Veldtman et al. (2006), USA [41] | n = 21 Mean age 45 ± 12 years Male 61.9% Marfan 9.5% AI > I 19.0% | Retrospective case series of aortic root repair or replacement with preservation of the BAV | Perioperative death Late death Reoperation | 0% 4.8% 9.5% |
| Author, year and country . | Demographicsa . | Study design . | Outcomesa . | Resultsa . |
|---|---|---|---|---|
| Aicher et al. (2004), Germany [15] | n = 60 Male 83.3% Mean age 53 ± 12 years Aortic dissection 6.7% | Retrospective cohort study comparing valve-sparing aortic root replacement in patients with TAV or BAV | In-hospital mortality Postoperative AI grade 5-year overall survival TE events/endocarditis during follow-up Reoperation during follow-up 5-year mean AV gradient 5-year freedom from AI > II 5-year freedom from reoperation | 0% 0.8 ± 0.7 82% 0% 1.7% 4.5 ± 2.3 mmHg 96% 98% |
| Aicher et al. (2007), Germany [16] | n = 81 Male 85.2% Aortic dissection 7.4% AI > II 55.6% Marfan 0% | Retrospective cohort study of aortic root remodelling in patients with BAV, AI and dilatation of the ascending aorta. Comparison made with patients with TAV | Hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0% 94% 87% 97% 99% |
| Aicher et al. (2010), Germany [17] | n = 205 | Retrospective cohort study comparing AV repair in patients with BAV and TAV, limited raw data presented for BAV | 5-year freedom from AI > II 10-year freedom from AI > II | 86% 83% |
| Aicher et al. (2011), Germany [4] | n = 316 Male 84.8% Age range 3–79 years Aortic dissection 8% Severe AI 72.8% | Retrospective case series evaluating the effect of valve configuration on outcomes after repair of BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > II 10-year freedom from reoperation 10-year freedom from AVR | 0.63% 92% 81% 81% 84% |
| Alsoufi et al. (2005), Canada [18] | n = 71 Male 87.3% Mean age 41.5 ± 13.2 years | Retrospective case series of prospectively collected data on AV repair for AI secondary to BAV, aortic remodelling/ replacement included | Hospital mortality Postoperative AI > II 8-year overall survival Freedom from TE/haemorrhage 8-year freedom from reoperation 8-year freedom from endocarditis 8-year freedom from AI > II | 0% 0% 96.7% 100% 82.3% 90% 44.2% |
| Ashikhmina et al. (2010), USA [19] | n = 108 Male 91% Mean age 41 years | Retrospective case series of BAV repair. Patients with valve-sparing aortic root replacements were excluded | In-hospital mortality 10-year overall survival 10-year freedom from reoperation 10-year freedom from AVR | 0% 87% 64% 49% |
| Badiu et al. (2010), Germany [20] | n = 11 Male 100% Mean age 37 ± 15.8 years Aortic dissection 0% Marfan 0% | Retrospective cohort study comparing BAV and TAV repair for AI | Operative mortality 5-year overall survival 5-year freedom from reoperation 5-year freedom from AI 5-year freedom from TE events | 0% 100% 100% 57.1% 95.9% |
| Bakhtiary et al. (2009), Germany [21] | n = 14 Male 92.9% Mean age 58 ± 5 years AI > I 78.6% | Retrospective case series of patients undergoing the modified David procedure for incompetent BAV. | Endocarditis NE Operative death In-hospital death Late death | 0% 0% 0% 0% 0% |
| Boodhwani et al. (2009), Belgium [12] | n = 90 | Retrospective cohort study comparing aortic valve repair for AI in BAV and TAV, limited raw data presented for BAV | Comparison of freedom from AI recurrence between BAV and TAV | P = 0.7 |
| Boodhwani et al. (2010), Belgium [22] | n = 122 Male 92% Mean age 44 ± 11 years AI > II 86.1% | Retrospective case series of patients undergoing surgery for BAV in association with either AI or dilatation of the proximal aorta | In-hospital mortality Discharge AI < II 8-year overall survival 5-year freedom from AI > II 8-year freedom from AV reoperation 8-year freedom from AVR 8-year freedom from TE and bleeding | 0% 93% 97 ± 2% 94 ± 3% 83 ± 5% 90 ± 5% 96 ± 2% |
| Casselman et al. (1999), USA [23] | n = 94 Male 93% Mean age 38 ± 10 years | Retrospective case series of aortic valve repair in patients with BAV and AI | Immediate reoperation Immediate postoperative AI > II 7-year freedom from AV reoperation | 8.5% 2.1% 84% |
| Davierwala et al. (2003), Canada [24] | n = 44 Mean age 39.3 ± 12.1 years Male 93.2% Preoperative CCF 13.6% | Retrospective cohort study of prospectively collected data comparing AV repair to AVR in patients with AI caused by BAV. Procedures on the aorta were included | Operative mortality Late death TE/Haemorrhagic events 5-year freedom from AI > I 5-year freedom from AV reoperation | 0% 0% 0% 79 ± 8% 91 ± 5% |
| de Kerchove et al. (2009), Belgium [25] | n = 54 | Retrospective cohort study comparing the impact of preoperative AI on outcome after aortic valve-sparing surgery, limited raw data presented for BAV | 5-year freedom from AI > II 8-year freedom from AV reoperation | 98 ± 2% 91 ± 9% |
| Delius et al. (1998), USA [26] | n = 16 | Retrospective cohort study of aortic valve repair in patients with either subvalvular or supravalvular aortic stenosis. Comparison made between BAV and TAV | 10-year overall survival 5-year freedom from reoperation 5-year freedom from AVR | 100% 43% 43% |
| Doss et al. (2010), Germany [27] | n = 66 (A) n = 49; (B) n = 17 Mean age 41.2 ± 12 years (A) 58 years; (B) 39 yrs Male 78.8% (A) 82.3%; (B) 77.6% AI > II 95.4% (A) 82.3%; (B) 100% | Retrospective cohort study of (A) patch augmentation plus reduction aortoplasty vs (B) modified David procedure in patients with BAV and AI | 5-year mortality 5-year reoperation 5-year endocarditis 5-year conduction disturbance/thromboembolism/AI > I | (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 2.0% (B) 0% (A) 0% (B) 0% |
| Fraser et al. (1994), USA [28] | n = 72 Male 94.4% Mean age 39 ± 11 years | Retrospective case series of AV repair for AI secondary to BAV | Operative death Late death 3-year freedom from AV reoperation | 0% 0% 89.5% |
| Kin et al. (2003), Japan [29] | n = 19 Male 98% Mean age 42 ± 17 years | Retrospective case series of AV repair for AI secondary to BAV, some patients underwent concomitant procedures on the aorta | Hospital death Early reoperation Reoperation at follow-up Late death 5-year overall survival 5-year freedom from AV reoperation | 5.2% 5.2% 15.8% 5.2% 90 ± 7% 76 ± 23% |
| Lausberg et al. (2006), Germany [30] | n = 89 n = 34; (B) n = 56 | Retrospective cohort study comparing (A) AV repair alone to (B) AV repair plus aortic root remodelling in patients with AI, limited data for BAV alone | Freedom from significant AI in (A) Freedom from reoperation in (A) | (A) 89.1% (A) 100% |
| Mangini et al. (2010), Italy [31] | n = 31 Mean age 49.9 ± 17.3 years Male 83.9% AI > I 96.8% | Prospective case series of patients undergoing repair of BAV for AI | 30-day operative mortality Discharge AI > I 5-year freedom from reoperation | 3.2% 3.2% 96.6% |
| McMullan et al. (2007), Australia [32] | n = 21 Median age 12.6 years Mean follow-up 36.4 months | Retrospective cohort study of tricuspidisation with cusp extension vs Ross procedure in children with AI or AS associated with BAV | Early reoperation Endocarditis during follow-up AVR during follow-up AI > 2 during follow-up | 9.5% 4.8% 9.5% 19.0% |
| Minakata et al. (2004), USA [14] | n = 54 | Retrospective case series of AV repair for AI, limited raw data presented for BAV | Reoperation during index admission Reoperation during follow-up 5-year reoperation rate | 3.7% 11.1% 9% |
| Moidl et al. (1995), Austria [33] | n = 14 Mean age 30.9 ± 12 years Male 92.9% Preoperative AI grade 3.5 ± 0.1 | Retrospective case series of valve-sparing correction of AI and BAV | Reoperation during index admission | 21.4% |
| Nash et al. (2004), USA [34] | n = 77 Mean age 38 ± 10 years Male 93% | Retrospective case series of the echocardiographic factors that predict successful AV repair in patients with BAV and AI | AVR during index admission Reoperation during index admission Perioperative death Thromboembolism Endocarditis | 2.6% 3.9% 0% 0% 0% |
| Odim et al. (2005), USA [35] | n = 39 | Retrospective case series of AV repair with pericardial leaflet extension | Early mortality 2-year freedom from reoperation | 2.6% 70–90% |
| Pretre et al. (2006), Switzerland [36] | n = 12 Median age 18 years Male 75% | Retrospective case series of AV repair with tricuspidization of the BAV | Postoperative morbidity AR > I | 0% 8.3% |
| Rao et al. (2000), Canada [37] | n = 23 | Retrospective case series of AV repair for multiple pathologies, limited raw data presented for BAV | Composite endpoint of reoperation or AI | No difference between BAV and TAV (P > 0.05) |
| Schafers et al. (2000), Germany [38] | n = 16 Age range 35–73 years Male 75% Aortic dissection 6.3% | Retrospective case series of AV repair and root replacement in patients with BAV, AI and aortic dilatation | In-hospital mortality AV reoperation during follow-up Reoperation during follow-up | 0% 0% 6.3% |
| Schafers et al. (2007), Germany [39] | n = 173 Mean age 48 ± 16 years Male 80.3% | Retrospective cohort study comparing (A) root remodelling, (B) AV repair + supracommissural aortic replacement, (C) AV repair alone | In-hospital mortality TE Endocarditis | (A) 0% (B) 2.6% (C) 1.8% 0% (A) 0% (B) 0% (C) 1.3% |
| Schafers et al. (2010), Germany [40] | n = 153 Mean age 51 ± 12 years Male 86.9% Preoperative AI grade 2.6 ± 0.8 Aortic dissection 3.9% | Retrospective case series of valve-preserving root replacement for AI and BAV | In-hospital mortality 10-year overall survival 10-year freedom from AI > I 10-year freedom from reoperation 10-year freedom from AVR TE events Endocarditis 10-year freedom from AV complications | 0.7% 91% 90% 95% 97% 2.6% 0% 91% |
| Veldtman et al. (2006), USA [41] | n = 21 Mean age 45 ± 12 years Male 61.9% Marfan 9.5% AI > I 19.0% | Retrospective case series of aortic root repair or replacement with preservation of the BAV | Perioperative death Late death Reoperation | 0% 4.8% 9.5% |
aWhere studies have included both patients with BAV and TAV repair, only demographics, outcomes and results of patients with BAV are reported.
AI: aortic insufficiency; AS: aortic stenosis; AV: aortic valve; AVR: aortic valve replacement; BAV: bicuspid aortic valve; CCF: congestive cardiac failure; TAV: tricuspid aortic valve; TE: thromboembolism.
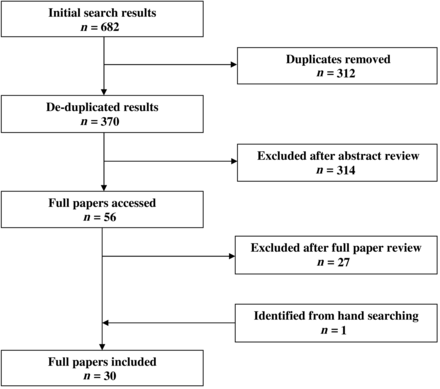
PRISMA diagram of studies considered for the systematic review.
Outcome reporting standard
A total of 163 different outcomes for valve-preserving BAV surgery were reported on 280 occasions in the included studies. The median number of outcomes reported in each study was 8.5 (range 1–22). The majority of endpoints were reported in only one study (n = 127, 77.9%). The most frequently reported endpoints were cardiopulmonary bypass time (CPB, n = 16, 53.3%), aortic cross clamp time (AXC, n = 16, 53.3%), 5-year freedom from reoperation (n = 9, 30.0%), 5-year overall survival (n = 7, 23.3%) and in-hospital mortality (n = 7, 23.3%). The timing of outcome assessment was recorded precisely on 152 occasions (54.3%).
Perioperative outcomes
Mortality in the early postoperative period was reported in 25 papers [4, 14–22, 24–26, 28–31, 34, 35, 37–41], although only 18 presented the results independently for patients with BAV [15–22, 24, 28, 29, 31, 34, 35, 38–41]. Nine different terms were used to describe mortality, including ‘in-hospital mortality’, ‘in-hospital death’, ‘hospital mortality’, ‘hospital death’, ‘operative mortality’, ‘operative death’, ‘perioperative death’, ‘early mortality’ and ‘30-day operative mortality’. Mortality rates in patients undergoing BAV procedures were generally good and ranged from 0 to 5.2% with a median of 0%. However, 5 studies failed to report any mortality outcomes [23, 27, 32, 33, 36]. In addition, just one study defined precisely the time point at which early postoperative mortality was measured [31].
Overall, 24 of the included publications reported the presence or absence of early complications and 18 of these explicitly described operative morbidity in patients with BAV. Six studies did not document whether or not any complications of surgery occurred [4, 19, 26, 35, 37, 41]. Early reoperation for bleeding was documented in 14 studies and the incidence ranged from 0 to 21.4% [18, 21–23, 27–29, 32–34, 38–40]. The incidence of early stroke or other neurological events was 0–6.3% and was described in four studies [18, 21, 22, 38]. Arrhythmia is a common complication of valve surgery, yet only 4 studies recorded its presence, with an incidence of between 0 and 25.0% [28, 36, 38, 39]. Permanent pacemaker insertion was reported in 2 articles and was necessary in 0.7–3.0% of patients [22, 40]. Complications reported in one study included early IE (0%), pulmonary embolism (2.3%), pericardial effusion (6.8%), atrial fibrillation (21.0%), myocardial infarction (0%) and prolonged ventilation (0%). Two articles also documented the incidence of ‘operative complications’, which ranged from 0 to 9%, although the exact nature of the complications was not specified [20, 36].
Sixteen studies reported the duration of CPB and AXC: mean pump times varied from 51 to 205 min and mean AXC values ranged from 38 to 168 min. Six articles described the mean length of hospital stay, and this varied from 5.5 to 13 days [24, 27, 28, 31, 36, 38]. The length of time spent on the intensive care unit (mean duration range from 18 h to 3 days) was additionally reported in 3 studies [21, 24, 31].
Long-term morbidity and mortality
Twenty-five publications provided at least one measure of medium or long-term survival [4, 14–29, 32, 35, 37–41]. Fifteen of these presented data exclusively for patients undergoing valve-preserving BAV surgery [4, 15, 16, 18–22, 24, 26–29, 40, 41]. Overall survival was measured at a range of intervals, including at 1, 3, 5, 8 and 10 years, while five publications reported ‘late death’, although the time point at which this was measured was not clearly defined [21, 24, 28, 29, 41]. Overall survival rates at 1, 3, 5, 8 and 10 years were 93–99% (n = 2 publications), 87% (n = 1 publication), 82–100% (n = 7 publications), 97% (n = 2 publications) and 87–100% (n = 5 publications), respectively. The incidence of ‘late death’ varied from 0 to 5.2%. One study reported a 5-year mortality rate of between 0 and 2% [27].
The incidence of thromboembolic events during the follow-up was recorded in seven studies [15, 18, 20, 22, 24, 27, 40]. Four of these did not demonstrate any cases of late thromboembolism [15, 18, 24, 27], while 3 reported incidences of between 2.6 and 4.1% [20, 22, 40]. Badiu et al. [20] also reported that freedom from neurological events was 94% at 5 years postoperatively. The incidence of postoperative IE of the BAV was described in five publications and ranged from 0 to 10% [15, 18, 27, 32, 40], although only 2 studies described precisely the timing of the assessment of this outcome. Alsoufi et al. [18] reported 90% freedom from IE at 8 years, while Doss et al. [27] described a 2% incidence of IE at 5 years following surgery. Major haemorrhage was described in three of the included articles. Freedom from haemorrhagic complications was between 96 and 100% at time intervals of up to 8 years [18, 22, 24]. Doss et al. [27] reported that 0% of patients had conduction disturbance 5 years post surgery.
The rate of reoperation was reported in 22 of the included publications [4, 14–16, 18–20, 22–32, 35, 38, 40, 41]. The overall reoperation rate for patients with BAV varied from 0 to 15.8% during the follow-up period. Freedom from cardiac reoperation was measured at 1, 2, 3, 5, 8 and 10 years, with the following respective results: 96.8–98% (n = 2 publications), 70–90% (n = 1 publication), 98% (n = 1 publication), 43–100% (n = 9 publications), 82.3% (n = 1 publication) and 49–99% (n = 4 publications) [4, 15, 16, 18–20, 26, 31, 35, 40]. Freedom from reoperation on the AV (redo repair and/or replacement) was 94–95%, 89.5%, 87–89.5%, 76–94%, 84, 91 and 83% at 1, 2, 3, 5, 7, 8 and 10 years postprimary surgery, respectively [22–25, 28, 29]. Freedom from AVR was also described in six articles. This was reported as 96, 43–99, 90 and 49–99% at 1, 5, 8 and 10 years, respectively [4, 16, 19, 22, 26, 40].
Echocardiographic and functional outcomes
The degree of AI in the immediate postoperative period was reported in 11 publications [3–18, 18–24, 28, 29, 31, 33, 36, 38, 41]. The proportion of patients with AI grade >1 was between 0 and 8.3% [22, 24, 31, 36, 38, 41], while AI grade > 2 was less frequently observed, with an incidence of between 0 and 2.1% [18, 23]. The mean early postoperative AI grade was noted in 3 studies to be between 0.39 and 1.1 [28, 29, 33]. Sixteen articles reported the degree of AI present in the medium or long-term follow-up period [4, 15–18, 20, 22, 24, 25, 27–30, 32, 33, 40]. This was recorded in a variety of ways. Freedom from AI > 1 was 85%, 79–100 and 90% at 1, 5 and 10 years, respectively [24, 27, 40]. At 1, 3, 5, 8 and 10 years after surgery, the proportion of patients with freedom from AI > 2 was reported as 94.7–98%, 96%, 71–98%, 44.2 and 81–87%, respectively [4, 15–18, 22, 25]. Badiu et al. [20] described freedom from any grade of AI of 57.1% at 5 years following surgery, while Lausberg et al. [30] reported 89.1% freedom from significant AI, although it is unclear how this outcome was defined. The mean follow-up AI grade was reported in three studies and varied from 0.89 to 1.8 [28, 29, 33]. In addition, Boodhwani et al. [12] found no difference in the rate of recurrence of AI in patients with TAV and BAV. In a study by Rao et al. [37], a composite endpoint of reoperation and/or AI was measured, and again no differences were found between patients with TAV or BAV.
Ten publications reported the gradient across the AV in the early postoperative period [15, 21, 24, 26, 28, 31, 32, 34, 38, 41]. The mean AV gradient varied from 4.7 to 16 mmHg [15, 21, 28, 31, 34, 38, 41], while the peak AV gradient ranged from 16.2 to 28.7 mmHg [24, 26, 31, 34]. McMullan et al. [14] noted that 14.3% of patients had an early postoperative peak AV gradient of >20 mmHg. Four articles reported medium- to long-term AV gradients [15, 18, 24, 27]. Values for the mean AV gradient were between 3.8 and 9.6 mmHg [15, 18, 27], while peak gradients ranged from 7.6 to 21 mmHg up to 5 years after initial surgery [18, 24, 27]. The follow-up effective orifice area of the AV was reported in 2 studies with figures of 1.86–2.9 cm2 [18, 27].
The effects of BAV-sparing surgery on left ventricular ejection fraction (LVEF) were commented on in four publications [18, 27, 29, 34]. Mean LVEF at hospital discharge was 50–55.8% [29, 34], and this was maintained throughout the follow-up. Values for the follow-up LVEF varied from 54.8–61.40% [27, 29], while Alsoufi et al. [18] demonstrated that 5.6% of patients had LVEF < 40% at the final follow-up. Left ventricular end-diastolic diameter (LVEDD) was 53.7–61.6 mm and decreased over time to values of 52.8–55 mm at final follow-up [29, 31, 33]. Left ventricular end-systolic diameter (LVESD) also decreased with time from 40.5–46.3 to 37.2–39.2 mm over the follow-up period [29, 33]. Five articles measured the postoperative NYHA classification of BAV patients [18, 22, 24, 28, 32]. Boodhwani et al. [22] reported that on discharge from hospital, 85% of patients were NYHA class I, 14% NYHA class II and 1% NYHA class III. In addition, Fraser et al. [28] demonstrated that 6% of patients were NYHA class II or worse at discharge. At final follow-up, 2 studies noted that 4.5–5.0% of patients were NYHA class III or IV [18, 24], although McMullan et al. [32] documented that all patients were asymptomatic (NYHA class I).
DISCUSSION
AI and AS are frequent complications of BAV anatomy. The proximal aorta is also prone to dilatation, which can result in fatal rupture or dissection. Surgical interventions aimed at correcting these sequelae include AVR, the Bentall operation, the Ross procedure and AV repair with or without concomitant aortic procedures (Fig. 2). AVR has proven effectiveness, although mechanical valves require life-long anticoagulation and bio-prosthetic implants are prone to degeneration requiring reoperation. The Ross procedure is used mainly in young patients with predominant stenosis. However, progressive autograft dilatation has been shown to occur in patients with previous BAV [42]. Although previously, AV repair has not been implemented as widely as reparative procedures on the mitral or tricuspid valves, it has emerged as a viable therapeutic alternative for patients with complicated BAV. In order to ensure comprehensive uptake of AV repair, it is first necessary to demonstrate that operative techniques are feasible and reproducible and have acceptable efficacy, safety and durability. This systematic review has summarized all available outcomes for valve-preserving surgery on the native BAV and has demonstrated that rates of perioperative morbidity and mortality are low, with excellent long-term survival and satisfactory freedom from reoperation. Echocardiographic and functional endpoints have also been shown to be acceptable both immediately after surgery and throughout follow-up.
Although it is beyond the scope of this review to systematically compare outcomes of BAV repair with those of AVR or pulmonary autografts, it is useful to put some of the major findings into context. Large series of patients undergoing AVR have reported 5-, 8- and 13-year overall survival rates of 80–88%, 70–81% and 31–53%, respectively [43–47]. These figures seem worse than those identified in the present review, although patients treated with AVR tend to be older and have more comorbidities [48]. Overall survival was 82% at the 16-year follow-up in a large cohort of patients undergoing the Ross procedure (median age 24 years) [49]. Freedom from reoperation in patients treated with bio-prosthetic AVR has been shown to be 96%, 90–99% and 96% at 5, 8 and 10 years, respectively, while mechanical AVR prostheses have even greater durability [43–47]. The 5- and 7-year freedom from reoperation for pulmonary autograft failure are 93 and 91% [49, 50]. In patients requiring concomitant aortic surgery, valve-sparing surgery may be a reason for a lower freedom from reoperation compared with aortic root replacement. These findings may give an impression that valve-sparing BAV surgery is less durable than either AVR or the Ross procedure. However, it must be borne in mind that AV repair is still in its infancy in terms of operator experience at many centres and reoperation figures are likely to improve considerably with time. Hence, we strongly advocate that valve-sparing surgery in BAV should exclusively be performed in most experienced centres having a large aortic valve-sparing experience. Based on the results of our review, it is necessary to fully inform the patient, including making them aware of the longer operative time and length of hospital stay and that it might be a safe alternative to valve replacement in experienced high-volume centres.
Another potential benefit of AV repair is that it leads to fewer incidents of thromboembolism, IE and major haemorrhage. Although there is some evidence to support this [12–14], a review by Carr et al. [48] found that there was little difference between AV repair, AVR and pulmonary autograft for these three major complications. Further work is required to understand better the relative safety of different aortic valve interventions, and this should ideally be accomplished through a systematic review and meta-analysis.
Our group advocates a systematic approach to BAV repair that addresses both cusp and ascending aortic pathology and is tailored to the specific anatomy encountered. We have demonstrated that BAV repair is feasible, patients have excellent long-term survival and repair durability remains acceptable at mid-term with excellent freedom from late AV reoperation and recurrent AI [12]. We have also shown that in the context of valve-sparing surgery for BAV, root replacement is associated with less AI recurrence compared with sub-commissural annuloplasty with or without ascending aortic replacement and the use of a pericardial patch for raphe repair is associated with increased recurrent AI [22]. Our approach is based on the principle that BAV repair needs to address the cusps, functional aortic annulus and the ascending aorta as one functional unit. Root replacement in this setting is performed not only to prevent the potentially fatal complications of aortic dissection and rupture but also to stabilize the repair procedure. Ongoing dilatation of the ventriculo-aortic junction (VAJ) or the sino-tubular junction can induce recurrent AI and render the cusp repair ineffective over the long-term. Thus, if a BAV repair is to be attempted, an aggressive stance should be taken towards root replacement. We replaced the aortic root based on size criteria and a visual assessment of the quality of the tissue of the aortic wall. All patients with an aortic root size of >4.5 cm and some patients with an aortic root diameter of <4.5 cm with fragile aortic wall tissue undergo root replacement at our centre. Another area of controversy relates to the use of a pericardial patch for valve repair. In the adult patient, a patch may be used for two reasons. First, for cusp restoration after the resection of a restrictive or calcified raphe. Secondly, to perform pericardial cusp extension to help increase the coaptation surface of the conjoint cusp by adding tissue to its free margin. The pericardium, however, may become fibrotic or calcified with time, leading to insufficiency, or the fact that these are complex valves which require challenging repairs makes them more prone to failure. The objective of cusp repair should be to attain uniform cusp coaptation at approximately the mid-height of the sinuses of Valsalva. It has been shown that the VAJ can dilate years after sub-commissural annuloplasty. To this effect, we have demonstrated that valve-sparing root replacement using the reimplantation technique can significantly increase the durability of BAV repair without additional morbidity when compared with less-aggressive annuloplasty. This leads to lower immediate postoperative transvalvular gradients when compared with the other techniques. Despite criticisms of the non-physiological cusp motion and potential cusp damage caused by the elimination of the Valsalva sinuses, the reimplantation technique is associated with excellent valve durability and long-term outcomes and the combination of the type of graft and the techniques used actually can recreate the Valsalva sinuses shape and improve cusp motion. In patients with recurrent AI requiring surgery after BAV repair, the choice of rerepair vs replacement is not simply a technical surgical decision, but also takes into account patient age and co-morbidities and most importantly, the patients' wishes regarding another attempt at repair. Since our understanding of the mechanism of failure in these patients has improved, we increasingly offer rerepair using the reimplantation technique to selected young patients.
The current review has also evaluated the standard of outcome reporting in studies of BAV surgery by considering the proportion of endpoints that were measured at clearly defined time points. Almost half of the endpoints reported (45.7%) did not provide sufficient information for the reader to ascertain the timing of outcome assessment. This makes it extremely difficult to combine data from multiple studies in systematic reviews and meta-analyses. To illustrate this problem, ‘late death’ was reported by 5 studies in this review and none of these defined precisely when it was measured. The incidence of ‘late death’ also varied between 0 and 5.2%. ‘Late death’ of 0% at 10 years postoperatively is a considerably better outcome than a 5.2% chance of death at 1 year. In addition, overall survival was reported at 1, 3, 5, 8 and 10 years, with different studies providing data at each time point. Heterogeneity in the timing of outcome assessment is a significant hindrance to data assimilation, but is not the only problem with endpoint evaluation. Different outcomes are invariably measured in studies of the same disease, as emphasized by the finding that 163 unique endpoints were reported in the publications included in this review. In addition, outcomes with the same name may not be defined in the same way or even defined at all. Although it was beyond the scope of this review to consider comprehensively endpoint definitions, it is interesting to note that there were 9 different terms used to describe early mortality after surgery. A final problem is outcome-reporting bias, which occurs when individual endpoints are reported selectively on the basis of the results, usually in favour of those that are significant statistically or against those that are negative. It is interesting to note that 5 articles included in this review (16.7%) failed to report any measure of perioperative mortality and 6 did not state whether complications occurred (20.0%). Although it is impossible to speculate about morbidity and mortality in these series or the reasons for their omission, it provides a useful illustration of how missing data might affect the quality of evidence synthesis in systematic reviews. Many of these problems can be partially overcome through the use of core outcome sets, which comprise agreed sets of standardized endpoints reported, as a minimum, in all studies of a given condition [51]. Although attempts have been made to standardize definitions of perioperative mortality in cardiac surgery [52], little work has considered other important endpoints, such as morbidity, reoperation or quality of life.
It is worthwhile considering that all of the publications included in this review were either cohort studies or case series and the vast majority was retrospective (96.7%). No randomized trials (RCTs) or prospective observational studies were identified comparing AV repair with either AVR or the Ross procedure. Given the growing body of evidence supporting the use of valve-sparing surgery as a viable alternative to valve-replacement surgery in a select cohort of patients with BAV, further well-designed prospective comparative studies are warranted. These may be difficult to undertake, not least because of the proven efficacy of valve replacement, a lack of equipoise amongst clinicians and problems with communicating trials and randomization to patients. However, the recent success of the PARTNER trial should encourage researchers to embark on RCTs in cardiac surgery. Further work is also warranted to improve the quality of outcome reporting in studies of AV surgery, possibly through the development of a core outcome set. This will improve the reliability and validity of the evidence synthesized in meta-analyses. Another striking feature of this review is that no patient-reported outcomes (PROs) were measured. Understanding the impact of different surgical procedures on quality of life and satisfaction with care from the patients' perspective is invaluable for shared decision-making when a range of treatment options exists. Further work embedding PROs into prospective studies of AV repair would be beneficial.
This review should be interpreted in light of its limitations. First, it did not systematically consider the evidence for AVR or pulmonary autografts in patients with BAV, which would have permitted a meta-analysis. However, it is the first systematic review to assess outcomes of valve-preserving surgery on the BAV. Another best-evidence review has compared aortic remodelling and reimplantation, although studies of TAV were also included and it was not conducted systematically [53]. A second limitation is that the review was restricted to articles indexed in MEDLINE and EMBASE and could have been expanded to include other medical databases and ‘grey’ literature, although it is unlikely that enough additional articles would have been identified to significantly affect the findings.
In conclusion, the present review has shown that valve-preserving surgery on the diseased BAV is feasible, safe and effective in both the short- and long-term. Some concerns exist regarding the durability of repairs and the need for reoperation, although it is likely that these outcomes will improve as more cardiac surgeons become more adept at performing AV repair. The review has also highlighted concerns about the standard of outcome reporting in studies of AV surgery and offers a partial solution. With such favourable outcomes, it is likely that the use of BAV repair will increase. Although the review goes some way to understanding the current evidence base, further prospective studies are warranted.
SUPPLEMENTARY MATERIAL
Supplementary material is available at EJCTS online.
Conflict of interest: none declared.




