-
PDF
- Split View
-
Views
-
Cite
Cite
Tetsuya Mizuno, Tetsuo Taniguchi, Yoshinori Ishikawa, Koji Kawaguchi, Takayuki Fukui, Futoshi Ishiguro, Shota Nakamura, Kohei Yokoi, Pulmonary metastasectomy for osteogenic and soft tissue sarcoma: who really benefits from surgical treatment?, European Journal of Cardio-Thoracic Surgery, Volume 43, Issue 4, April 2013, Pages 795–799, https://doi.org/10.1093/ejcts/ezs419
Close - Share Icon Share
Abstract
Surgical resection is widely accepted as a beneficial treatment of pulmonary metastases originating from osteogenic and soft tissue sarcomas despite adequate validation. The factors associated with the selection of patients who receive pulmonary metastasectomy (PM) are controversial and not well known. In this study, we aimed to identify the prognostic factors associated with survival after treatment with PM and to disclose the candidates who profit from PM being performed on patients with osteogenic and soft tissue sarcomas.
We retrospectively reviewed the variables and survival outcomes in 52 consecutive patients who underwent PM to treat lung metastases originating from osteogenic and soft tissue malignancies from April 1996 to January 2011. Prognostic factors associated with overall survival after the first PM were evaluated using univariate and multivariate analyses.
Fifty-eight PM procedures were performed in 52 patients as the first PM including 6 bilateral diseases. Wedge resection was the most frequently performed PM procedure (84%), and video-assisted thoracic surgery was introduced in 34 (59%). The median follow-up of the patients was 33 months and the 5-year survival rate after the first PM was 50.9%. Forty-eight (92%) patients underwent complete resection during the first PM. Thirty-three patients (62%) experienced relapse after the first PM. Among those patients, 20 received redo surgeries for pulmonary relapse, and the 5-year survival rate in this group was 49.7%. According to univariate analyses, the use of complete resection, the number of metastatic nodules (one or two) and the length of the disease-free interval prior to the first PM were each found to be significant favourable factors. According to a multivariate analysis, the use of complete resection and the number of metastatic nodules were both found to be independent prognostic factors associated with overall survival. Although our cohort included 15 patients with poor prognostic factors (29%), 5 patients who underwent redo surgery survived >22 months.
The survival of those patients with one or two pulmonary nodules and those who underwent complete resection was favourable following the treatment of osteogenic and soft tissue sarcomas with PM. Redo surgery may also provide some survival benefit in patients with poor prognostic factors.
INTRODUCTION
The lungs are one of the most common organs targeted by metastatic malignancies. It is widely believed that malignant diseases with pulmonary metastases are so advanced that the prognoses of patients are unfavourable. In cases of orthopaedic sarcoma, especially those originating in the extremities, it has been reported that ∼20% relapse in the lungs [1, 2]. Patients with osteogenic and soft tissue sarcomas sometimes develop metastatic nodules in peripheral lung areas, which cannot be sufficiently treated with therapies other than surgical resection. Because of surgical accessibility concerns and drug-resistant characteristics in the selected population, pulmonary metastasectomy (PM) has come to be accepted as a potentially effective treatment.
Although a number of retrospective analyses have reported the outcomes and efficacy of PM, the true benefits of this treatment have not been disclosed due to the heterogeneity of the disease. Others have reported 5-year survival rates of 15–52% and have suggested that the number of nodules, the length of the disease-free interval (DFI) prior to PM, the histology of the primary tumour and the size of the pulmonary nodule are each prognostic factors associated with survival outcomes [3–5]. Controversies still remain among the reports regarding these prognostic factors. In addition, we have acknowledged that ∼40% of patients who underwent PM to treat osteogenic and soft tissue sarcomas experienced relapses in the lungs. Treatment with repeated PM has also recently been introduced without an adequate investigation of its efficacy.
We review here our experience with PMs performed for osteogenic and soft tissue sarcomas and aim to investigate the prognostic factors associated with the selection of patients.
PATIENTS AND METHODS
Patients
From April 1996 to January 2011, 52 consecutive patients underwent surgical resection at Nagoya University Hospital to treat pulmonary metastases originating from orthopaedic sarcomas. In all patients, the primary tumours were pathologically diagnosed prior to pulmonary resection. However, preoperative diagnoses of the pulmonary nodules were made based on the radiological findings of chest computed tomography (CT).
Whether or not surgery was deemed to be indicated was determined based on the background, respiratory function, the length of the DFI after treatment of the primary site, number of pulmonary nodules and location of the tumour in the lungs of each patient. In all 52 patients, the primary and distant sites, except for the lungs, were controlled, and PMs were performed with the intention of completing radical resections.
Investigated variables
The data regarding gender, age, histology and sites of the primary tumours were reviewed as variables for the patient characteristics. Furthermore, the following six clinical variables were investigated for each patient: the length of the pre-PM DFI before the first PM (pre-DFI), the laterality of the lung nodules, the type of PM procedure, the number of metastatic pulmonary nodules, the completeness of PM, the frequency of PM, the length of the post-PM DFI after PM (post-DFI) and the prognosis (survival, recurrence). In the univariate analysis of the number of metastatic pulmonary nodules, the patients were classified into two groups: patients with one or two nodules, and patients with three or more nodules.
Statistical analyses
The overall survival rate of each patient was measured from the date of the first PM until either death or the last day of the follow-up. The length of the DFI (pre-DFI) was calculated from the day of treatment of the primary tumour to the day of the first PM. Survival curves were created using the Kaplan–Meier method. Univariate analyses were performed using the log-rank test based on the Kaplan–Meier method to assess the prognostic significance of the individual factors. A multivariate analysis was performed on the significant variables of the univariate analyses using the Cox regression hazard model to access the independent prognostic values of the potential factors. These statistical analyses were completed using the SPSS software for Windows (version 12.0; Chicago, IL, USA). In both univariate and multivariate analyses, P < 0.05 was considered to be significant.
RESULTS
The characteristics of the 52 patients are shown in Table 1. The majority of the patients were male (65%). The median age of the patients was 41 years. Forty-two percent of the patients were younger than 40 years of age, and only four patients older than 70 years of age were included. The most dominant histological type of primary tumour observed was osteosarcoma (42%), and the remaining half of the patients suffered from soft tissue sarcomas. Most of the patients (85%) had unilateral diseases, and approximately half of the patients (54%) had solitary lung nodules. The maximum number of metastatic nodules occurring in one patient was 10. During surgery, two unexpected pulmonary nodules were found in two cases, and multiple pleural disseminated nodules were disclosed in three cases. Fifty-eight PM procedures were performed in 52 patients as the first PM including six bilateral diseases of one-staged surgery. Wedge resection was the most frequently performed PM procedure (84%). Video-assisted thoracic surgery was introduced in 34 procedures (59%), and the rest were performed through thoracotomy. Complete resection was accomplished in 92% of the patients. On the other hand, PMs were incomplete in four patients. Three were due to unexpected pleural dissemination, and one was due to a positive surgical margin in the bronchus. Postoperative complications were identified in four patients; pneumonia, prolonged air leakage, wound infection and arrhythmia in one each. Thirty-six (69%) of 52 patients received chemotherapy after the first PM, and 49 (94%) experienced chemotherapy during their overall treatment course. The median length of the DFI before the first PM (pre-DFI) was 13.3 months, including one synchronous case.
| Variables . | n = 52 . | % . |
|---|---|---|
| Gender (male/female) | 34/18 | |
| Age (year, median/range) | 41/7–74 | |
| Histology | ||
| Osteosarcoma | 22 | 42 |
| MFH | 7 | 14 |
| Liposarcoma | 6 | 12 |
| Synovial sarcoma | 4 | 8 |
| Others | 13 | 24 |
| Primary site | ||
| Extremities | 37 | 72 |
| Trunk | 15 | 28 |
| Unilateral | 44 | 85 |
| Number of nodules (1/2/3/4/5/10) | 28/9/8/4/2/1 | |
| First PM procedure | ||
| Wedge resection | 49 | 84 |
| Segmentectomy | 5 | 9 |
| Lobectomy or more | 4 | 7 |
| Complete resection | 48 | 92 |
| Pre-DFI (months; median/range) | 13.3/0–130 | |
| Recurrence after the first PM | 33 | 62 |
| Variables . | n = 52 . | % . |
|---|---|---|
| Gender (male/female) | 34/18 | |
| Age (year, median/range) | 41/7–74 | |
| Histology | ||
| Osteosarcoma | 22 | 42 |
| MFH | 7 | 14 |
| Liposarcoma | 6 | 12 |
| Synovial sarcoma | 4 | 8 |
| Others | 13 | 24 |
| Primary site | ||
| Extremities | 37 | 72 |
| Trunk | 15 | 28 |
| Unilateral | 44 | 85 |
| Number of nodules (1/2/3/4/5/10) | 28/9/8/4/2/1 | |
| First PM procedure | ||
| Wedge resection | 49 | 84 |
| Segmentectomy | 5 | 9 |
| Lobectomy or more | 4 | 7 |
| Complete resection | 48 | 92 |
| Pre-DFI (months; median/range) | 13.3/0–130 | |
| Recurrence after the first PM | 33 | 62 |
| Variables . | n = 52 . | % . |
|---|---|---|
| Gender (male/female) | 34/18 | |
| Age (year, median/range) | 41/7–74 | |
| Histology | ||
| Osteosarcoma | 22 | 42 |
| MFH | 7 | 14 |
| Liposarcoma | 6 | 12 |
| Synovial sarcoma | 4 | 8 |
| Others | 13 | 24 |
| Primary site | ||
| Extremities | 37 | 72 |
| Trunk | 15 | 28 |
| Unilateral | 44 | 85 |
| Number of nodules (1/2/3/4/5/10) | 28/9/8/4/2/1 | |
| First PM procedure | ||
| Wedge resection | 49 | 84 |
| Segmentectomy | 5 | 9 |
| Lobectomy or more | 4 | 7 |
| Complete resection | 48 | 92 |
| Pre-DFI (months; median/range) | 13.3/0–130 | |
| Recurrence after the first PM | 33 | 62 |
| Variables . | n = 52 . | % . |
|---|---|---|
| Gender (male/female) | 34/18 | |
| Age (year, median/range) | 41/7–74 | |
| Histology | ||
| Osteosarcoma | 22 | 42 |
| MFH | 7 | 14 |
| Liposarcoma | 6 | 12 |
| Synovial sarcoma | 4 | 8 |
| Others | 13 | 24 |
| Primary site | ||
| Extremities | 37 | 72 |
| Trunk | 15 | 28 |
| Unilateral | 44 | 85 |
| Number of nodules (1/2/3/4/5/10) | 28/9/8/4/2/1 | |
| First PM procedure | ||
| Wedge resection | 49 | 84 |
| Segmentectomy | 5 | 9 |
| Lobectomy or more | 4 | 7 |
| Complete resection | 48 | 92 |
| Pre-DFI (months; median/range) | 13.3/0–130 | |
| Recurrence after the first PM | 33 | 62 |
Of 48 patients who underwent complete resection of the pulmonary metastases, 15 survived to be free of disease after the first PMs. Each of these patients had unilateral diseases with one or two metastatic nodules, and the median survival time (MST) of this group was 50 months. However, 33 patients experienced relapses of the lung metastases. Among these patients, 20 underwent repeated PM for recurrent lung disease. Despite such aggressive surgical treatment, re-recurrence was observed in 13 patients (65%) with short DFI intervals after the first PMs were performed (post-DFI, median: 6.9 months). Thirteen patients did not meet the criteria for repeated PM, including patients with three or more metastatic nodules and shorter pre-DFI (median: 9.7 months) at the first PMs (Fig. 1). Redo PMs were not performed for these patients due to extrathoracic recurrence in four, multiple pulmonary metastases in four, pleural dissemination in two, intrathoracic recurrence involving the subclavian vessels in one, and hilar lymph node metastasis in one. One patient refused redo surgery for her metastatic pulmonary disease.
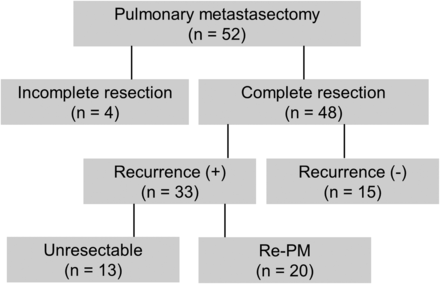
The enrolment and survival outcomes of the patients with pulmonary metastases originating from osteogenic and soft tissue sarcomas.
The MST and 5-year survival rate of all 52 patients was 33.3 months and 50.9%, respectively (Fig. 2a). The MST and 5-year survival rate of the 20 patients who underwent repeated PM was 38.3 months and 49.7%, respectively, while those of the 13 patients who did not undergo repeated PM was 8.7 months and 10.3%, respectively (Fig. 2b).
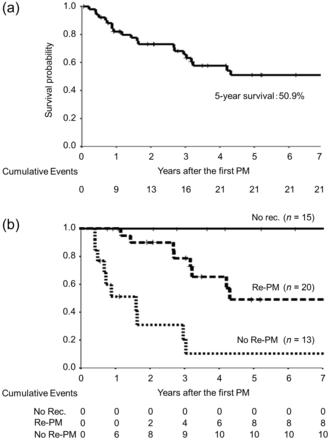
(a) The overall survival of the patients after the first PMs who received surgery to treat lung metastases originating from osteogenic and soft tissue sarcomas. (b) The overall survival curves according to the frequency with which PM was performed. No rec.: no recurrence; Re-PM: repeated PM.
The univariate analyses demonstrated that the number of metastatic nodules, the length of the pre-DFI and the use of complete resection were each significant prognostic factors associated with the overall survival after the first PMs were performed (Table 2). A multivariate analysis showed that the number of metastatic nodules and the completeness of resection were both independent prognostic factors, while the length of the pre-DFI was not significant (Table 3).
Univariate analyses of the factors associated with overall survival after PM
| Variables . | Survival (month) . | 3-year survival (%) . | 5-year survival (%) . | P-value . |
|---|---|---|---|---|
| Gender | ||||
| Male | 33.3 | 63.6 | 50.3 | 0.99 |
| Female | 22.8 | 70.7 | 53.0 | |
| Age | ||||
| <40 | 36.4 | 70.7 | 50.0 | 0.94 |
| ≥40 | 35.5 | 62.5 | 51.6 | |
| Histology | ||||
| Osteosarcoma | 38.0 | 65.2 | 50.3 | 0.62 |
| Non-osteosarcoma | 32.0 | 64.7 | 49.9 | |
| Primary site | ||||
| Extremities | 41.9 | 63.1 | 52.6 | 0.84 |
| Trunk | 13.9 | 75.7 | 28.4 | |
| Laterality | ||||
| Unilateral | 36.4 | 70.1 | 53.2 | 0.28 |
| Bilateral | 19.2 | 38.1 | 38.1 | |
| Number of nodules | ||||
| <2 | 38.3 | 78.6 | 63.1 | <0.001 |
| ≥3 | 10.5 | 31.2 | 15.6 | |
| PM procedure | ||||
| Limited resection | 36.4 | 69.1 | 51.7 | 0.45 |
| Lobectomy or more | 32.2 | 40.0 | 40.0 | |
| Complete resection | ||||
| Yes | 27.6 | 70.0 | 54.2 | <0.001 |
| No | 6.2 | 0.0 | 0.0 | |
| Pre-DFI | ||||
| <12 months | 17.2 | 35.8 | 30.5 | 0.003 |
| ≥12 months | 38.3 | 85.1 | 65.1 | |
| Redo PM | ||||
| Yes | 38.3 | 77.0 | 53.8 | 0.34 |
| No | 19.2 | 59.7 | 49.6 | |
| Variables . | Survival (month) . | 3-year survival (%) . | 5-year survival (%) . | P-value . |
|---|---|---|---|---|
| Gender | ||||
| Male | 33.3 | 63.6 | 50.3 | 0.99 |
| Female | 22.8 | 70.7 | 53.0 | |
| Age | ||||
| <40 | 36.4 | 70.7 | 50.0 | 0.94 |
| ≥40 | 35.5 | 62.5 | 51.6 | |
| Histology | ||||
| Osteosarcoma | 38.0 | 65.2 | 50.3 | 0.62 |
| Non-osteosarcoma | 32.0 | 64.7 | 49.9 | |
| Primary site | ||||
| Extremities | 41.9 | 63.1 | 52.6 | 0.84 |
| Trunk | 13.9 | 75.7 | 28.4 | |
| Laterality | ||||
| Unilateral | 36.4 | 70.1 | 53.2 | 0.28 |
| Bilateral | 19.2 | 38.1 | 38.1 | |
| Number of nodules | ||||
| <2 | 38.3 | 78.6 | 63.1 | <0.001 |
| ≥3 | 10.5 | 31.2 | 15.6 | |
| PM procedure | ||||
| Limited resection | 36.4 | 69.1 | 51.7 | 0.45 |
| Lobectomy or more | 32.2 | 40.0 | 40.0 | |
| Complete resection | ||||
| Yes | 27.6 | 70.0 | 54.2 | <0.001 |
| No | 6.2 | 0.0 | 0.0 | |
| Pre-DFI | ||||
| <12 months | 17.2 | 35.8 | 30.5 | 0.003 |
| ≥12 months | 38.3 | 85.1 | 65.1 | |
| Redo PM | ||||
| Yes | 38.3 | 77.0 | 53.8 | 0.34 |
| No | 19.2 | 59.7 | 49.6 | |
DFI: disease-free interval; PM: pulmonary metastasectomy.
Univariate analyses of the factors associated with overall survival after PM
| Variables . | Survival (month) . | 3-year survival (%) . | 5-year survival (%) . | P-value . |
|---|---|---|---|---|
| Gender | ||||
| Male | 33.3 | 63.6 | 50.3 | 0.99 |
| Female | 22.8 | 70.7 | 53.0 | |
| Age | ||||
| <40 | 36.4 | 70.7 | 50.0 | 0.94 |
| ≥40 | 35.5 | 62.5 | 51.6 | |
| Histology | ||||
| Osteosarcoma | 38.0 | 65.2 | 50.3 | 0.62 |
| Non-osteosarcoma | 32.0 | 64.7 | 49.9 | |
| Primary site | ||||
| Extremities | 41.9 | 63.1 | 52.6 | 0.84 |
| Trunk | 13.9 | 75.7 | 28.4 | |
| Laterality | ||||
| Unilateral | 36.4 | 70.1 | 53.2 | 0.28 |
| Bilateral | 19.2 | 38.1 | 38.1 | |
| Number of nodules | ||||
| <2 | 38.3 | 78.6 | 63.1 | <0.001 |
| ≥3 | 10.5 | 31.2 | 15.6 | |
| PM procedure | ||||
| Limited resection | 36.4 | 69.1 | 51.7 | 0.45 |
| Lobectomy or more | 32.2 | 40.0 | 40.0 | |
| Complete resection | ||||
| Yes | 27.6 | 70.0 | 54.2 | <0.001 |
| No | 6.2 | 0.0 | 0.0 | |
| Pre-DFI | ||||
| <12 months | 17.2 | 35.8 | 30.5 | 0.003 |
| ≥12 months | 38.3 | 85.1 | 65.1 | |
| Redo PM | ||||
| Yes | 38.3 | 77.0 | 53.8 | 0.34 |
| No | 19.2 | 59.7 | 49.6 | |
| Variables . | Survival (month) . | 3-year survival (%) . | 5-year survival (%) . | P-value . |
|---|---|---|---|---|
| Gender | ||||
| Male | 33.3 | 63.6 | 50.3 | 0.99 |
| Female | 22.8 | 70.7 | 53.0 | |
| Age | ||||
| <40 | 36.4 | 70.7 | 50.0 | 0.94 |
| ≥40 | 35.5 | 62.5 | 51.6 | |
| Histology | ||||
| Osteosarcoma | 38.0 | 65.2 | 50.3 | 0.62 |
| Non-osteosarcoma | 32.0 | 64.7 | 49.9 | |
| Primary site | ||||
| Extremities | 41.9 | 63.1 | 52.6 | 0.84 |
| Trunk | 13.9 | 75.7 | 28.4 | |
| Laterality | ||||
| Unilateral | 36.4 | 70.1 | 53.2 | 0.28 |
| Bilateral | 19.2 | 38.1 | 38.1 | |
| Number of nodules | ||||
| <2 | 38.3 | 78.6 | 63.1 | <0.001 |
| ≥3 | 10.5 | 31.2 | 15.6 | |
| PM procedure | ||||
| Limited resection | 36.4 | 69.1 | 51.7 | 0.45 |
| Lobectomy or more | 32.2 | 40.0 | 40.0 | |
| Complete resection | ||||
| Yes | 27.6 | 70.0 | 54.2 | <0.001 |
| No | 6.2 | 0.0 | 0.0 | |
| Pre-DFI | ||||
| <12 months | 17.2 | 35.8 | 30.5 | 0.003 |
| ≥12 months | 38.3 | 85.1 | 65.1 | |
| Redo PM | ||||
| Yes | 38.3 | 77.0 | 53.8 | 0.34 |
| No | 19.2 | 59.7 | 49.6 | |
DFI: disease-free interval; PM: pulmonary metastasectomy.
A multivariate analysis of the factors associated with overall survival rates after the first PM
| Variables . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Number of nodules | 1.16 | 1.10–2.503 | 0.016 |
| Pre-DFI | 0.997 | 0.98–1.015 | 0.759 |
| Complete resection (yes/no) | 0.15 | 0.035–0.596 | 0.007 |
| Variables . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Number of nodules | 1.16 | 1.10–2.503 | 0.016 |
| Pre-DFI | 0.997 | 0.98–1.015 | 0.759 |
| Complete resection (yes/no) | 0.15 | 0.035–0.596 | 0.007 |
DFI: disease-free interval; CI: confident interval.
A multivariate analysis of the factors associated with overall survival rates after the first PM
| Variables . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Number of nodules | 1.16 | 1.10–2.503 | 0.016 |
| Pre-DFI | 0.997 | 0.98–1.015 | 0.759 |
| Complete resection (yes/no) | 0.15 | 0.035–0.596 | 0.007 |
| Variables . | Hazard ratio . | 95% CI . | P-value . |
|---|---|---|---|
| Number of nodules | 1.16 | 1.10–2.503 | 0.016 |
| Pre-DFI | 0.997 | 0.98–1.015 | 0.759 |
| Complete resection (yes/no) | 0.15 | 0.035–0.596 | 0.007 |
DFI: disease-free interval; CI: confident interval.
In our present population, we identified 15 patients with unfavourable factors. The MST and 5-year survival rate in this group was 11 months and 15.6%, respectively. Although all of these patients experienced recurrences after the first PMs were performed, five patients (33%) who underwent repeated PM achieved longer survival times. We did not identify any trends in characteristics, including histology, history of chemotherapy, length of pre-DFI or length of post-DFS in these patients (data not shown).
DISCUSSION
It is widely accepted that treatment with PM offers a potential cure for patients with metastatic malignancies, despite a lack of validation with prospective studies. The large cohort study conducted by the International Registry of Lung Metastases reported a 5-year survival rate of 36% in patients who underwent complete resection of pulmonary metastases compared with that of 7% in patients who underwent incomplete resection [6]. Regarding PM performed to treat metastases of sarcomatous histology, limited numbers of retrospective studies report beneficial effects of PM or increased patient survival rates. Although others have reported 5-year survival rates of 15–52% in patients selected to received PM and have also identified several prognostic factors, including the use of complete resection of the pulmonary metastases, the length of the DFI prior to PM (pre-DFI), the number of nodules, tumour diameter and the use of redo surgery, controversies remain among these reports [3–5, 7–12]. A report from Massachusetts General Hospital (MGH), which is one of the most recent retrospective studies and included 97 patients, showed that resectability, laterality, the length of the DFI, the number of metastases and the use of redo surgery were important factors in determining whether patients were selected to receive PM [13]. This report documented a 50.1% overall survival rate in carefully selected patients and also commented on the presence of selection bias.
In the present study, we reported that having a small number of nodules, especially fewer than three, and undergoing complete resection, were found to be favourable prognostic factors associated with overall survival in PM patients. Concerning the number of metastatic nodules, some reports have supported our results [3, 11, 14], while others have demonstrated that fewer than two or five nodules predict favourable outcomes [13, 15]. Our cohort included 15 patients who underwent resection of three or more metastatic nodules and who had 5-year survival rates as low as 15.6%. The majority of these patients had short pre-DFI and post-DFI of <12 months, and all of these patients experienced relapse. After conducting statistical analyses and detailed case investigations, we conclude that whether PM is performed should be carefully decided in patients in whom three or more nodules are diagnosed prior to surgery.
The completeness of resection is another independent prognostic factor that has been identified by previous reports. Suzuki et al. [12] reported that the 5-year survival rate in patients who underwent incomplete resection was 8.3%, and no patients survived 5 years in the report published by Kim et al. [13] from MGH. There were no 3-year survivors in our four cases of incomplete resection. Because resectability is a factor confirmed by surgical or postoperative pathological findings, it may not be useful for surgeons to utilize resectability as a prognostic factor to predict a patient's survival preoperatively. However, since an accurate preoperative diagnosis of resectability is not given in some cases even with current diagnostic modalities, our results promote the use of PM in patients with potentially resectable disease.
Several reports have demonstrated the DFI interval between the treatment of the primary tumour and the first PM to be one of the factors predicting a patient's survival outcome [4, 13]. Kim etal. [13] have suggested that a DFI exceeding 12 months is an independent prognostic factor. On the other hand, Smith et al. and Garcia et al. have reported DFI of >25 months in patients with soft tissue sarcomas and >20 months in patients with bone sarcomas, respectively [4, 16]. In the present study, although the survival of patients with pre-DFI of >12 months, who consisted of half of our population, was favourable, this factor did not reach a level of statistical significance due to a lack of power. A larger series study may prove this factor to be significant. Unfortunately, the cut-off values for the pre-DFI interval used to determine whether or not PM should be performed still remain controversial.
Currently, redo surgery is also considered to be an important treatment for achieving longer survival times for PM patients. Several studies have suggested that re-recurrent patients who undergo redo PM have a survival advantage compared with those who undergo single PM [13, 17, 18]. Furthermore, Blackmon etal. [19] have demonstrated that survival rates improve in accordance with the frequency with which PM is performed. According to those reports, it is possible that cases of redo surgery include patients with longer DFI, who might have less-aggressive tumours. Although our analysis did not demonstrate a statistically significant impact of redo surgery on the survival of PM patients, five patients who underwent redo surgery survived >22 months despite having unfavourable factors.
Our data showed that undergoing complete resection and having fewer than three metastatic nodules were both independent favourable predictors of overall survival. Although our study included some limitations of patient selection bias and a small series, our results suggest that whether PM is performed in patients with three or more pulmonary nodules should be carefully considered and strong efforts for complete resection should be made. Even if patients who undergo PM to treat osteogenic and soft tissue sarcomas experience relapse in the lungs, aggressively performing redo PM in selected patients may also improve survival.
Conflict of interest: none declared.




