-
PDF
- Split View
-
Views
-
Cite
Cite
Arie Pieter Kappetein, Stuart J. Head, Philippe Généreux, Nicolo Piazza, Nicolas M. van Mieghem, Eugene H. Blackstone, Thomas G. Brott, David J. Cohen, Donald E. Cutlip, Gerrit-Anne van Es, Rebecca T. Hahn, Ajay J. Kirtane, Mitchell W. Krucoff, Susheel Kodali, Michael J. Mack, Roxana Mehran, Josep Rodés-Cabau, Pascal Vranckx, John G. Webb, Stephan Windecker, Patrick W. Serruys, Martin B. Leon, Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2), European Journal of Cardio-Thoracic Surgery, Volume 42, Issue 5, November 2012, Pages S45–S60, https://doi.org/10.1093/ejcts/ezs533
Close - Share Icon Share
Abstract
The aim of the current Valve Academic Research Consortium (VARC)-2 initiative was to revisit the selection and definitions of transcatheter aortic valve implantation (TAVI) clinical endpoints to make them more suitable to the present and future needs of clinical trials. In addition, this document is intended to expand the understanding of patient risk stratification and case selection.
A recent study confirmed that VARC definitions have already been incorporated into clinical and research practice and represent a new standard for consistency in reporting clinical outcomes of patients with symptomatic severe aortic stenosis (AS) undergoing TAVI. However, as the clinical experience with this technology has matured and expanded, certain definitions have become unsuitable or ambiguous.
Two in-person meetings (held in September 2011 in Washington, DC, USA, and in February 2012 in Rotterdam, Netherlands) involving VARC study group members, independent experts (including surgeons, interventional and non-interventional cardiologists, imaging specialists, neurologists, geriatric specialists, and clinical trialists), the US Food and Drug Administration (FDA), and industry representatives, provided much of the substantive discussion from which this VARC-2 consensus manuscript was derived. This document provides an overview of risk assessment and patient stratification that need to be considered for accurate patient inclusion in studies. Working groups were assigned to define the following clinical endpoints: mortality, stroke, myocardial infarction, bleeding complications, acute kidney injury, vascular complications, conduction disturbances and arrhythmias, and a miscellaneous category including relevant complications not previously categorized. Furthermore, comprehensive echocardiographic recommendations are provided for the evaluation of prosthetic valve (dys)function. Definitions for the quality of life assessments are also reported. These endpoints formed the basis for several recommended composite endpoints.
This VARC-2 document has provided further standardization of endpoint definitions for studies evaluating the use of TAVI, which will lead to improved comparability and interpretability of the study results, supplying an increasingly growing body of evidence with respect to TAVI and/or surgical aortic valve replacement. This initiative and document can furthermore be used as a model during current endeavours of applying definitions to other transcatheter valve therapies (for example, mitral valve repair).
INTRODUCTION
The first Valve Academic Research Consortium (VARC) consensus manuscript was published in January 2011 with the goal of achieving consensus for (i) selecting appropriate clinical endpoints reflecting device, procedure and patient-related effectiveness and safety, and (ii) standardizing definitions for single and composite clinical endpoints, for transcatheter aortic valve implantation (TAVI) clinical trials [1, 2]. A recent pooled analysis, which included 3519 patients from 16 unique studies, confirms that VARC definitions have already been incorporated into clinical and research practice and represent a new standard for consistency in reporting clinical outcomes of patients with symptomatic severe aortic stenosis (AS) undergoing TAVI [3]. However, as the clinical experience with this technology has matured and expanded, certain definitions have become unsuitable or ambiguous [3–7]. The aim of the current VARC was therefore to revisit the selection and definitions of TAVI-related clinical endpoints to make them more suitable to the present and future needs of clinical trials. In addition, this document is intended to expand the understanding of patient risk stratification and case selection.
Similar to the VARC-1 process, two in-person meetings (held in September 2011 in Washington, DC, USA, and in February 2012 in Rotterdam, Netherlands) involving VARC study group members, independent experts (including surgeons, interventional and non-interventional cardiologists, imaging specialists, neurologists, geriatric specialists, and clinical trialists), the US Food and Drug Administration (FDA), and industry representatives, provided much of the substantive discussion from which this VARC-2 consensus manuscript was derived (see Appendixes).
RISK SCORES AND COMORBIDITIES
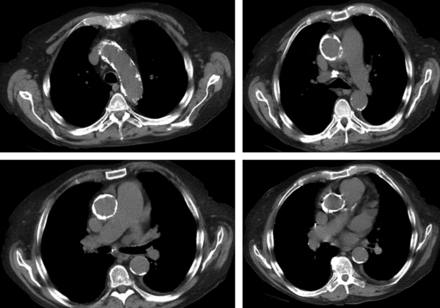
Risk stratification of patients is crucial to identifying appropriate candidates for specific cardiac procedures. The EuroSCORE and Society of Thoracic Surgeons (STS) score are the most widely used risk scores to predict operative mortality in cardiac surgery. These models were developed and validated in a standard surgical risk population. The predictive power of both models is therefore suboptimal in high-risk patients with valvular disease, although the STS score has shown to outperform the Logistic EuroSCORE [8]. These models are even more limited in application to patients who are considered at prohibitive risk for cardiac surgery, a cohort that could particularly benefit from TAVI. Current models could be improved by the addition of specific clinical and anatomical variables that affect mortality [9]. As an example, the presence of a porcelain aorta and frailty are important factors not included in either risk model but are routinely considered during patient evaluation (Fig. 1 and Table 1).
| Co-morbidities . | Definition/criteria . | Diagnostic modalities . |
|---|---|---|
| Porcelain aorta or severely atherosclerotic aorta | Heavy circumferential calcification or severe atheromatous plaques of the entire ascending aorta extending to the arch such that aortic cross-clamping is not feasible | Non-contrast axial CT at levels:
|
| Frailty | Slowness, weakness, exhaustion, wasting and malnutrition, poor endurance and inactivity, loss of independence Criteria:
|
|
| Severe liver disease/cirrhosis | Any of the following:
|
|
| Hostile chest | Any of the following or other reasons that make redo operation through sternotomy or right anterior thoracotomy prohibitively hazardous:
|
|
| IMA or other critical conduit(s) crossing midline and/or adherent to posterior table of sternum | A patent IMA graft that is adherent to the sternum such that injuring it during re-operation is likely A patient may be considered at extreme risk if any of the following are present:
|
|
|
|
|
| Co-morbidities . | Definition/criteria . | Diagnostic modalities . |
|---|---|---|
| Porcelain aorta or severely atherosclerotic aorta | Heavy circumferential calcification or severe atheromatous plaques of the entire ascending aorta extending to the arch such that aortic cross-clamping is not feasible | Non-contrast axial CT at levels:
|
| Frailty | Slowness, weakness, exhaustion, wasting and malnutrition, poor endurance and inactivity, loss of independence Criteria:
|
|
| Severe liver disease/cirrhosis | Any of the following:
|
|
| Hostile chest | Any of the following or other reasons that make redo operation through sternotomy or right anterior thoracotomy prohibitively hazardous:
|
|
| IMA or other critical conduit(s) crossing midline and/or adherent to posterior table of sternum | A patent IMA graft that is adherent to the sternum such that injuring it during re-operation is likely A patient may be considered at extreme risk if any of the following are present:
|
|
|
|
|
CT: computed tomography; MELD: Model for End-Stage Liver Disease; INR: international normalized ratio; IMA: internal mammary artery; PA: pulmonary artery.
aVariable with respect to age and gender without validated scientific thresholds.
bRudski et al. [71].
| Co-morbidities . | Definition/criteria . | Diagnostic modalities . |
|---|---|---|
| Porcelain aorta or severely atherosclerotic aorta | Heavy circumferential calcification or severe atheromatous plaques of the entire ascending aorta extending to the arch such that aortic cross-clamping is not feasible | Non-contrast axial CT at levels:
|
| Frailty | Slowness, weakness, exhaustion, wasting and malnutrition, poor endurance and inactivity, loss of independence Criteria:
|
|
| Severe liver disease/cirrhosis | Any of the following:
|
|
| Hostile chest | Any of the following or other reasons that make redo operation through sternotomy or right anterior thoracotomy prohibitively hazardous:
|
|
| IMA or other critical conduit(s) crossing midline and/or adherent to posterior table of sternum | A patent IMA graft that is adherent to the sternum such that injuring it during re-operation is likely A patient may be considered at extreme risk if any of the following are present:
|
|
|
|
|
| Co-morbidities . | Definition/criteria . | Diagnostic modalities . |
|---|---|---|
| Porcelain aorta or severely atherosclerotic aorta | Heavy circumferential calcification or severe atheromatous plaques of the entire ascending aorta extending to the arch such that aortic cross-clamping is not feasible | Non-contrast axial CT at levels:
|
| Frailty | Slowness, weakness, exhaustion, wasting and malnutrition, poor endurance and inactivity, loss of independence Criteria:
|
|
| Severe liver disease/cirrhosis | Any of the following:
|
|
| Hostile chest | Any of the following or other reasons that make redo operation through sternotomy or right anterior thoracotomy prohibitively hazardous:
|
|
| IMA or other critical conduit(s) crossing midline and/or adherent to posterior table of sternum | A patent IMA graft that is adherent to the sternum such that injuring it during re-operation is likely A patient may be considered at extreme risk if any of the following are present:
|
|
|
|
|
CT: computed tomography; MELD: Model for End-Stage Liver Disease; INR: international normalized ratio; IMA: internal mammary artery; PA: pulmonary artery.
aVariable with respect to age and gender without validated scientific thresholds.
bRudski et al. [71].
Perhaps the most important patient characteristic not included in current risk models is frailty [10]. Frailty is frequently assessed subjectively based upon an informal ‘eyeball test’. However, physical performance assessments such as gait speed and grip strength are more objective performance measures that may capture an individual's overall functional status [11]. These continuous measures are reproducible and can be re-assessed at various time points. In addition, they require no language translation. Assessments of cognition, weight (loss), activity level, and independence in the activities of daily living provide additional information on the overall health state of the individual [11]. These limitations are more often found in patients with a high comorbidity burden and may co-exist with certain laboratory findings (e.g. low serum albumin, elevated inflammatory markers, anaemia) that further reflect the health state and physiological reserve of the frail patient.
Baseline evaluation of the presence of cognitive dysfunction (mild cognitive impairment or dementia) has also emerged as an essential part of the initial risk stratification, especially in older populations, where the risk, benefit, and cost-effectiveness of invasive procedures must be weighed judiciously. Pre-procedural cognitive assessment may also help avoid attributing post-procedural mental status changes to stroke categories. Among the several clinically established rating scales [e.g. Mini-Mental State Examination, modified Telephone Interview of Cognitive Status (TICS-M), Clinical Dementia Rating Scale], [12] there is no particular standard for TAVI. Nevertheless, some systematic cognitive assessment by neuropsychological experts should be a part of the initial heart team evaluation.
Table 1 provides an overview of these and other risk factors (Figs 1–3) and VARC-2 recommendations on how each should be assessed. In clinical trials, it will be important to capture variables that predict extreme operative risk and to standardize the evaluation criteria and process. This will help to determine which subsets of patients are likely to benefit from TAVI treatment.
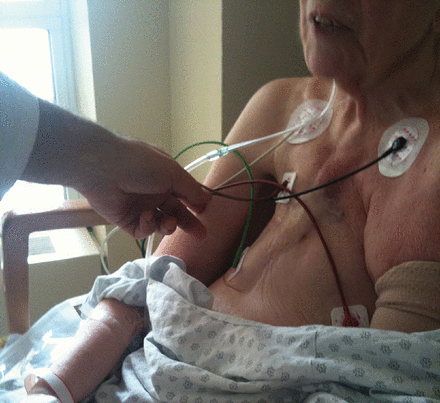

Patent IMA graft crossing midline and/or adherent to the posterior table of sternum.
PATIENT STRATIFICATION: THE HEART TEAM APPROACH
Valve Academic Research Consortium-2 recommends the use of a heart team for patient evaluation. The heart team should consist of at least (interventional) cardiologists, cardiovascular surgeons, and imaging specialists, but its composition is dynamic and can also include anaesthesiologists, geriatricians, neurologists, etc. This multi-disciplinary team should convene as a group on a regular basis to review and interpret clinical data to arrive at a consensus on the optimal treatment strategy for each patient. The heart team approach also allows for the adjustment of the decision-making process according to local experience and circumstances.
The heart team should agree on an estimated 30-day mortality risk for each patient based upon integrating a careful clinical assessment and utilizing appropriate risk prediction scoring systems, preferably the STS score. Surgical mortality risk strata are difficult to precisely assign, but an estimated 30-day mortality of <4% is considered low risk, 4–10% is intermediate risk, >10% is high risk, and >15% is very high risk. A patient is considered at extreme risk if at least two cardiovascular surgeons from a tertiary centre of excellence deny surgery because of prohibitive operative risks, estimated to be a combined >50% risk of irreversible morbidity or mortality [13]. In addition to the specific risk factors that can prohibit patients from undergoing TAVI or surgical aortic valve replacement (SAVR) (Table 1), the operative risk assessment is also important to identify patients who are likely not to benefit from either TAVI or SAVR (the so-called ‘futility’ category of high-risk patients). An expected improvement in the quality of life (QOL) may further be necessary to identify treatment responders vs. non-responders. Individualized life expectancy assumptions should be incorporated by the heart team in the clinical decision-making process as a central factor in weighing the risk–benefit ratio. Prognostic indices of life expectancy may play a central role in moving beyond arbitrary age-based cut-offs [14].
The most important role of the heart team is to provide customized management decisions for common and unusual clinical scenarios in terms of patient selection, procedural performance, and complication management. An example is the frequent situation of severe AS and concomitant coronary artery disease (CAD). The complexity of CAD and appropriate revascularization strategies in the setting of AS should be determined by consensus from interventional cardiologists and cardiovascular surgeons [15, 16]. In new TAVI clinical trials, angiographic risk scores (e.g. SYNTAX score) may be utilized to help determine the complexity of CAD, as a basis for the inclusion in the trial. Thresholds for coronary revascularization and the choice for a staged or concomitant PCI with TAVI should be guided by the complexity of the CAD and other factors as determined by the heart team [17, 18]. In general, the plan to deal with other co-existing conditions [such as atrial fibrillation (AF), other valvular lesions, and other congenital lesions] should be pre-specified and all complications encountered in the treatment of associated conditions (including treatment after the TAVI procedure) should be captured. Such thorough pre-procedural assessment is also valuable in discriminating new post-procedural complications from simple exacerbations of pre-existing conditions.
CLINICAL ENDPOINTS
Mortality
In addition to the original VARC definitions, VARC-2 recommends the collection of immediate procedural mortality to capture intra-procedural events that result in immediate or consequent death ≤72 h post-procedure. Taking into account the surgical literature, procedural mortality consists of all-cause mortality within 30 days or during index procedure hospitalization—if the postoperative length of stay is longer than 30 days.
The cause of death should be captured, based on a careful review of narrative summaries and source material. All-cause, cardiovascular, and non-cardiovascular mortality should be reported after 30 days during the follow-up (Table 2). In determining the cause of death, the adjudication committee should consider the clinical context at the time of the index procedure and during the time interval leading up to death. All efforts (including the use of national death registries) should be made to identify, precisely characterize, and appropriately classify any death.
| All-cause mortality . |
|---|
Cardiovascular mortality
|
| Non-cardiovascular mortality Any death in which the primary cause of death is clearly related to another condition (e.g. trauma, cancer, suicide) |
| All-cause mortality . |
|---|
Cardiovascular mortality
|
| Non-cardiovascular mortality Any death in which the primary cause of death is clearly related to another condition (e.g. trauma, cancer, suicide) |
| All-cause mortality . |
|---|
Cardiovascular mortality
|
| Non-cardiovascular mortality Any death in which the primary cause of death is clearly related to another condition (e.g. trauma, cancer, suicide) |
| All-cause mortality . |
|---|
Cardiovascular mortality
|
| Non-cardiovascular mortality Any death in which the primary cause of death is clearly related to another condition (e.g. trauma, cancer, suicide) |
Myocardial infarction
Myocardial injury as determined by a significant rise in cardiac biomarkers occurs frequently following TAVI, and a significant magnitude of myocardial injury has been associated with worse outcomes [19]. Valve Academic Research Consortium-2 recommends the systematic collection of biomarkers of myocardial injury prior to the procedure, within 12–24 h after the procedure, at 24 h thereafter, at 72 h or at discharge, and, if still elevated, daily until values show a decline. Similar to the previous VARC recommendations, the definition of peri-procedural (≤72 h following TAVI) MI will be based on a combination of clinical criteria and cardiac biomarkers. However, the threshold values have been adjusted (Table 3). Acute ischaemic events occurring after 72 h should be considered spontaneous myocardial infarctions and defined in accordance with the universal MI guidelines [20].
Peri-procedural MI (≤72 h after the index procedure)
|
Spontaneous MI (>72 h after the index procedure) Any one of the following criteria
|
Peri-procedural MI (≤72 h after the index procedure)
|
Spontaneous MI (>72 h after the index procedure) Any one of the following criteria
|
aPreviously in the original VARC it was 10× and 5× for troponin and CK-MB, respectively.
Peri-procedural MI (≤72 h after the index procedure)
|
Spontaneous MI (>72 h after the index procedure) Any one of the following criteria
|
Peri-procedural MI (≤72 h after the index procedure)
|
Spontaneous MI (>72 h after the index procedure) Any one of the following criteria
|
aPreviously in the original VARC it was 10× and 5× for troponin and CK-MB, respectively.
Stroke
With increasing attention to stroke as an important peri-procedural complication of TAVI, [21] the FDA has emphasized the need for an accurate assessment of stroke and has participated actively in recommending specific details of the VARC-2 definitions. In an attempt to further align with the fundamental definitions now endorsed by the FDA, [22] consensus was reached at VARC-2 to further refine the definition of stroke and recommend the use of these definitions in future TAVI clinical trials (Table 4). The definitions endorsed by the FDA are intended to apply to a wide range of clinical trials and to enable those trials to assess the clinically relevant consequences of vascular brain injury for determining the safety or effectiveness of an intervention.
Diagnostic criteria
|
Stroke classification
|
Stroke definitionsb
|
Diagnostic criteria
|
Stroke classification
|
Stroke definitionsb
|
mRS: modified Rankin Scale.
aPatients with non-focal global encephalopathy will not be reported as a stroke without unequivocal evidence of cerebral infarction-based upon neuroimaging studies (CT scan or brain MRI).
bModified Rankin Scale assessments should be made by qualified individuals according to a certification process [23–25].
Diagnostic criteria
|
Stroke classification
|
Stroke definitionsb
|
Diagnostic criteria
|
Stroke classification
|
Stroke definitionsb
|
mRS: modified Rankin Scale.
aPatients with non-focal global encephalopathy will not be reported as a stroke without unequivocal evidence of cerebral infarction-based upon neuroimaging studies (CT scan or brain MRI).
bModified Rankin Scale assessments should be made by qualified individuals according to a certification process [23–25].
Stroke is defined as an acute episode of focal or global neurological dysfunction caused by the brain, spinal cord, or retinal vascular injury as a result of haemorrhage or infarction. Stroke may be classified as ischaemic or haemorrhagic with appropriate subdefinitions. Ischaemic stroke is defined as an acute episode of focal cerebral, spinal, or retinal dysfunction caused by infarction of central nervous system tissue. Haemorrhagic stroke is defined as an acute episode of focal or global cerebral or spinal dysfunction caused by intraparenchymal, intraventricular, or subarachnoid haemorrhage. A stroke may be classified as ‘undetermined’ if there is insufficient information to allow the categorization as ischaemic or haemorrhagic.
An entity closely related to an ischaemic stroke that should be assessed is a transient ischaemic attack (TIA). Transient ischaemic attack is defined as a transient episode of focal neurological dysfunction caused by the brain, spinal cord, or retinal ischaemia, without acute infarction. The difference between TIA and ischaemic stroke is the presence of tissue damage on neuro-imaging studies or new sensory–motor deficit persisting >24 h. By definition, a TIA does not produce a lasting disability.
Valve Academic Research Consortium-2 recognizes that an assessment of stroke is incomplete without an appropriate measurement of the disability resulting from the stroke. Valve Academic Research Consortium-2 recommends the use of the modified Rankin Scale (mRS) to assess this clinical disability [23–25]. The assessment of the mRS should occur at all scheduled visits in a trial and at 90 days after the onset of any stroke. This approach will maximize the detection of new or recurrent strokes, assist in the ongoing evaluation of events previously determined as TIAs, and provide an accepted and reliable indicator of the long-term impact of a given stroke.
Previously, VARC recommended categorizing strokes as ‘major’ and ‘minor’ based upon mRS scores. To enhance the accuracy in the description of a given stroke and to provide accurate categorization of strokes within a given trial, VARC-2 now recommends the use of the terms ‘disabling’ and ‘non-disabling’. A disabling stroke is one that results (at 90 days after stroke onset) in an mRS score of ≥2 and an increase in ≥1 mRS category from an individual's pre-stroke baseline. A non-disabling stroke is one that results (at 90 days after stroke onset) in an mRS score of <2 or that does not result in an increase in ≥1 mRS category from an individual's pre-stroke baseline. In addition to this categorization of disabling and non-disabling strokes, the endpoint of all strokes should be reported.
Although brain imaging (typically, MRI for acute and chronic ischaemia and haemorrhage, and CT for acute and chronic haemorrhage and chronic ischaemia) is often used to supplement the clinical diagnosis of stroke, [26] a diagnosis of stroke may be made on clinical grounds alone. Valve Academic Research Consortium-2 recognizes that stroke symptoms are protean and not well suited to a pre-specified itemized listing. Accordingly, VARC-2 recommends that a vascular neurologist experienced in clinical trials involving stroke be included in all phases of trial planning, execution, and monitoring, including involvement in the Clinical Events Committee and the Data and Safety Monitoring Board.
New insights into the timing of events show delayed or late occurrence of strokes, beyond the early post-implantation phase [27]. This may suggest that the cause of stroke is additionally related to other factors or patient susceptibilities and should necessitate active investigation of devices and adjunctive pharmacotherapy to reduce the frequency and severity of strokes after TAVI, including precise documentation of the use and dosage of antithrombotic and antiplatelet medication. Patient baseline characteristics (e.g. carotid stenosis) and postoperative complications (e.g. AF) need to be carefully documented to be able to identify the contributing causes of stroke.
Invasive stroke management (catheter-based intracranial intervention) is gaining an increasingly important role and may impact morbidity and mortality. Valve Academic Research Consortium-2 therefore recommends the ascertainment of any acute stroke management strategy (e.g. aspiration, thrombolysis, or conservative management).
Bleeding complications
Valve Academic Research Consortium-2 acknowledges the fact that the Bleeding Academic Research Consortium (BARC) recently convened and established standardized bleeding definitions for patients receiving antithrombotic therapy and undergoing coronary revascularization (PCI or CABG) [28, 29]. However, because the current definitions have been well adopted and shown to be accurate in predicting adverse events, [30] VARC-2 has chosen to maintain the original VARC definitions with BARC classifications (Table 5), recognizing that future validation of BARC criteria in this population may warrant revision of the current recommendations.
Life-threatening or disabling bleeding
|
Major bleeding (BARC type 3a)
|
Minor bleeding (BARC type 2 or 3a, depending on the severity)
|
Life-threatening or disabling bleeding
|
Major bleeding (BARC type 3a)
|
Minor bleeding (BARC type 2 or 3a, depending on the severity)
|
BARC: Bleeding Academic Research Consortium [29]; RBC, red blood cell.
aGiven that one unit of packed RBC typically will raise the haemoglobin concentration by 1 g/dl, an estimated decrease in haemoglobin will be calculated.
Life-threatening or disabling bleeding
|
Major bleeding (BARC type 3a)
|
Minor bleeding (BARC type 2 or 3a, depending on the severity)
|
Life-threatening or disabling bleeding
|
Major bleeding (BARC type 3a)
|
Minor bleeding (BARC type 2 or 3a, depending on the severity)
|
BARC: Bleeding Academic Research Consortium [29]; RBC, red blood cell.
aGiven that one unit of packed RBC typically will raise the haemoglobin concentration by 1 g/dl, an estimated decrease in haemoglobin will be calculated.
With respect to blood transfusions, it is critical to acknowledge that a bleeding complication has to be the result of overt bleeding and cannot be adjudicated based on blood transfusions alone.
Acute kidney injury
The original VARC definitions recommended the use of a modified version of the RIFLE classification. However, we now recommend using the AKIN system (Table 6), which is a modified version of RIFLE that has been adopted by many in the nephrology community, including the KDIGO initiative [31, 32]. As a result, acute kidney injury (AKI) can also be diagnosed according to urine output measures (Table 6).
Stage 1
|
Stage 2
|
Stage 3b
|
Stage 1
|
Stage 2
|
Stage 3b
|
The increase in creatinine must occur within 48 h.
aMehta et al. [31].
bPatients receiving renal replacement therapy are considered to meet Stage 3 criteria irrespective of other criteria.
Stage 1
|
Stage 2
|
Stage 3b
|
Stage 1
|
Stage 2
|
Stage 3b
|
The increase in creatinine must occur within 48 h.
aMehta et al. [31].
bPatients receiving renal replacement therapy are considered to meet Stage 3 criteria irrespective of other criteria.
In comparison with the original VARC, the timing for the diagnosis of AKI is extended from 72 h to 7 days. Patients who experience AKI should have follow-up renal function assessments after 7 days until stabilization.
Vascular complications
Table 7 lists VARC-2 definitions for major and minor vascular complications. Further clarifications of these definitions to supplement the original VARC document are as follows. Pre-planned surgical access or a planned endovascular approach to vascular closure (e.g. ‘pre-closure’) [33, 34] should be considered as part of the TAVI procedure and not as a complication, unless untoward clinical consequences are documented (e.g. bleeding complications, limb ischaemia, distal embolization, or neurological impairment). Unplanned endovascular stenting or surgical repair for any vascular complications during the index procedure without other clinical sequelae should be considered a minor vascular complication, except if associated with qualifying consequences (Table 7). Complications related to alternative access sites, including the left-ventricular apex, subclavian artery, or aorta should be systematically recorded. To ensure accurate capture of these elements, VARC-2 strongly recommends that detailed information regarding the access site and pre-planned vascular closure technique be recorded as well as the use of any additional unplanned access or closure techniques (surgical repair, endovascular stenting, or endovascular balloon therapy). Since many vascular complications will also result in a bleeding complication, events that meet VARC-2 definitions for both categories should be reported in both categories. Finally, VARC-2 recommends that all vascular complications be recorded as either access (e.g. iliac rupture) or non-access site-related (e.g. ascending aorta dissection or rupture unless aortic access is used and the event originates from the cannulation site).
Major vascular complications
|
Minor vascular complications
|
Percutaneous closure device failure
|
Major vascular complications
|
Minor vascular complications
|
Percutaneous closure device failure
|
aRefers to VARC bleeding definitions.
Major vascular complications
|
Minor vascular complications
|
Percutaneous closure device failure
|
Major vascular complications
|
Minor vascular complications
|
Percutaneous closure device failure
|
aRefers to VARC bleeding definitions.
Conduction disturbances and arrhythmias
Valve Academic Research Consortium-2 proposes the systematic collection of data on the frequency of implant-related new and/or worsened conduction disturbances and the incidence and indication for permanent pacemaker implantation (Table 8). In addition, the frequency of specific arrhythmias following TAVI should be recorded as they may result in prolonged hospitalization and impaired clinical outcomes. New-onset AF (or flutter) is diagnosed as any arrhythmia within hospitalization that has the ECG characteristics of AF and lasts sufficiently long to be recorded on a 12-lead ECG, or for at least 30 s on a rhythm strip [35]. The therapeutic approach to new-onset AF (spontaneous conversion, electrical or medical cardioversion, initiation of oral anticoagulation, and rate or rhythm control medications) and any clinical consequences should be thoroughly documented in the case report form.
| Up to 72 h, continuous rhythm monitoring is recommended in order to maximize the detection of arrhythmias . |
|---|
Data elements to be collected should include
|
| Up to 72 h, continuous rhythm monitoring is recommended in order to maximize the detection of arrhythmias . |
|---|
Data elements to be collected should include
|
aType of permanent pacemaker should be recorded (e.g. defibrillator, single vs. dual chamber, biventricular).
bNew-onset atrial fibrillation (or flutter) is diagnosed as any arrhythmia within hospitalization that has the ECG characteristics of atrial fibrillation (or flutter) and lasts sufficiently long to be recorded on a 12-lead ECG, or at least 30 s on a rhythm strip.
cTherapy includes electrical/medical cardioversion or initiation of a new medication (oral anticoagulation, rhythm, or rate controlling therapy).
| Up to 72 h, continuous rhythm monitoring is recommended in order to maximize the detection of arrhythmias . |
|---|
Data elements to be collected should include
|
| Up to 72 h, continuous rhythm monitoring is recommended in order to maximize the detection of arrhythmias . |
|---|
Data elements to be collected should include
|
aType of permanent pacemaker should be recorded (e.g. defibrillator, single vs. dual chamber, biventricular).
bNew-onset atrial fibrillation (or flutter) is diagnosed as any arrhythmia within hospitalization that has the ECG characteristics of atrial fibrillation (or flutter) and lasts sufficiently long to be recorded on a 12-lead ECG, or at least 30 s on a rhythm strip.
cTherapy includes electrical/medical cardioversion or initiation of a new medication (oral anticoagulation, rhythm, or rate controlling therapy).
Other TAVI-related complications
The original VARC document recommended the collection of a number of TAVI-related complications, but did not provide specific endpoint definitions for several endpoints. Valve Academic Research Consortium-2 recommends reporting any other complications related to the TAVI procedure, even those occurring less frequently, and provides formal VARC-2 definitions (Table 9) [36–38]. 3
Conversion to open surgery
|
Unplanned use of cardiopulmonary bypass (CPB)
|
Coronary obstruction
|
Ventricular septal perforation
|
Mitral valve apparatus damage or dysfunction
|
Cardiac tamponade
|
Endocarditis
|
Valve thrombosis
|
Valve malpositioning
|
TAV-in-TAV deployment
|
Conversion to open surgery
|
Unplanned use of cardiopulmonary bypass (CPB)
|
Coronary obstruction
|
Ventricular septal perforation
|
Mitral valve apparatus damage or dysfunction
|
Cardiac tamponade
|
Endocarditis
|
Valve thrombosis
|
Valve malpositioning
|
TAV-in-TAV deployment
|
TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve.
aDurack et al. [72].
Conversion to open surgery
|
Unplanned use of cardiopulmonary bypass (CPB)
|
Coronary obstruction
|
Ventricular septal perforation
|
Mitral valve apparatus damage or dysfunction
|
Cardiac tamponade
|
Endocarditis
|
Valve thrombosis
|
Valve malpositioning
|
TAV-in-TAV deployment
|
Conversion to open surgery
|
Unplanned use of cardiopulmonary bypass (CPB)
|
Coronary obstruction
|
Ventricular septal perforation
|
Mitral valve apparatus damage or dysfunction
|
Cardiac tamponade
|
Endocarditis
|
Valve thrombosis
|
Valve malpositioning
|
TAV-in-TAV deployment
|
TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve.
aDurack et al. [72].
Additional considerations
For studies or trials where the occurrence, prevention, or treatment of cerebral infarction is a fundamental feature (e.g. embolic protection devices) additional appropriate imaging in all or a subset of patients may be necessary to allow determination of effectiveness.
VALVULAR FUNCTION
Valve Academic Research Consortium-2 maintains the original recommendations to use echocardiography as the primary imaging modality for the assessment of prosthetic valve function [39]. This should include the valve position, morphology, function, and evaluation of the left ventricle (LV) and right ventricle (RV) size and function. The suggested time points for routine follow-up transthoracic echocardiography (TTE) following valve implantation are: immediately (before discharge) following the implantation for transarterial approaches or within 30 days for transapical or transaortic approaches, 6 months following implantation, 1 year following implantation, and yearly thereafter. At these endpoints, prosthetic aortic valve stenosis and regurgitation should be reported.
Transcatheter valve stenosis
The assessment of prosthetic valve stenosis should be an integrative process utilizing multiple parameters of valve function. Table 10 outlines the primary parameters used for assessing prosthetic valve function based on published guidelines [40]. Divergence from the guidelines is based on a number of studies,[41, 42] as well as methods used in large randomized control trials of TAVI [43, 44]. In addition, VARC-2 does not recommend using acceleration time, which is dependent on ventricular function and heart rate [42]. The limitation of flow-dependent parameters such as peak jet velocity or mean transprosthetic gradient is obvious, however, even flow-independent parameters such as the effective orifice area (EOA) and the Doppler velocity index (DVI) have limitations: (i) the absolute EOA does not account for the cardiac output requirements in relation to the patient's body size; thus lower criteria should be used to define prosthetic valve stenosis in patients with BSA <1.6 m2 (Table 10), (ii) the indexed EOA may overestimate the valve-related haemodynamic burden in obesity; hence, lower criteria may be more appropriate in patients with a body mass index ≥30 kg/m2, (iii) DVI severity criteria are dependent on the left ventricular outflow tract (LVOT) size; thus a lower threshold may be more appropriate in patients with LVOT diameters of >25 mm. The EOA should generally be calculated with the use of the LVOT diameter and the velocity measured just underneath the apical margin of the valve stent [45, 46]. In cases where the landing zone of the stent is low in the LVOT, the diameter and velocity may both be measured in the proximal portion of the stent. Unlike the surgically implanted valve, the transcatheter prosthetic valve EOA is defined not only by the size of the valve but also by the patient's aortic valve/annular anatomy and procedural variables. Thus, well-established normal transcatheter valve gradients and EOAs based on pre-implant aortic annular dimensions do not currently exist. Clinicians should be aware of this variability when assessing a patient for transcatheter valve function and VARC-2 strongly recommends that the patient's own initial post-implant study be used as a reference for serial comparisons.
| . | Prosthetic aortic valve stenosisa . | ||
|---|---|---|---|
| . | Normal . | Mild stenosis . | Moderate/severe stenosis . |
| Quantitative parameters (flow-dependent)b | |||
| Peak velocity (m/s) | <3 m/s | 3–4 m/s | >4 m/s |
| Mean gradient (mmHg) | <20 mmHg | 20–40 mmHg | >40 mmHg |
| Quantitative parameters (flow-independent) | |||
| Doppler velocity indexc | >0.35 | 0.35–0.25 | <0.25 |
| Effective orifice aread | >1.1 cm2 | 1.1–0.8 cm2 | <0.8 cm2 |
| Effective orifice areae | >0.9 cm2 | 0.9–0.6 cm2 | <0.6 cm2 |
| Prosthesis–patient mismatch (PPM) | |||
| Insignificant | Moderate | Severe | |
| Indexed effective orifice areaf (cm2/m2) | >0.85 cm2/m2 | 0.85–0.65 cm2/m2 | <0.65 cm2/m2 |
| Indexed effective orifice areag (cm2/m2) | >0.70 cm2/m2 | 0.90–0.60 cm2/m2 | <0.60 cm2/m2 |
| Prosthetic aortic valve regurgitation | |||
| Mild | Moderate | Severe | |
| Semi-quantitative parameters | |||
| Diastolic flow reversal in the descending aorta—PW | Absent or brief early diastolic | Intermediate | Prominent, holodiastolic |
| Circumferential extent of prosthetic valve paravalvular regurgitation (%)h | <10% | 10–29% | ≥30% |
| Quantitative parametersc | |||
| Regurgitant volume (ml/beat) | <30 ml | 30–59 ml | ≥60 ml |
| Regurgitant fraction (%) | <30% | 30–49% | ≥50% |
| EROA (cm2) | 0.10 cm2 | 0.10–0.29 cm2 | ≥0.30 cm2 |
| . | Prosthetic aortic valve stenosisa . | ||
|---|---|---|---|
| . | Normal . | Mild stenosis . | Moderate/severe stenosis . |
| Quantitative parameters (flow-dependent)b | |||
| Peak velocity (m/s) | <3 m/s | 3–4 m/s | >4 m/s |
| Mean gradient (mmHg) | <20 mmHg | 20–40 mmHg | >40 mmHg |
| Quantitative parameters (flow-independent) | |||
| Doppler velocity indexc | >0.35 | 0.35–0.25 | <0.25 |
| Effective orifice aread | >1.1 cm2 | 1.1–0.8 cm2 | <0.8 cm2 |
| Effective orifice areae | >0.9 cm2 | 0.9–0.6 cm2 | <0.6 cm2 |
| Prosthesis–patient mismatch (PPM) | |||
| Insignificant | Moderate | Severe | |
| Indexed effective orifice areaf (cm2/m2) | >0.85 cm2/m2 | 0.85–0.65 cm2/m2 | <0.65 cm2/m2 |
| Indexed effective orifice areag (cm2/m2) | >0.70 cm2/m2 | 0.90–0.60 cm2/m2 | <0.60 cm2/m2 |
| Prosthetic aortic valve regurgitation | |||
| Mild | Moderate | Severe | |
| Semi-quantitative parameters | |||
| Diastolic flow reversal in the descending aorta—PW | Absent or brief early diastolic | Intermediate | Prominent, holodiastolic |
| Circumferential extent of prosthetic valve paravalvular regurgitation (%)h | <10% | 10–29% | ≥30% |
| Quantitative parametersc | |||
| Regurgitant volume (ml/beat) | <30 ml | 30–59 ml | ≥60 ml |
| Regurgitant fraction (%) | <30% | 30–49% | ≥50% |
| EROA (cm2) | 0.10 cm2 | 0.10–0.29 cm2 | ≥0.30 cm2 |
aIn conditions of normal or near normal stroke volume (50–70 ml).
bThese parameters are more affected by flow, including concomitant aortic regurgitation.
cFor LVOT >2.5 cm, significant stenosis criteria is <0.20.
dUse in setting of BSA ≥1.6 cm2 (note: dependent on the size of the valve and the size of the native annulus).
eUse in setting of BSA <1.6 cm2.
fUse in setting of BMI <30 kg/cm2.
gUse in setting of BMI ≥30 kg/cm2.
hNot well-validated and may overestimate the severity compared with the quantitative Doppler.
| . | Prosthetic aortic valve stenosisa . | ||
|---|---|---|---|
| . | Normal . | Mild stenosis . | Moderate/severe stenosis . |
| Quantitative parameters (flow-dependent)b | |||
| Peak velocity (m/s) | <3 m/s | 3–4 m/s | >4 m/s |
| Mean gradient (mmHg) | <20 mmHg | 20–40 mmHg | >40 mmHg |
| Quantitative parameters (flow-independent) | |||
| Doppler velocity indexc | >0.35 | 0.35–0.25 | <0.25 |
| Effective orifice aread | >1.1 cm2 | 1.1–0.8 cm2 | <0.8 cm2 |
| Effective orifice areae | >0.9 cm2 | 0.9–0.6 cm2 | <0.6 cm2 |
| Prosthesis–patient mismatch (PPM) | |||
| Insignificant | Moderate | Severe | |
| Indexed effective orifice areaf (cm2/m2) | >0.85 cm2/m2 | 0.85–0.65 cm2/m2 | <0.65 cm2/m2 |
| Indexed effective orifice areag (cm2/m2) | >0.70 cm2/m2 | 0.90–0.60 cm2/m2 | <0.60 cm2/m2 |
| Prosthetic aortic valve regurgitation | |||
| Mild | Moderate | Severe | |
| Semi-quantitative parameters | |||
| Diastolic flow reversal in the descending aorta—PW | Absent or brief early diastolic | Intermediate | Prominent, holodiastolic |
| Circumferential extent of prosthetic valve paravalvular regurgitation (%)h | <10% | 10–29% | ≥30% |
| Quantitative parametersc | |||
| Regurgitant volume (ml/beat) | <30 ml | 30–59 ml | ≥60 ml |
| Regurgitant fraction (%) | <30% | 30–49% | ≥50% |
| EROA (cm2) | 0.10 cm2 | 0.10–0.29 cm2 | ≥0.30 cm2 |
| . | Prosthetic aortic valve stenosisa . | ||
|---|---|---|---|
| . | Normal . | Mild stenosis . | Moderate/severe stenosis . |
| Quantitative parameters (flow-dependent)b | |||
| Peak velocity (m/s) | <3 m/s | 3–4 m/s | >4 m/s |
| Mean gradient (mmHg) | <20 mmHg | 20–40 mmHg | >40 mmHg |
| Quantitative parameters (flow-independent) | |||
| Doppler velocity indexc | >0.35 | 0.35–0.25 | <0.25 |
| Effective orifice aread | >1.1 cm2 | 1.1–0.8 cm2 | <0.8 cm2 |
| Effective orifice areae | >0.9 cm2 | 0.9–0.6 cm2 | <0.6 cm2 |
| Prosthesis–patient mismatch (PPM) | |||
| Insignificant | Moderate | Severe | |
| Indexed effective orifice areaf (cm2/m2) | >0.85 cm2/m2 | 0.85–0.65 cm2/m2 | <0.65 cm2/m2 |
| Indexed effective orifice areag (cm2/m2) | >0.70 cm2/m2 | 0.90–0.60 cm2/m2 | <0.60 cm2/m2 |
| Prosthetic aortic valve regurgitation | |||
| Mild | Moderate | Severe | |
| Semi-quantitative parameters | |||
| Diastolic flow reversal in the descending aorta—PW | Absent or brief early diastolic | Intermediate | Prominent, holodiastolic |
| Circumferential extent of prosthetic valve paravalvular regurgitation (%)h | <10% | 10–29% | ≥30% |
| Quantitative parametersc | |||
| Regurgitant volume (ml/beat) | <30 ml | 30–59 ml | ≥60 ml |
| Regurgitant fraction (%) | <30% | 30–49% | ≥50% |
| EROA (cm2) | 0.10 cm2 | 0.10–0.29 cm2 | ≥0.30 cm2 |
aIn conditions of normal or near normal stroke volume (50–70 ml).
bThese parameters are more affected by flow, including concomitant aortic regurgitation.
cFor LVOT >2.5 cm, significant stenosis criteria is <0.20.
dUse in setting of BSA ≥1.6 cm2 (note: dependent on the size of the valve and the size of the native annulus).
eUse in setting of BSA <1.6 cm2.
fUse in setting of BMI <30 kg/cm2.
gUse in setting of BMI ≥30 kg/cm2.
hNot well-validated and may overestimate the severity compared with the quantitative Doppler.
The assessment of transcatheter valve dysfunction includes the immediate post-TAVI haemodynamics and the follow-up evaluation. The immediate post-TAVI evaluation documents initial valve appearance (position and circularity of the stent, and leaflet morphology and motion) and a comprehensive haemodynamic evaluation. Valve Academic Research Consortium-2 advocates using the integrative approach outlined in the algorithm shown in Figure 4 as part of a comprehensive haemodynamic evaluation by initially using one flow dependent (e.g. mean gradient) and one flow independent criterion (e.g. EOA) for the initial haemodynamic evaluation. If there is discordance between these measurements, then the DVI should be calculated. An abnormal DVI indicates possible prosthetic valve dysfunction. A normal DVI indicates intrinsically normal prosthetic valve function, and the indexed EOA can then be used to determine the reason for the initial measurement discordance. When the indexed EOA is low in the setting of a normal DVI, the patient probably has a prosthesis–patient mismatch (PPM), an indicator of the intrinsic relationship of the implanted valve to the cardiac output requirements of the patient [47]. Prosthesis–patient mismatch occurs in the setting of a morphologically normal valve and is considered to be haemodynamically insignificant if the indexed EOA is >0.85 cm2/m2, moderate if between 0.65 and 0.85 cm2/m2, and severe if <0.65 cm2/m2. However, for obese patients (body mass index ≥30 kg/m2) lower criteria may be more appropriate (Table 10).
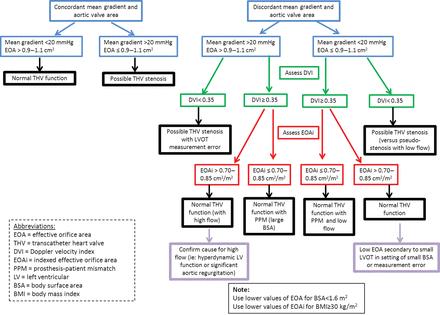
Transcatheter valve regurgitation
There is growing evidence suggesting a significant association of post-procedural paravalvular aortic regurgitation (AR) with short- and long-term mortality [48, 49]. As the duration of implanted transcatheter heart valves increases, valve durability and dysfunction become more crucial issues. Evaluating the presence and severity of regurgitation should include an assessment of both central and paravalvular components, with a combined measurement of ‘total’ aortic regurgitation (AR) reflecting the total volume load imposed on the LV (Table 10). The quantitative and semi-quantitative haemodynamic assessment of AR severity should be performed with Doppler echocardiography according to the guidelines [39, 50, 51]. Colour Doppler evaluation should be performed just below the valve stent for paravalvular jets, and at the coaptation point of the leaflets for central regurgitation. Although all imaging windows should be used, the parasternal short-axis view is critical in assessing the number and severity of paravalvular jets. Whenever possible, the quantification of the prosthetic regurgitant volume, effective regurgitant orifice area, and regurgitant fraction (Table 10) should be performed [40, 51, 52]. The regurgitant volume may be calculated as the difference between the stroke volume across any non-regurgitant orifice (RVOT or mitral valve) and the stroke volume across the LVOT.
It is important to realize that at this time the body of evidence supporting the numerical criteria used in Table 10 as well as Fig. 4 may be limited. These criteria should be used as guidelines for clinical decision-making and require further validation as our experience continues to expand.
Follow-up assessments
The follow-up assessment should also begin with valve imaging and documentation of changes in morphology. When determining whether a patient has developed haemodynamically significant structural valve failure, the patient's own baseline echocardiographic parameters should be used as a reference. An increase in the mean gradient >10 mmHg, a decrease in the EOA >0.3–0.4 cm2, or a reduction in the DVI >0.1–0.13 probably indicates a change in valve function and should trigger a comprehensive haemodynamic evaluation. Whenever valve dysfunction is suspected, the careful evaluation of valve morphology should confirm a structurally abnormal valve. In addition, measurement error must be excluded; the use of a consistent LVOT diameter for more accurate follow-up study comparisons is recommended. Finally, changes in ventricular morphology would be expected in the setting of long-standing significant valvular dysfunction and this parameter may support the clinical assessment of severity.
Although the rate of moderate or severe regurgitation may appear to be less at the follow-up, this may be the result of attrition of the sickest patients. To assess such time trends, it is recommended to report an individual patient's progression of regurgitation, in a table that provides changes between short-term and long-term regurgitation, including mortality [48].
QUALITY OF LIFE
Quality of life evaluation in aortic stenosis
New York Heart Association (NYHA) classification is limited by the discrete nature of the scale, which provides only modest resolution to detect clinically relevant changes. Moreover, since the NYHA class is assessed by an external body rather than the patient, it does not reflect the patient's perspective. Thus, the NYHA class is more properly considered a measure of the functional status than the QOL.
The Minnesota Living with Heart Failure Questionnaire (MLHF) [53] and the Kansas City Cardiomyopathy Questionnaire (KCCQ) [54, 55] have a number of desirable properties for the evaluation of health-related QOL (HRQOL) in the setting of AS. Both instruments produce outcomes on a continuous scale, which improves responsiveness and sensitivity. Although only the MLHF has been specifically validated in patients with aortic valve disease, [56] preliminary experience with the KCCQ in patients undergoing TAVI has also demonstrated a high degree of responsiveness and internal consistency [57].
Recommended endpoints and timing of assessment
Valve Academic Research Consortium-2 recommends that a comprehensive assessment of HRQOL for patients undergoing TAVI incorporate both a heart failure-specific measure (such as the KCCQ or MLHF) as well as one or more generic measures [such as the Medical Outcomes Study Short-Form 36 (SF-36), the Short-Form 12 (SF-12), or the EuroQOL (EQ-5D)] [58–60]. The disease-specific measures offer improved sensitivity/responsiveness as well as clinical interpretability, whereas the inclusion of a generic health status measure is useful because it captures some additional domains. Furthermore, generic measures can enhance the comparability across different diseases and populations and can be used to compare patients with population-level benchmarks.
For the comparison of TAVI vs. SAVR (or for the comparison of alternative access sites for TAVI), we recommend that early QOL assessment be performed at 2 weeks, 1 month, and 3 months using a combination of generic instruments and pain scales (e.g. visual analogue scale) to assess the early recovery process. The evaluation of the QOL at an intermediate time point (e.g. 6 months) could also be considered in order to confirm that QOL recovery is complete by this stage. At later time points (1–5 years), the use of heart failure-specific instruments to identify the consequences of long-term valve performance may be more useful. Finally, the assessment of cognitive function at later time points (1–5 years) may be valuable for the comparison of surgical vs. catheter-based techniques, although these endpoints generally require highly specialized and demanding neuropsychiatric testing [61]. In contrast, for the comparison of alternative TAVI systems (as may be expected in the near future), HRQOL assessment should focus mainly on heart failure-specific endpoints at intermediate and later time points (1–5 years), wherein between-device differences in the haemodynamic performance or structural valve deterioration may emerge. The inclusion of disease-specific QOL measures in these studies can also provide insight into the consequences of valve-related complications such as the need for pacemaker insertion.
Additional considerations
It is essential to ensure complete ascertainment of HRQOL at each time point, as missing data cannot be retrieved retrospectively and statistical adjustment techniques (e.g. multiple imputation) that assume that data are ‘missing at random’ may not be adequate. Differential mortality between two treatments may complicate the interpretation of QOL results since the QOL may appear to ‘improve’ over time even with an ineffective therapy simply because of attrition of the sickest patients. The use of categorical endpoints that characterize outcomes as favourable (e.g. survival AND improvement of QOL endpoints) [44, 57] or endpoints that integrate survival and the QOL (e.g. quality-adjusted life expectancy) may provide more interpretable results. In such cases, reporting the outcomes in both ways (i.e. among the entire study cohort and separately among only the surviving patients) will provide the most complete description of the results.
COMPOSITE ENDPOINTS
Rationale and caveats
Comparisons of the success, safety, and effectiveness with achievable study cohort sample sizes may at times require the use of composite endpoints. However, it is important that composites contain components that have roughly similar impacts on the patient. A family of single endpoints tending in the same direction may, as a family of hypotheses, be statistically significant when individual endpoints are not.
Each post-procedural event has a different temporal risk profile (hazard function) modulated by different risk factors. Therefore, traditionally, the evaluation of the safety and efficacy of procedures has focused on in-hospital events (complications and morbidity), events within 30 days of the procedure, and ‘late’ events.
Specific composite endpoints
The assessment of TAVI, SAVR, and their alternatives or new devices should include device, procedure, and patient-oriented endpoints. These endpoints have been devised to be applicable to both TAVI and SAVR. Previous clinical trials have used the all-cause mortality at 1 year as the primary clinical endpoint. Owing to the emergence of stroke as an important clinical event, future trials should also require the composite of all-cause mortality and disabling stroke as a primary or secondary endpoint.
The first VARC document proposed three composite endpoints: device success, early safety, and clinical efficacy. Valve Academic Research Consortium-2 goes beyond the early and intermediate experience of TAVI, drawing upon prior surgical AVR guidelines to include time-related safety endpoints [62]. Therefore, VARC-2 recommends a new composite endpoint, time-related valve safety, which combines valve dysfunction, endocarditis, and thrombotic complications of the prosthesis (Table 11).
Device success
|
Early safety (at 30 days)
|
Clinical efficacy (after 30 days)
|
Time-related valve safety
|
Device success
|
Early safety (at 30 days)
|
Clinical efficacy (after 30 days)
|
Time-related valve safety
|
BAV: balloon aortic valvuloplasty; TAVI: transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement.
aRefers to VARC definitions.
bAs a basis for calculation of ‘days alive outside the hospital’ endpoint. Supplementary appendix of Leon et al. [43]. Includes heart failure, angina, or syncope due to aortic valve disease requiring intervention or intensified medical management; clinical symptoms of CHF with objective signs including pulmonary oedema, hypoperfusion, or documented volume overload AND administration of IV diuresis or inotropic therapy, performance of aortic valvuloplasty, institution of mechanical support (IABP or ventilation for pulmonary oedema) or haemodialysis for volume overload; clear documentation of anginal symptoms AND no clinical evidence that angina was related to CAD or ACS; documented loss of consciousness not related to seizure or tachyarrhythmia.
cDepending on the body surface area.
Device success
|
Early safety (at 30 days)
|
Clinical efficacy (after 30 days)
|
Time-related valve safety
|
Device success
|
Early safety (at 30 days)
|
Clinical efficacy (after 30 days)
|
Time-related valve safety
|
BAV: balloon aortic valvuloplasty; TAVI: transcatheter aortic valve implantation; SAVR: surgical aortic valve replacement.
aRefers to VARC definitions.
bAs a basis for calculation of ‘days alive outside the hospital’ endpoint. Supplementary appendix of Leon et al. [43]. Includes heart failure, angina, or syncope due to aortic valve disease requiring intervention or intensified medical management; clinical symptoms of CHF with objective signs including pulmonary oedema, hypoperfusion, or documented volume overload AND administration of IV diuresis or inotropic therapy, performance of aortic valvuloplasty, institution of mechanical support (IABP or ventilation for pulmonary oedema) or haemodialysis for volume overload; clear documentation of anginal symptoms AND no clinical evidence that angina was related to CAD or ACS; documented loss of consciousness not related to seizure or tachyarrhythmia.
cDepending on the body surface area.
DISCUSSION
Although the original VARC standardized endpoint definitions were fundamentally useful and have been widely adopted, growing experience with TAVI studies has identified some definitions as ambiguous, of limited clinical utility, or in need of updating or extension [5, 6, 63, 64]. This need provided the rationale for a VARC-2 document with such improvements and additions. As was the case with the original VARC process, it should be emphasized that this consensus manuscript is not intended to be a guidelines document, but rather a practical tool to facilitate and inform clinical research in TAVI.
Current clinical trials are focusing more on intermediate risk patients, and more studies are comparing TAVI with surgical AVR. Therefore, it becomes increasingly important to identify those patients who benefit from either treatment. Specific risk categories have been defined to allow universal clinical study designs and outcome comparisons.
Changes and additions that have been applied to improve the interpretation of clinical endpoint definitions and provide further insights on TAVI-related outcomes are as follows: (i) risk stratification should be done by a dedicated ‘heart team’ and include other factors (e.g. frailty, porcelain aorta) beyond the traditional risk scores, and should take into account co-existing conditions; (ii) immediate procedural death has been added to capture intra-procedural events that result in immediate or consequent death; (iii) stroke ascertainment requires the use of precise definitions, standardized assessments, close collaboration with neurology experts including the consideration of acute stroke management, and has been re-categorized as non-disabling or disabling; (iv) detailed documentation of the aetiology of strokes and concomitant therapies is needed to provide insights into the multi-factorial nature of acute, early, and late strokes; (v) closure device failure is now a separate category within vascular complications, and if unplanned percutaneous or surgical intervention does not lead to adverse outcomes, these are not considered as a major vascular complication per se; (vi) the time for AKI diagnosis has been extended from 72 h to 7 days; (vii) AKI is diagnosed according to AKIN guidelines, which include classification by the urine output to detect a wider range of aetiologies; (viii) peri-procedural myocardial infarction is defined by troponin or CK-MB elevation and the troponin threshold has changed from 10× ULN to 15× ULN based on recent data; [19] (ix) assessment of conduction disturbances and arrhythmias has been reinforced; [65–68] (x) new definitions for several TAVI-related complications and valve malpositioning are reported; (xi) echocardiography parameters of prosthetic valve stenosis and regurgitation have been updated and now include the assessment of the prosthesis–patient mismatch; (xii) for the QOL assessment, VARC-2 recommends the use of both heart failure-specific and generic measures during the follow-up between 30 days and 5 years to fully assess the impact of the procedure and the durability of clinical benefit. These definitions can be used in studies comparing TAVI to surgical AVR, as well as in future trials comparing first generation to next generation TAVI devices.
The composite endpoint of device success has specifically been criticized for being too strict with regard to valve performance; for example, an AVA >1.2 cm2 seems unachievable in patients with smaller body habitus [5]. The current VARC-2 definition therefore corrects for the body surface area so that valve performance is now assessed through the indexed EOA. It is notable that valve-in-valve procedures for failing bioprostheses will frequently have a low device success, even with this modified definition [69]. Considering that stroke has emerged as an important concern, the composite of all-cause mortality and disabling stroke should be considered as a primary or secondary endpoint in future trials. Two ongoing large randomized trials [PARTNER II (NCT01314313) and SURTAVI (NCT01586910)] are already incorporating these composite endpoints.
With longer follow-up duration, it becomes more critical to include time-related valve safety composite endpoints. This will eventually provide linearized rates of complications with transcatheter valves, known as ‘objective performance criteria’, as has been used to evaluate surgical valves [70].
With this VARC-2 document, we have provided further standardization of endpoint definitions and hope that the adoption of these criteria will continue to increase, ultimately leading to improved comparability and interpretability of the study results.
Funding
Grants have been provided to the ARC Board including representatives of Cardialysis, the Cardiovascular Research Foundation, Duke Clinical Research Institute, and Harvard Clinical Research Institute to cover the costs of travel, meeting rooms, and lodging for academic attendees at the Washington and Rotterdam meetings by Abbott Vascular, Boston Scientific, Direct Flow Medical, Edwards Lifesciences, Heart Leaflet Technologies, Medtronic Corporation, and St. Jude Medical.
Conflicts of interest: N. Piazza has received consultancy fees from Medtronic, his institution has received grants/grants pending from Medtronic. E.H. Blackstone has received study support from Edwards Lifesciences consultancy fees from Edwards Lifesciences. D.J. Cohen has received consultancy fees from Medtronic, his institution has received grants/grants pending from Medtronic and Edwards Lifesciences. G.A van Es is an employee of Cardialysis BV, Netherlands. M.W. Krucoff has received study support from Edwards Lifesciences. S. Kodali is a Board member of St. Jude Medical and Thubrikar Aortic Valve and has received consultancy fees from Medtronic and Edwards Lifesciences. R. Mehran has received consultancy fees from Astra Zeneca, Janssen (Johnson & Johnson), Regado, Abbott Laboratories, Merck Sharpe & Dohme Corp., Maya Medical. Her institution has received grants/grants pending from BMS/Sanofi, The Medicines Company, Lilly/Daiichi Sanko. J. Rodés-Cabau has received consultancy fees from Edwards Lifesciences, St. Jude Medical. J.G. Webb has received consultancy fees from Edwards Lifesciences, his institution has received grants/grants pending from Edwards Lifesciences. S. Windecker has received speakers bureaus fees from Edwards Lifesciences and Medtronic, his institution has received a Swiss National Science Foundation Grant (32003B_135807) and has grants/grants pending from Edwards Lifesciences and Medtronic. M.B. Leon is on the advisory Board for Edwards Lifesciences. The others authors have declared to have no conflict of interests for this paper. The VARC meetings involved members of the Interventional Cardiology Devices Branch, of the Office of Device Evaluation, Center for Devices and Radiological Health, USFDA. The opinions or assertions herein are the private views of the authors and are not to be construed as reflecting the views of the FDA.
REFERENCES
APPENDIX 1
VARC contributing groups
(1) Academic Research Organizations (2) Societies (3) US Food and Drug Administration
Cardialysis (Rotterdam, Netherlands)
Cardiovascular Research Foundation (New York, NY, USA)
Duke Clinical Research Institute (Durham, NC, USA)
Harvard Clinical Research Institute (Boston, MA, USA)
American College of Cardiology
European Association for Cardio-Thoracic Surgery
European Society of Cardiology
Society of Thoracic Surgeons
(4) Industry representatives
APPENDIX 2
VARC participants
(1) Clinical Research Organizations (2) Cardiologists (3) Surgeons (4) Echocardiographers (5) Neurologists (6) Statisticians (7) US Food and Drug Administration (8) Industry representatives (9) Observers: other
Cardialysis/Erasmus MC, Rotterdam, Netherlands
Head, SJ
Morel, MA
Serruys, PW
Van Es, GA
Van Mieghem, NM
Vranckx, P
Cardiovascular Research Foundation, New York, NY, USA
Généreux, P
Hahn, RT
Kirtane, AJ
Kodali, SK
Leon, MB
Maxwell, Y
Mehran, R
Duke Clinical Research Institute, Durham, NC, USA
Alexander, KP
Douglas, DS
Krucoff, MW
Petersen, J
Harvard Cardiovascular Research Institute, Boston, MA, USA
Cutlip, DE
Borer, JS: Howard Gilman Institute for Heart Valve Diseases, Brooklyn, NY, USA
Cohen, DJ: Saint Luke's Mid America Heart Institute, Kansas City, MO, USA
Holmes, DR Jr: Mayo Clinic Rochester, Rochester, MN, USA
Iung, B: CHU Bichat, Paris, France
Makkar, RR: Cedars-Sinai Heart Institute, Los Angeles, CA, USA
Piazza, N: German Heart Center, Munich, Germany; and McGill University Health Center, Montreal, Canada
Popma, JJ: Beth Israel—Deaconess Medical Center, Boston, MA, USA
Rodès-Cabau, J: Quebec Heart and Lung Institute, Quebec, Canada
Thomas, M: Guys and St Thomas Hospital, London, UK
Tuzcu, EM: Cleveland Clinic Foundation, Cleveland, OH, USA
Vahanian, A: CHU Bichat, Paris, France
Webb, JG: St Paul's Hospital, Vancouver, BC, Canada
Windecker, S: University Hospital Bern, Bern, Switzerland
Adams, DH: Mount Sinai Medical Center, New York, NY, USA
Cameron, DE: The Johns Hopkins Medical Institutions, Baltimore, MD, USA
Fontana, GP: Lenox Hill Heart and Vascular Institute, New York, NY, USA
Kappetein, AP: Erasmus MC, Rotterdam, Netherlands
Mack, MJ: Baylor Health Care Systems, TX, USA
Maisano, F: San Raffaele Hospital, Milan, Italy
Miller, DC: Stanford University, CA, Stanford, USA
Moat, NE: Royal Brompton and Harefield National Health Service (NHS) Foundation Trust, London, UK
Walther, T: Kerckhoff Heartcenter Bad Nauheim, Bad Nauheim, Germany
Geleijnse, ML: Erasmus University Medical Center, Rotterdam, Netherlands
Brott, TG: Mayo Clinic, Jacksonville, FL, USA
Van der Worp, HB: University Medical Center Utrecht, Utrecht, Netherlands
Blackstone, EH: Cleveland Clinic Foundation, Cleveland, OH, USA
Aguel, F
Dunn, B
Getzoff, N
Laschinger, J
Patel, S
Sansing, V
Sastry, A
Swain, J
Zuckerman, B
Akin, J: Edwards Lifesciences, Orange, CA, USA
Allocco, D: Boston Scientific, Minneapolis, MN, USA
Armitage, T: Medtronic CardioVascular, Minneapolis, MN, USA
Bebeau, V: St Jude Medical, Minneapolis, MN, USA
Concepcion, B: Boston Scientific, Minneapolis, MN, USA
Fonseca, T: Medtronic CardioVascular, Minneapolis, MN, USA
Robb, R: Medtronic CardioVascular, Minneapolis, MN, USA
Schroeder R: Heart Leaflet Technologies, Minneapolis, MN, USA
Sethuraman, B: Abbott Vascular, Santa Clara, CA, USA
Sheahan, B: Direct Flow Medical, Santa Rosa, CA, USA
Tatarek, N: St Jude Medical, Minneapolis, MN, USA
Fitzgerald, S
Carroll, JD
Edwards, FH
Lansky, AJ
Prager, RL
Author notes
The Valve Academic Research Consortium (VARC) consists of representatives from several independent Academic Research Organizations, several Surgery and Cardiology Societies, members of the US Food and Drug Administration (FDA), and several independent experts. However, it is not a society document. Neither the societies nor the FDA has been asked to endorse the document.
- aortic valve stenosis
- cardiac arrhythmia
- myocardial infarction
- hemorrhage
- cerebrovascular accident
- ischemic stroke
- renal failure, acute
- netherlands
- surgical procedures, operative
- united states food and drug administration
- diagnostic imaging
- mortality
- quality of life
- stratification
- surrogate endpoints
- vascular complications
- transcatheter aortic-valve implantation
- consensus




