-
PDF
- Split View
-
Views
-
Cite
Cite
Alfredo Mussi, Olivia Fanucchi, Federico Davini, Marco Lucchi, Alessandro Picchi, Marcello Carlo Ambrogi, Franca Melfi, Robotic extended thymectomy for early-stage thymomas, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages e43–e47, https://doi.org/10.1093/ejcts/ezr322
Close - Share Icon Share
Abstract
The aim of this study was to report a single referral centre experience in robotic extended thymectomy for clinical early-stage thymomas, evaluating its safety, feasibility and efficacy, with special regard to oncological outcomes.
Between April 2002 and February 2011, we retrospectively selected all those patients who underwent robotic thymectomy for clinical early-stage thymomas. Operative time, morbidity, mortality, duration of hospitalization and overall and disease-free survival were analysed.
There were 14 patients (8 males, 6 females) with a mean age of 65.2 years (range 23–81). One patient suffered from myasthenia gravis. The WHO classifications were: A in two cases, AB in four cases, B1 in three cases, B2 in two cases and B3 in three cases. The Masaoka stages were: I in seven cases, IIA in four cases, IIB in two cases and III in one case. The mean operative time was 139 min. No intra-operative complication or death occurred. Conversion to open surgery was required in two cases. Minor complications occurred in two patients (14.2%) due to pleural effusion. The mean hospitalization was 4.0 days. Five patients underwent adjuvant radiotherapy. All patients were alive with no disease recurrence, with a median follow-up of 14.5 months (range 1–98).
Robotic thymectomy is a safe and feasible technique, with a short operative time and low morbidity. Even on a small series with short follow-up, robotic extended thymectomy for thymoma appeared to be an effective treatment for early-stage thymomas.
INTRODUCTION
Thymoma represents a rare tumour of the thymic gland, with a typical slow growth, associated with a spreading by local extension to the pleura, pericardium or diaphragm, although extrathoracic metasteses are uncommon [1]. The complete resection of thymoma en-bloc, with all thymic and peri-thymic tissue is the main intent of surgery, which represents the best opportunity of cure. A recent review reported that the 5-year overall survival rates of patients with Stage I or II thymoma, who underwent surgical resection, ranged from 89 to 100% and 71 to 95%, respectively [2]. However, some criticisms with regard to the surgical approaches still remain. Over the last decades, video-assisted thoracoscopic surgery (VATS), and more recently robot-assisted surgery, has been introduced in the thoracic field. These minimally invasive approaches have frequently been described for treatment of benign diseases of the mediastinum or for thymectomy in cases of myasthenia gravis (MG) [3–8]. However, with regard to thymomatous disease, few published data are available describing the outcomes of minimally invasive approaches, which still remain controversial. Thus, after long experience with robotic surgery, we decided to apply this approach for thymectomy in patients with a clinical early-stage thymoma. The main aim of this study was to assess the safety and the feasibility of robotic thymectomy for an early-stage thymoma. The secondary aim was the evaluation of the oncological outcomes.
MATERIALS AND METHODS
We retrospectively reviewed all patients undergoing robotic thymectomy for clinical early-stage thymoma at the University of Pisa. The Institutional Review Board approved the study. All patients signed a detailed informed consent.
Age, gender, presence of MG, comorbidities were recorded for each patient. MG was stratified according to the Myasthenia Gravis Foundation of America (MGFA) classification [9]. Comorbidity scores were recorded using the Adult Co-Morbidity Evaluation scoring system (ACE-27). In addition, the operative time (defined as the time between skin incision and skin closure), docking time (defined as the time necessary for surgical cart positioning and robotic arms placement in the surgical field), conversion rate, morbidity and mortality were recorded. The safety assessment included identification of treatment-related complications. These were those occurring within 30 days of treatment. Complications were stratified according to the Clavien–Dindo classification [10]. The Masaoka staging system was used to define clinical and pathological stage of the thymoma [11], whereas the new World Health Organization classification was used for histological classification [12].
Robotic system
The da VinciTM Robotic System (Surgical Intuitive, Sunnyvale, CA, USA) consists of a master console, a computer controller and a three-arm surgical manipulator with fixed remote centre kinematics connected via electrical cables and optic fibres. The master console was connected to a surgical manipulator with instrument arms and an endoscope arm. The endoscope (12 mm in diameter) consisted in two separate 5 mm high-resolution cameras that showed two separate images. Specific robotic surgical instruments were introduced through special ports (8 mm) and attached to the arms of the robot. The movements of surgeon's hand are transmitted to the tips of the robotic instruments.
Surgical technique
All patients underwent general anaesthesia with double-lumen intubation. The patient was positioned left side up at 30°. The first incision was generally performed in the fifth intercostal space at the anterior–axillary line. Thus, the camera was inserted to explore the chest cavity, and CO2 was inflated (ranging between 4 and 8 mmHg) to enlarge the operating space and safely perform the other port incisions: at the anterior–axillary line at the third intercostal and at the fifth intercostal space at the mid-clavicular line (Fig. 1). The right arm had a Spatula (EndoWrist; Intuitive Surgical) with electric cautery function to perform dissection, whereas the left arm had a Cadiere forcep (EndoWrist; Intuitive Surgical), an atraumatic instrument for grasping the normal thymus. The 30° scope permitted an excellent visualization of normal thymic tissue and thymoma's capsule. The normal thymic tissue and peri-thymic fat were used for grasping and for traction of the tumour, avoiding the direct manipulation of the tumour, to minimize the risk of a capsule damage.
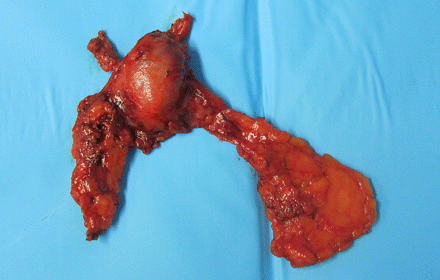
After inspection of the thymus gland and individuation of the thymoma, the dissection generally started from inferiorly, first from the left side, at the pericardiophrenic angle. Then it continued on the right side, from the retrosternal area, finding the right mediastinal pleura and the right phrenic nerve, permitting a safe dissection of the right inferior horn under direct vision of the nerve. Consequently, the dissection continued upward to the neck until the superior horns were identified. The thymic veins were identified, and separately clipped. The lesions were removed with Endoscopic Pouches (InziiTM, Applied Medical) from the cavity through the port incision in the mid-axillary region. If necessary, the incision was enlarged (3 cm maximum). No additional utility incision was used. A drainage tube was inserted, generally 32Ch. All thymus and perithymic fat was dissected, according to the Masaoka criteria [13], and the completeness of the thymectomy was assessed by macroscopic inspection of the thymic bed and specimen (Figs 2 and 3).
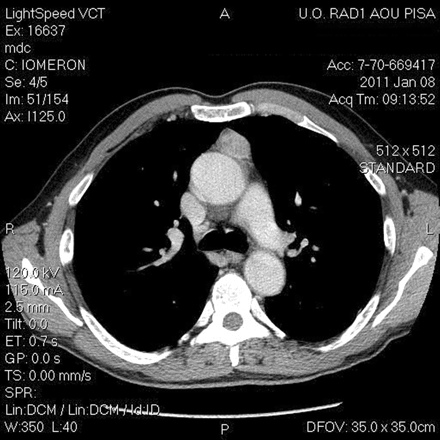
CT imaging of a 59-year old man, with a suspected well-capsulated thymoma.
Follow-up
The clinical and radiological follow-up was performed at our consulting room. It consisted of physical examination and CT scan imaging at 3, 6 and 12 months after surgery, and then every year. The patient affected by MG also underwent neurological evaluation and dosage of acetylcholine receptor antibodies.
Statistical analysis
Statistical analysis was performed using Statistica software version 7.0 for PC (Stat-Soft, Inc., Tulsa, OK, USA). Statistical analysis was expressed in terms of frequency, mean and range. Disease-free survival was defined as the time from surgery to the first diagnosis of recurrence. The overall survival was defined as the period of time from treatment to death or last follow-up.
RESULTS
We operated on eight males and six females, with a mean age of 65.2 years, ranging between 23 and 81. One patient suffered from MG, class I according to the MGFA clinical classification. The most important comorbidities are summarized in Table 1. The ACE-27 score resulted Grade 0 in five cases, Grade 1 in five cases, Grade 2 in two cases and Grade 3 in two cases. The mean operative time was 139 min, ranging between 85 and 180. The mean docking time was 6.5 min, ranging between 5 and 15 min. No intra-operative complications occurred, nor vessel or nerve injury. No mortality occurred. In two cases, an unexpected degree of tumour invasion, which was not evident at the pre-operative CT scan, was macroscopically observed during thoracoscopic inspection. In one case, the tumour was infiltrating the pericardium infiltration, thus, according to our policy, conversion to open surgery was performed. Histopathological examination confirmed the pericardium infiltration (Stage III). In the other case, the tumour seemed to infiltrate the pericardium at thoracoscopic inspection, but after conversion to open approach, the lesion resulted only adherent to the pericardium. These two cases occurred at the beginning of our experience, and were excluded from the survival analysis. Morbidity (Grade II complications according to Clavien–Dindo classification) occurred in two patients, due to pleural effusion treated and resolved with medical therapy. The mean chest drainage duration was 1.6 days (range 1–3). The mean post-operative stay was 4.0 days (range 3–5). Most of the patients were fit for discharged on third post-operative day, but stayed longer for social reasons (i.e. long distance from home). The mean size of the thymomas was 3.3 cm (range 1.4–4.5 cm). The Masaoka stages were I in seven cases, IIA in four cases, IIB in two cases and III in one case (pericardium infiltration). The WHO classifications were: A in two cases, AB in four cases, B1 in three cases, B2 in two cases and B3 in three cases. Five patients underwent adjuvant radiotherapy. Two patients refused it.
| Comorbidities . | Number of patients . |
|---|---|
| Hypertension | 3 |
| Coronary artery disease | 2 |
| Chronic atrial fibrillation | 1 |
| Nephrosic syndrome | 1 |
| Chronic renal failure | 1 |
| Previous TIA | 1 |
| MG | 1 |
| Others | 3 |
| Comorbidities . | Number of patients . |
|---|---|
| Hypertension | 3 |
| Coronary artery disease | 2 |
| Chronic atrial fibrillation | 1 |
| Nephrosic syndrome | 1 |
| Chronic renal failure | 1 |
| Previous TIA | 1 |
| MG | 1 |
| Others | 3 |
Five patients have two or more comorbidities (coronary artery disease was defined as a positive exercise testing or prior myocardial infarction or prior coronary bypass.
TIA: transitory ischaemic attack; MG: myasthenia gravis.
| Comorbidities . | Number of patients . |
|---|---|
| Hypertension | 3 |
| Coronary artery disease | 2 |
| Chronic atrial fibrillation | 1 |
| Nephrosic syndrome | 1 |
| Chronic renal failure | 1 |
| Previous TIA | 1 |
| MG | 1 |
| Others | 3 |
| Comorbidities . | Number of patients . |
|---|---|
| Hypertension | 3 |
| Coronary artery disease | 2 |
| Chronic atrial fibrillation | 1 |
| Nephrosic syndrome | 1 |
| Chronic renal failure | 1 |
| Previous TIA | 1 |
| MG | 1 |
| Others | 3 |
Five patients have two or more comorbidities (coronary artery disease was defined as a positive exercise testing or prior myocardial infarction or prior coronary bypass.
TIA: transitory ischaemic attack; MG: myasthenia gravis.
No patient was lost at follow-up. At the time of analysis (mean follow-up 14.5 months, range 1–98), no patients had local or pleural disease recurrence. For the patient affected by MG, at the time of analysis, the MGFA change in status results improved.
DISCUSSION
The open surgical approach was generally accepted as a gold standard for resection of thymoma [3–5]. However, over the last decades, both thoracoscopic and, more recently, robotic approaches have been introduced into the thoracic field and also applied for thymectomy. Several data were published regarding minimally invasive thymectomy for MG, reporting interesting outcomes, emphasising less operative trauma, the shorter hospital stay, preserved pulmonary function and cosmetic results [8, 14–16]. With regard to neurological outcomes, Cakar et al. [17] demonstrated that robotic thymectomy provides at least the same positive effect as open transsternal thymectomy, whereas Ruckert et al. [18] reported an improved outcome for the robotic thymectomy in respect to thoracoscopic thymectomy.
Although these are encouraging data, few studies have been published for minimally invasive treatment of thymoma. Since 1992, some papers have attested to the possibility of performing thymectomy for Masaoka Stage I and II thymomas with VATS [3, 4, 19]. Moreover, although the first paper describing the robotic resection of a Masaoka Stage I thymoma was published by Yoshino et al. in 2001 [20], few data focusing on the robotic approach for thymoma exist. Thus, we want to retrospectively report our experience on robotic thymectomy for treatment of clinical early-stage thymomas.
After an initial training phase, necessary to improve our skill with the master console and robotic instrumentations, and as we gained experience with the removal of mediastinal lesions, we reasoned that the utilization of CO2 inflation and 30° scope permit an excellent visualization of the mediastinal field, of all thymic and peri-thymic tissue and thymoma's capsule. Additionally, the articulation of the tip of the robotic instrument inside the chest cavity allows the surgeon to operate around corners and in the remote areas, such as the neck. These facts have led us to evaluate the robotic approach for well-capsulated thymomas.
In our study, the mean docking time and the mean operative time were adequately low. Unfortunately, there is no comparable paper solely focusing on robotic thymectomy for thymoma. Bodner et al. [21] and Savitt et al. [22] reported a similar operative time on a heterogeneous series of resections of mediastinal lesions, including thymomas. However, the operative time resulted similar with those reported in other studies for robotic thymectomy in the case of MG [8, 14], and for VATS thymectomy in the case of thymoma [23, 24]. Moreover, in our series, the morbidity rate resulted low and comparable with the 6–10% reported for robotic thymectomy for MG [8, 14]. Agasthain et al., on a large series of VATS thymectomies, reported one case of phrenic nerve injury, which, however, was not observed in other papers [14, 24, 25], nor in this study.
Nevertheless, with regard to oncological outcomes, some criticisms of minimally invasive approaches still exist. First, the possibility of tumour spreading into chest cavity was reported in some papers [3]. Agasthian et al. [25] reported a local recurrence rate of 3.4% in patients who underwent VATS thymectomy for thymoma, while two recent papers compared the VATS and the open approaches for thymomas. Cheng et al. [24] observed no local or pleural disease recurrence for Stage II thymoma, either in the open or in the VATS groups, and similar results were published by Pennathur et al. [25] in a larger comparative study, reporting no significant difference in disease recurrence and overall survival between the two groups. In our small series, even if the follow-up period is short, we observed no local or pleural recurrence. We believe that the robotic system facilitates the individuation of thymoma capsule and normal thymic tissue, allows safe manipulation. Nevertheless, an important factor that affected the success of minimally invasive approach is probably represented by the dimension of the lesion. In our series, the mean thymoma dimension was 3.3 cm, similar to those reported in the above-mentioned comparative studies for the VATS approach [24, 25]. However, the indolent nature of thymomatous disease requires a long follow-up, 10 years, in order to evaluate survival and disease-free rate, thus further multi-institutional studies, on larger series, are necessary.
In addition, with regard to the robotic approach, an important criticism is represented by the cost (cost of robotic system, of annual maintenance and of the disposable materials). At our department, this problem was in part resolved with a multidisciplinary organization of the dedicated ‘robotic’ operating theatre, which is utilized by different surgical divisions during the week.
The present study has some limitations, including its retrospective nature, which may have resulted in a selection bias. Moreover, the study sample was small, with a short follow-up period. However, this is an initial experience, resulting from gained skill in robotic surgery for mediastinal lesions, and the main aim was to analyse the safety and the technical feasibility of the robotic approach for early-stage thymomas.
In fact, few data regarding the robotic approach focusing on thymoma are published, and our small series is the largest one to our knowledge. However, this preliminary study demonstrates the safety and feasibility of robotic thymectomy for thymoma, with no mortality, low morbidity and no nerve or vessel injury. Nevertheless, it is hoped that randomized multi-institutional trials with long follow-up will be designed to compare the transsternal and robotic approaches and evaluate the oncological outcomes.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr R. Schmid(Berne, Switzerland): This series dates from 2002 to 2011 and you have 14 cases; I know you are a high-volume centre, so your selection criteria were quite rigid. Is it only size, as you mentioned, or is it also location of the thymoma? Can you outline your criteria a bit more?
Dr Fanucchi: Generally, the criteria were mainly the dimension and the absence of tumour invasion of contiguous structures. The patient number is small, I know, but at the beginning of our experience we were reluctant to introduce this kind of approach for oncologic disease. So, we began with thymic hyperplasia, and then we decided to apply this approach also for thymoma, for only early-stage thymoma, clinical early stage.
Dr Schmid: So, the limiting factor was more oncologic?
Dr Fanucchi: Yes, we selected only early stage.
Dr Schmid: I have another question, and I don’t know if you mentioned it, the extent of resection. I think there is not much evidence that you have to do a total thymectomy. I know this topic is controversial. What is your policy in thymoma?
Dr Fanucchi: Our policy is to do an extended thymectomy, so from the phrenic nerve to phrenic nerve, following the Masaoka criteria.
Dr Schmid: If you have a patient with myasthenia gravis, it might be correct, but do you have strong evidence from the literature that you have to do a complete thymectomy?
Dr Fanucchi: These are really a few cases. We don’t yet have results with regard to the oncologic outcomes.
Dr Schmid: Not from you. I mean we do many things and we don’t realise that there is not much supporting evidence. We went through the literature and found that there is not much evidence. I know, I do it the same way. In addition, you mentioned operation time. When discussing robotic surgery, one major topic is operation time and OR time. Can you tell us the operation and OR usage times?
Dr Fanucchi: In the slides, you saw the operation time skin-to-skin, so from the first incision to the closure of all the ports, but we have to consider that there is a docking time, the time necessary for the machine to be attached to the patient so that the surgeon can really start the operation. Generally, it takes some minutes. At the beginning of our experience, it took 15 or 20 minutes, but now we have standardized the technique and we rapidly attach the robot and begin the procedure.
Dr Schmid: But maybe it is better to also give the OR usage time when you discuss minimally invasive surgery. In your manuscript, you stated that the patients were all ready to leave on the third postoperative day, but they remained in hospital for social reasons. When you do minimally invasive surgery, you want to save money. You spend the money in the OR and you want to save the money by reducing the hospitalization time. These patients were ready to go on the third day but were kept until the fifth or sixth day. Can you discuss this?
Dr Fanucchi: I said that many patients live far away from the hospital and our neurologists suggested prolonging their hospitalization in order to be sure that everything went well. It has changed a little bit in the last year, and generally all the patients are discharged on the third postoperative day. I know it is a contradiction, minimally invasive surgery and prolonged hospitalization, but this is what happened.
Dr P. Van Schil(Antwerp, Belgium): I would like to come back to Professor Schmid's first two questions. We also are doing robotic thymectomies for thymoma, and I think that location is equally as important as size. It's not very difficult to remove a 4-cm thymoma invading the pericardium on the left side. You can easily open up the pericardium. But a 4-cm thymoma lying just in front of the left brachiocephalic vein with invasion of the vein is another issue. Do you agree that that's more difficult and such cases should be better done by sternotomy?
Dr Fanucchi: I agree with you. I think it's more difficult. Our patients were really well-selected patients and we used the left-sided approach, and all the thymomas generally were on the medial region or on the left side.
Dr Van Schil: Secondly, indeed we don’t have enough data on thymoma and the extent of resection, so within the EACTS we created the Thymic Working Group, with Dr. Marco Lucchi from your institution as current chair. In addition, ITMIG (the International Thymic Malignancies Interest Group) has now created a prospective database to obtain more good quality data from centres all over the world. So, I would like to encourage you to include patients in that database or to contact Dr. Lucchi if you are interested in participating.
Dr Fanucchi: Yes. We work together in the same Thoracic Division.
Dr K. Yamamoto(Kyoto, Japan): We normally perform this kind of resection through the thoracoscopic approach. I would like to know the advantage of robotic resection over thoracoscopic resection. The cost-effectiveness is a little bit different between the two approaches, so I would like to know the advantage of it.
Dr Fanucchi: The advantage of the robotic system compared to the traditional VATS approach is firstly the three-dimensional vision. It's really useful. The second advantage, which is really important, is the 7 degrees of movement of the instrumentation inside the chest cavity. The surgeon has a really high degree of movement inside the chest cavity.
Dr Yamamoto: So, you mean with the three-dimensional image, you can skip videothoracoscopy?
Dr Fanucchi: Yes. The surgeon who is performing the operation is at the console and has three-dimensional vision inside the console.
Dr T. Folliquet(Paris, France): To answer your question, there have been some very well-executed studies done on 3-D imaging and 2-D. If you take, for example, experienced surgeons in video, or beginners, medical students, the 3-D vision with the da Vinci decreases the learning curve. If you are an experienced video surgeon, then you can cope with it, but really the da Vinci for any type of operation, with the 3-D and the extra 7 degrees of freedom, allows you to do these operations more quickly and with a lesser learning curve than with video.
Author notes
Presented at the 25th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 1–5 October 2011.




