-
PDF
- Split View
-
Views
-
Cite
Cite
Murat Tavlasoglu, Artan Jahollari, Anar Amrahov, Mehmet Ali Sahin, An instrument facilitates mitral valve repair training at home, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages 940–941, https://doi.org/10.1093/ejcts/ezr087
Close - Share Icon Share
Abstract
The importance of surgical simulation has grown in the quickly changing climate of modern surgical training. Prior to operating on human hearts, practice in appropriate experimental models is necessary to attain adequate experience. Nowadays, training of surgery residents has shifted to simulation workshops and residency programmes outside the operating theatre. We have experience in training our residents to perform mitral valve repair techniques in bovine hearts. Previously, the heart would be fixed on the tablecloth with simple stitches, which proved to be a complex and difficult technique while performing surgery. Moving forwards, we have built a successful ‘surgical table’ to achieve better stabilization and to simplify the surgery. This paper describes our model, which could be a helpful tool for any cardiac surgeon performing surgical techniques successfully at home.
INTRODUCTION
In recent years, simulation in surgery has been discussed by many educators. A bovine or porcine model of cardiac surgical simulation can be performed in surgical laboratories. However, in our study model, a surgical table was designed to give the opportunity to surgery residents to learn by oneself at home. With this table, mitral, aortic and tricuspid repair or coronary anastomotic techniques can be studied and experimented home alone. It is a cheap tool, and the presence of a second person for assistance is not necessary. It can be used in hospitals to train cardiac surgical residents who do not have surgical laboratories.
TECHNIQUE
Two chromium steel rods were fixed on to two corners of a tea tray (30 × 40 × 2 cm), and two left atrial retractors were mounted on each rod, capable of three-dimensional movements. A smaller table was fixed obliquely in the centre of the surgical table to hold the bovine heart (Fig. 1a).
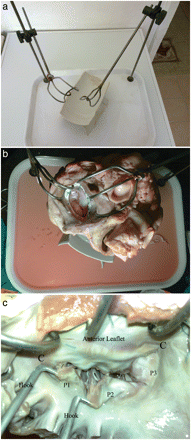
(a) Surgical table: each atrial retractor can be moved upwards, downwards, right, left, backwards, forwards and circumferentially. A smaller table is fixed obliquely in the centre. (b) A bovine heart is placed on the small table within the surgical table and the left atrial walls are retracted; the posterior part of the left atrium is removed and the aorta is closed with a 3-0 silk suture. (c) In this examination, there is an elongation in the P2 scallop next to P1.
The heart set-up begins by assessing the heart for non-anatomic defects from the butchering process. To perform tricuspid valve repair, the right ventricle and right atrium must be intact. To perform David procedures, the aortic root, sinuses of valsalva and aortic cusps must be undamaged. In this study, we attempted mitral valve repair procedures.
The aorta was closed with a running 3-0 silk suture to prevent leakage from the aortic valve during the saline test after mitral repair. Pulmonary veins were ligated with 3-0 silk sutures and a left atriotomy was performed. The atrial retractors were fixed to the atrial wall with three or four simple sutures. The gravity force of the heart and the opposite forces of the retractors provide stabilization (Fig. 1b). The Mitral valve apparatus was examined by starting from the P1 scallop with nerve hooks in order to assess any chordal rupture or elongation (Fig. 1c). If there were no leaks in the valve, a lesion (chordal cut, resection or perforation of the leaflets) was created in the mitral apparatus and then the valve was examined again to select the most suitable repair technique.
After repair, the left ventricle was filled with water to perform a saline leakage test (Fig. 2a–c).
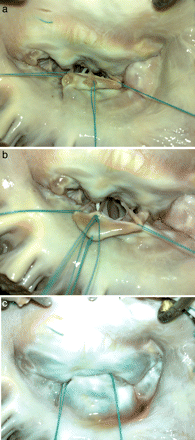
(a) A healthy secondary chorda is cut beneath the P2 scallop to be transferred to the free margin of the elongated part. Note the stay suture at each side of the elongated part of P2. (Note that a white subject like catheter is visible inside the left ventricle in (a) and (b) is the flashing of another secondary chorda while taking the photograph.) (b) The secondary chorda is sutured to the free margin with three interrupted 5-0 polyester sutures. (c) Following completion of the repair, the left ventricle is filled with water and no leaks are observed.
COMMENT
Valve repair offers a distinct event-free survival advantage compared with replacement with a bioprosthetic or mechanical valve [1–3]. Despite a consensus in guidelines encouraging mitral valve repair [4], it is interesting to note that a significant number of patients with degenerative mitral-valve disease still undergo planned mitral valve replacement all over the world, including in the USA [5]. This results in many patients receiving a valve replacement, not because the valves are irreparable, but because they are operated on by surgeons who do not have the specific expertise required to complete a successful repair [5].
In surgical education, a desire to move basic skills-acquisition out of the operating theatre still exists [6]. Therefore, to increase the experience in heart surgery, performing on porcine or bovine heart models is essential.
Our cardiac surgical table was designed to accomplish valve surgery (mitral valve repair, aortic valve sparing procedures, tricuspid valve repair etc.). Not only surgical residents, but also staff members and cardiac surgeons who wish to make training in complex surgical techniques such as mitral or aortic valve repair without any assistance, can use this surgical table.
Conflict of interest: none declared.




