-
PDF
- Split View
-
Views
-
Cite
Cite
Hitendu Dave, Barbara Rosser, Kim Reineke, Sylvie Nguyen-Minh, Walter Knirsch, René Prêtre, Aortic arch enlargement and coarctation repair through a left thoracotomy: significance of ductal perfusion, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages 906–912, https://doi.org/10.1093/ejcts/ezr110
Close - Share Icon Share
Abstract
To analyse the technique of neonatal aortic arch enlargement without cardiopulmonary bypass through a left posterior thoracotomy, as an adjunct to extended resection for Coarctation and severe arch hypoplasia.
Ten neonates with coarctation, severe arch hypoplasia and a persistent ductus arteriosus (PDA) were subjected to arch repair through a left posterior thoracotomy. Nine of these patients had associated significant intracardiac anomalies; three of them received pulmonary artery (PA) banding. After exclusion from circulation, the roof of the intervening arch between left carotid and left subclavian was enlarged using a patch. After adequate reperfusion, a classic resection and extended end-to-end anastomosis was performed. Median age and weight were 5.5 (1–10) days and 3.3 (2.2–4.1) kg respectively. The median preoperative arch diameter was 1.07 (0.75–1.32) mm/kg body weight.
All patients could be successfully operated with this approach. The non-ischaemic and ischaemic aortic clamp times were 40 (15–68) and 23 (18–32) min, respectively. The median postoperative arch diameter achieved was 1.43 (1.06–1.46) mm/kg body weight. None of the patients had significant gradient early postoperatively. Two patients with recurrent stenosis were successfully treated with balloon dilatation (1) or surgery with cardiopulmonary bypass (CPB) (1). One patient has a corrected gradient of 16 mmHg in the proximal arch which is being observed. The remaining patients are free from stenosis at a median follow-up of 30.1 (13.2–57.8) months.
Use of PDA for lower body perfusion allows complex reconstruction of the arch without incurring lower body ischaemia. The extended resection could then be performed without excessive stretch. This modification saves these patients from undergoing a complex arch reconstruction with CPB in the early neonatal period.
INTRODUCTION
Coarctation of aorta, loco classico is a common malformation occurring in 5% of the patients born with congenital heart defects. It occurs in isolation, as well in combination with other defects, most commonly ventricular septal defect/s, atrio-ventricular septal defect, bicuspid aortic valve, transposition of great arteries (TGA) and occasionally with double inlet left ventricle and (DILV) forme fruste Shone's complex, etc. [1]. Up to 40% of patients with aortic coarctation have variable degrees of arch hypoplasia, notably at the intervening segment between the origin of the left common carotid and the left subclavian arteries [2]. This segment is also discretely longer, which in fact has been described as an indirect sign of the existence of coarctation in neonates where it is difficult to define due to a large persistent infantile duct [3]. While the first cut and sew technique to repair aortic coarctation was described in 1954 by Clarence Crafoord in Stockholm [4], the so-called excision of coarctation with extended and radically extended end-to-end anastomosis were evolved to treat coexistent aortic arch hypoplasia [5, 6]. While an extended anastomosis deals with mild hypoplasia of distal arch, there is a limit to which the extension can be performed, not to forget the Japanese finger-type stretch of the adjoining aorta caused by excision of about a centimetre or more of length of the aorta. While it is mostly hoped and believed that the remaining hypoplasia copes in growth with improved flow, severe hypoplasia of the arch is not always treatable by the best possible and aggressive extension of the anastomosis [7]. Any small residual gradient at the arch, especially in patients with coexistent intra-cardiac shunt, typically a ventricle septal defect (VSD) or an atrioventricular septal defect (AVSD), causes increasing left-to-right shunt through the VSD, leading to excessive pulmonary flow and deficient cardiac reserve. Some of these patients may need an early intracardiac repair with simultaneous correction of the aortic arch hypoplasia. While even neonatal repair of such a constellation of defects is possible [8], it involves use of either deep hypothermic circulatory arrest [9], or hypothermia with antegrade cerebral perfusion [10], both of which are quite invasive, to say the least [11–13]. Since most of these anomalies present to us in the early neonatal period, with a logical consequence that the ductus arteriosus Botalli is either spontaneously open or maintained open with prostaglandin E1 (PGE1), we pursued a technique [1, 14] to perform arch enlargement and coarctation repair, without cardiopulmonary bypass.
PATIENTS AND METHODS
Ten neonates with coarctation and severe arch hypoplasia and a persistent ductus arteriosus (PDA) were subjected to arch repair through a left posterior thoracotomy from 2006 to 2010. Preoperative written consent for the operation was obtained from all patients. These cases constituted 12.2% of the total of 82 patients operated for coarctation at our institute in the corresponding period. Nine of these 10 patients had significant intracardiac anomalies which constituted 29% of the 31 with significant intracardiac structural malformations, having been operated for coarctation repair during this period. Of the nine patients having associated intracardiac anomalies (three perimembranous VSD—one of them with atrioventricular (AV) discordance, two AVSD, one interrupted aortic arch Type A with Truncus Arteriosus, one borderline left ventricle (LV) with bicuspid aortic valve—progressive valvar and sub-valvar stenosis, two apical muscular VSD), three received pulmonary artery banding. Median age and weight of the children were 5.5 (1–10) days and 3.0 (2.2–3.9) kg, respectively. The median preoperative arch diameter was 3.4 (2.7–4) mm, equivalent to a median of 1.1 (0.8–1.3) mm/kg body weight. The arch diameter was always less than the widely held definition of a hypoplastic arch being equivalent to weight in kilograms plus 1 in millimetres.
Technique
A left posterior thoracotomy through the fourth intercostal space and an extrapleural approach to the aortic isthmus and arch is used [2]. Patients with an echo/magnetic resonance imaging (MRI) diagnosis of significant arch hypoplasia are subjected to a patch augmentation of the aortic arch. The need to achieve optimal correction of the hypoplastic arch is all the more compelling in patients with coexisting intracardiac shunts or malformations. Thorough mobilization of the isthmus, aortic arch, the neck vessels including the truncus brachiocephalicus and the proximal descending aorta is performed. The distal ascending aorta is also mobilized from its pericardial reflection to facilitate placement of the proximal clamp. An autologous pericardial patch (used in four patients) is prepared by opening the anterior part of the parietal pleura, which is partially suture closed at the end. The patch is treated with 0.2% glutaraldehyde for about 4 min and washed 5 min each in three water baths. Bovine xenopericardial patch (Edwards Lifesciences Inc., Irvine, USA) was used in four patients; while a carotid flap in situ using a slit in the inner curvature of the left common carotid was used in two patients.
Thin vascular clamps are placed between truncus brachiocephalicus and the left common carotid artery (Cooley clamp) as well as between the left subclavian and the PDA (straight vascular clamp) (Fig. 1a), ensuring that the right radial pressure tracing demonstrates unobstructed ejection from the LV to the truncus. The left common carotid and the left subclavian arteries are temporarily clamped high up with vascular clips. The neonatal pulmonary hypertension ensures that part of the right ventricular (RV) output perfuses the lower body through the PDA. This strategy decongests the left heart, and bestows better security against spinal cord ischaemia.
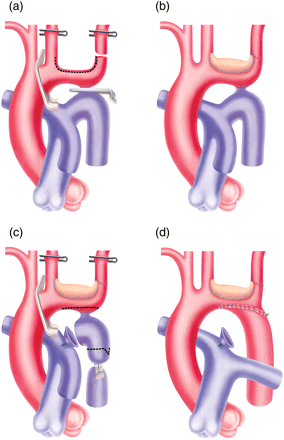
Technique of aortic arch enlargement, while maintaining ductal perfusion to lower torso (a and b). A classic resection and extended end-to-end anastomosis is then performed (c and d).
Augmentation patch
It is evident when looking at the morphology of these arches (Fig. 2), that there is a scooped-out loss of volume of the arch, which needs to be augmented with additional tissue. We preferred to augment the roof of the intervening arch between left carotid and left subclavian using a glutaraldehyde-treated autologous pericardial patch. A thin pliable xenopericardial patch could also be used, especially in heavier kids. To facilitate this augmentation, we more often transect the left subclavian artery about a centimetre above the origin (Fig. 1a) and the straightened arch is slit open up to about a centimetre onto the left common carotid origin. The patch is trimmed to fit the gap and aortic roof enlargement plasty is performed. The patch anastomosis is started backhand (7.0 polypropylene) from the farthest and the highest point on the left common carotid artery, running along the posterior anastomosis first. The anterior anastomosis is performed thereafter. The detached left subclavian is slit open medially, and re-anastomosed to achieve an oblique ‘extended’ anastomosis. All the clamps are removed and the circulation is restored (Fig. 1b).
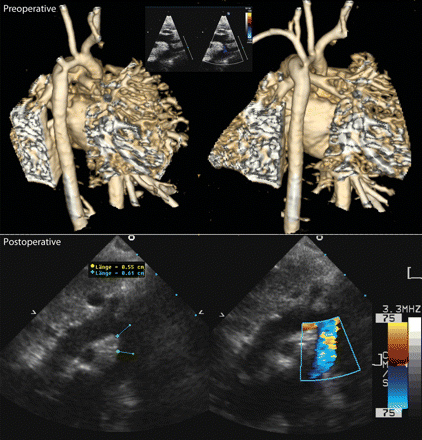
Preoperative three-dimensional magnetic resonance reconstruction of a severely hypoplastic ‘scooped out’ arch in a 10 day old 2.97 kg child, associated with a complete AV septal defect (upper panel); echo pictures nearly 6 months postoperatively showing a widely enlarged aortic arch (lower panel).
Reperfusion, coarctation resection and extended end-to-end anastomosis
After reperfusion for about 15 min, a classic resection and extended end-to-end anastomosis is performed (Fig. 1c and d). Near infra-red spectroscopy (NIRS) pads placed on the scalp (carotid territories) as well as on the abdominal flanks (descending aortic territory) (Somanetics Invos cerebral/somatic Oxymeter, Michigan, USA), are used to monitor regional tissue oxygen saturation (Fig. 3).
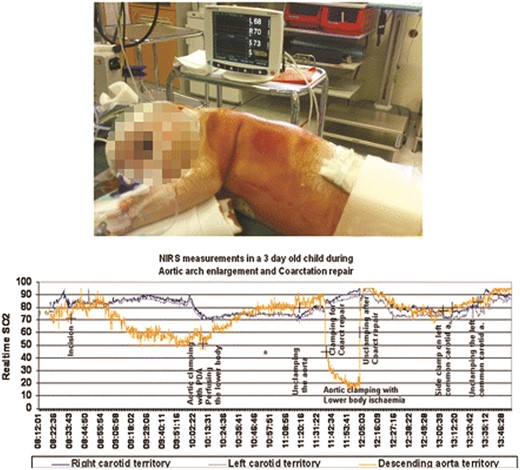
NIRS (intraoperative pad placement—upper panel) showing regional tissue oxygen saturation, in both carotids and descending aortic territories. Note the high lower torso oxygen saturation during non-ischaemic clamping of the aortic isthmus with maintained ductal perfusion (lower panel).
RESULTS
There was no early or late death. All patients could be successfully operated upon by means of this approach. The non-ischaemic and ischaemic aortic clamp times were 48 (15–62) and 23 (20–32) min, respectively. None of the patients had a significant gradient early postoperatively. The median arch diameter could be augmented from a preoperative median 3.4 (2.7–4) mm to a postoperative median 4.3 (3.8–5) mm. This corresponded to a preoperative diameter of 1.1 (0.8–1.3) mm/kg body weight and a postoperative diameter of 1.4 (1.1–1.5) mm/kg body weight. Two patients had left subclavian artery problems—thrombosis in the perioperative phase. In one patient (observed intraoperatively), the left subclavian artery was transected from its origin and reimplanted end to side to the left common carotid artery. In another patient, a postoperative (1 day) left subclavian artery thrombosis was treated with embolectomy and re-implantation at its native position. No recurrent laryngeal palsy was observed. One Trisomy 21 AVSD child had spontaneous milky secretion from the pericardium (opened to harvest the patch) which resolved postoperatively with conservative measures. No neurological complication was observed.
Two patients had late recurrence of gradient after discharge. One underwent surgical arch enlargement using cardiopulmonary bypass (CPB) (3 months postoperatively) and one was treated with balloon angioplasty (6 months postoperatively). A third patient had a corrected gradient of 16 mmHg in the proximal arch (Fig. 4), which is under observation. The remaining patients are free of stenosis at a median follow-up of 30.1 (13.2–57.8) months.
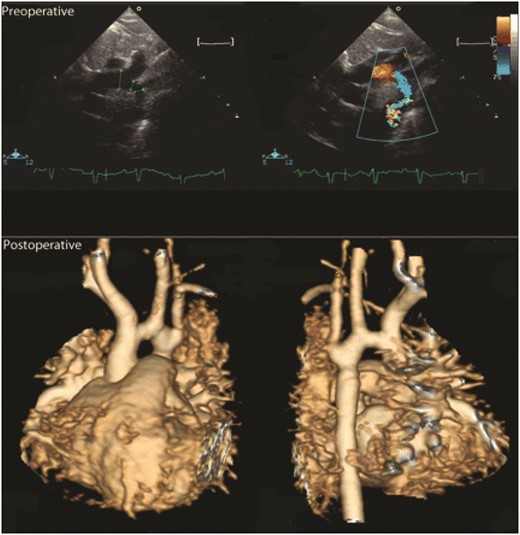
Preoperative echo (upper panels) showing severely hypoplastic arch. Postoperative MRI (lower panel) shows a widened distal arch; the mild hypoplasia in the proximal arch cannot be enlarged with this technique. This child has a corrected gradient of 16 mmHg and is being observed.
The NIRS measurements [15] in the flanks confirmed maintained lower body perfusion as evidenced by normal somatic saturation values when the ductal perfusion was maintained, in contrast to a significant fall (Fig. 3) during the phase of ischaemic aortic cross clamping.
Six patients have successfully undergone intracardiac repair till date, without need for intervention of the arch. Kaplan–Meier freedom from reoperation on the aortic arch and/or the isthmus was 90 (+10,−18.6)% at 48 months (Fig. 5) [16].
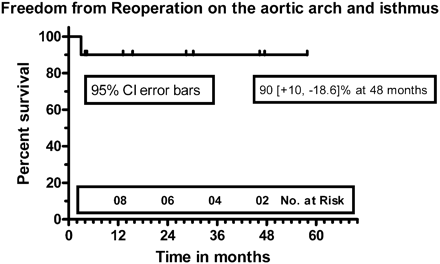
Kaplan–Meier freedom from reoperation on the aortic arch and/or the aortic isthmus.
DISCUSSION
While advancements in cardiac surgery, perfusion and intensive care have made neonatal cardiac surgery possible, the impact of complex neonatal surgery using CPB, on neurocognitive development of the child is not yet clear [17, 18]. While it is compelling that extreme aortic arch anomalies need handling in the neonatal period, very contrasting philosophies and practices such as repair under deep hypothermia and circulatory arrest, repair under hypothermia and antegrade cerebral perfusion—with high flows versus moderate flows, continue to be ardently advocated [19, 20]. What all these mean to the brain structures early on, as well as to the motoric and cognitive functions later on in life, are just beginning to be unraveled [21]. In addition, coexisting anomalies such as atrioventricular septal defect and Swiss cheese VSD carry high risk when operated simultaneously with the arch through a sternotomy in the early neonatal period. As far as the efficacy of aortic arch repair is concerned, Hager et al. [16] and Kaushal et al. [22] among many others have shown hypoplastic arch to be an independent risk factor for death or restenosis following coarctation repair. Similarly, a Great Ormond Street Hospital study [5] found coexisting major intracardiac lesions, preoperative resuscitation and postoperative residual gradient to be associated with higher mortality. The operative mortality for coarctation repairs ranges from 0 to 8% [22], with a recoarctation rate for neonatal and early infant repairs ranging from 10 to 18% [23]. A recent study from Children's Memorial Hospital, Chicago found sternotomy, VSD closure and hypoplastic aortic arch to be risk factors significant for death [22]. Not only can the mortality of neonatal repair through a sternotomy be as high as 18.8% [24], but it is also not immune to recurrent arch obstruction [22], not to mention the unknowns about the complex CPB strategies involved. It is in this backdrop that we pursued a two-stage strategy while trying to ensure a radical correction of the arch.
The surgical approach described herein is not new. Various groups starting with Tiraboschi et al. [1, 14] have described the so-called reverse subclavian artery flap to enlarge the arch between the left common carotid to the left subclavian artery. Many have in the process accepted sacrificing antegrade flow to the left subclavian artery. While many subsequent reports have shown increasing improvements in the results of coarctation repair, the role of adjunctive techniques for arch enlargement appear to have been understated, in deference to single-stage correction of all malformations through a sternotomy and using cardiopulmonary bypass.
Significance of maintained ductal perfusion
This paper elucidates the technique of arch augmentation while highlighting the significance of ductal perfusion. This technique bestows high safety quotient which performing complex arch repair, making it reproducible and transferable [18]. Considering that the reported median duration of ischaemic aortic clamping in patients developing paraplegia was 49 min [25], the safety factor inherent in this technique, especially for young surgeons need not be overemphasized. This technique potentially saves almost 12% of the cohort of coarctation with arch hypoplasia patients from being subjected to a complex arch and intracardiac repair using CPB in the neonatal period. For the cohort of patients having arch hypoplasia coexistent with intracardiac malformations this proportion is nearly 29%.
Augmenting the roof and maintaining perfusion to the left subclavian artery
The significance of this strategy also lies in the fact that it adds tissue to a ‘scooped out’ hypoplasic arch (Fig. 2), thus preventing the Japanese finger-type stretch effect. Considering the arch segment between the left common carotid and the left subclavian artery as a cylinder, the calculated volume (4πr2l) increased from a median preoperative 0.06 (0.03–0.08) cm3 to a postoperative 0.09 (0.08–0.28) cm3 without subjecting to complex CPB. Moreover, it aimed to maintain antegrade left subclavian artery perfusion, while not at all impinging on the archaic space under the arch through which the branch pulmonary arteries, the trachea and the left main bronchus pass. The overall freedom from reoperation on the isthmus of 90% at 48 months is very gratifying, in spite of the learning curve involved. Of course, the growth of this arch with time will have to be critically followed.
Pitfalls
The most critical step in this procedure is the optimal placement of the proximal clamp. A natural fold protruding from the lateral aspect of the origin of the left common carotid artery could be many millimetres thick and needs to be carefully negotiated. It is therefore imperative to have the left carotid artery completely free from the clamp (Fig. 6). This becomes difficult in the so-called truncus bicaroticus morphology where both the carotids stem together from the arch, thus making it a relative contraindication for such an approach.
White arrow points at the thickened intimal fold at the lateral margin of the origin of the left common carotid artery, which must be clearly negotiated to be able to perform this technique.
Although it has been performed, we would rather avoid using this approach when the right subclavian artery is a Lusoria, which means that antegrade perfusion to both the subclavian arteries is occluded by the clamps while performing the coarctation repair. This subjects the lower torso to higher risk of ischaemia.
The other pitfall is the sizing of the length of the patch. A perfect geometric three-dimensional understanding is required so as not to oversize the length of the patch resulting in stenosis of the origin of the left subclavian artery. A xenopericardial patch when used for repair is too stiff for a neonatal aorta, which means that a millimetre of error can impinge on the origin of the left subclavian artery. In a difficult situation, the left subclavian artery can be transected and anastomosed end to side to the left carotid artery (as we did in one case). Glutaraldehyde-treated autologous pericardium is more forgiving, however is not immune. These all mean that, there exists a learning curve, while pursuing such an approach.
A hypoplastic proximal arch between the truncus brachiocephalicus and the left common carotid cannot be enlarged using this technique (Fig. 4). It is, however, expected that with improved flow dynamics, this part of the arch copes with the growth.
Once the roof is enlarged, the excision and end-to-end repair of coarctation can be performed without trying to effect too much of an extension of the anastomosis underneath the arch. With this approach, the need for neonatal correction of arch through a sternotomy using antegrade cerebral perfusion is limited to a seldom isolated case where proximal clamping is not possible or one of the above-mentioned contraindications exist. MRI and three-dimensional reconstructions come in handy while planning this approach.
CONCLUSION
In conclusion, aortic roof enlargement in neonates, while using the PDA to perfuse the lower torso, provides an efficient, minimally invasive way to perform complex arch reconstructions in a significant number of patients with arch hypoplasia. The safety quotient of this technique makes it reproducible and transferable. Proactive use of this approach can possibly reduce the rate of residual obstruction that may occur while using the classic approaches in patients with severe arch hypoplasia coexisting with significant intracardiac malformations, where a residual gradient is often poorly tolerated.
http://ejcts.oxfordjournals.org/lookup/suppl/doi:10.1093/ejcts/ezr110/-/DC1
ACKNOWLEDGEMENTS
We thank Emaneula Valsangiacomo and Marco Bosshardt/Christoph Burki/Dubravka Deanovic for providing the MRI reconstructions and the NIRS graphics, respectively. We thank Stefan Schwyter for the graphic illustrations.
Conflict of interest: none declared.




