-
PDF
- Split View
-
Views
-
Cite
Cite
Saina Attaran, Hesham Z. Saleh, Matthew Shaw, Andrew Ward, Mark Pullan, Brian M. Fabri, Does the outcome improve after radiofrequency ablation for atrial fibrillation in patients undergoing cardiac surgery? A propensity-matched comparison, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages 806–811, https://doi.org/10.1093/ejcts/ezr107
Close - Share Icon Share
Abstract
Preoperative atrial fibrillation (AF) significantly reduces the survival rate post cardiac surgery. It has been shown that patients in persistent or paroxysmal AF have higher mid- and long-term mortality post cardiac surgery compared with those in sinus rhythm. In this study we aimed to assess whether radiofrequency (RF) ablation during cardiac surgery in these patients improves the survival.
For a period of 5 years (2005–10), we studied all the patients who underwent ablation for AF during cardiac surgery for persistent/paroxysmal AF in our institution. We used RF ablation on 113 patients who had AF for <5 years and where the atrial dimension measured <5.5 cm. A 1:2 propensity matching was performed to adjust for the preoperative and operative characteristics with a group in persistent/paroxysmal AF, who had cardiac surgery during the same period of time (2005–10) and did not undergo ablation. We compared the postoperative outcome and survival rates between the two groups.
Before and after adjusting for the preoperative and operative characteristics, inotropic support, renal failure, stroke, intensive care unit and hospital stay, as well as in-hospital mortality were similar between the two groups. After 5 years the difference in the survival was significant between the groups; 91.1 and 83.2%, with and without ablation, respectively (P value = 0.038).
Despite, the similar postoperative outcome with or without ablation in persistent/paroxysmal AF, 5-year survival was found to be significantly higher with the ablation during cardiac surgery. This improvement can be due to the fall in the incidence of cerebro-vascular events or bleeding with AF or warfarin. Ablation during cardiac surgery is a simple and quick procedure and should be considered if indicated.
INTRODUCTION
Atrial fibrillation (AF) is a progressive disease secondary to continuous structural remodelling of the atria due to AF itself, ageing or deterioration of underlying heart disease [1]. With an ageing population increasingly requiring cardiac surgery, the incidence of preoperative AF has risen over the last decade and is reported to be as high as 20% in patients undergoing coronary artery bypass grafting (CABG) and up to 60% in mitral valve disease [2–4].
It has been shown that preoperative AF, significantly reduces the survival rate post cardiac surgery, and patients in persistent or paroxysmal AF have higher mid- and long-term mortality post cardiac surgery compared with those in sinus rhythm [5–7]. The Cox–Maze procedure, which was designed to interrupt macro-reentrant circuits, has been successful in restoring sinus rhythm (70–97%) and improving postoperative outcome and survival rates [8–10]. Despite its success, the maze procedure is time consuming, increases cross-clamp time and the risk of bleeding [11]. Therefore, in concomitant cardiac procedures, the cut-and-sew Cox–Maze procedure has been widely replaced by radiofrequency (RF) ablation [12], and demonstrated comparable results both in short- and long term in restoring sinus rhythm [13].
With preoperative AF being an independent risk factor of postoperative mortality [5–7], treatment of this arrhythmia is expected to improve the outcome and increase the postoperative survival rate. However, data on this subject are limited. In this study we aimed to assess whether RF ablation during cardiac surgery in these patients improves survival.
METHODS
Patients
In this retrospective study, we reviewed prospectively collected data on patients who underwent concomitant cardiac surgery and RF ablation from 2005 to 2010. Out of 1384 patients with preoperative AF, RF ablation was performed on 158 (11.5%) patients. Selection criteria for performing the ablation were: (i) history of AF (paroxysmal/persistent) for <5 years, (ii) atrial dimensions measured <5.5 cm and (iii) age <75 years old [14, 15].
Patients’ details, registry database, medical records and national death index were reviewed. Mean age at the operation was 66.8 years (57.9–73.9 years) and 35% (n = 56) were female. Patient characteristics are summarized in Table 1.
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Age at operation (years) | 73.0 (67.0–77.6) | 66.8 (57.9–73.9) | <0.001 | 69.7 (63.6–74.9) | 69.9 (63.7–74.5) | 0.62 |
| BMI (kg/m2), % (n) | 26.9 (24.1–30.5) | 27.6 (24.6–31.2) | 0.13 | 27.6 (24.7–31.6) | 27.6 (24.6–31.2) | 0.86 |
| Female gender, % (n) | 38.8 (476) | 35.4 (56) | 0.41 | 39.8 (90) | 39.8 (45) | >0.99 |
| Angina Class IV, % (n) | 6.8 (83) | 1.9 (3) | 0.02 | 2.2 (5) | 2.7 (3) | >0.99 |
| Recent MI, % (n) | 6.3 (77) | 1.9 (3) | 0.03 | 2.2 (5) | 2.7 (3) | >0.99 |
| Current smoker, % (n) | 6.4 (79) | 3.8 (6) | 0.19 | 2.2 (5) | 3.5 (4) | 0.49 |
| Diabetes, % (n) | 21.9 (269) | 13.9 (22) | 0.02 | 16.8 (38) | 15.0 (17) | 0.68 |
| Hypercholesterolaemia, % (n) | 69.7 (855) | 58.2 (92) | 0.003 | 62.4 (141) | 64.6 (73) | 0.69 |
| Hypertension, % (n) | 58.3 (715) | 51.9 (82) | 0.12 | 54.4 (123) | 58.4 (66) | 0.49 |
| Respiratory disease, % (n) | 45.4 (556) | 38.0 (60) | 0.08 | 39.8 (90) | 42.5 (48) | 0.64 |
| Cerebrovascular disease, % (n) | 15.8 (194) | 10.1 (16) | 0.06 | 8.0 (18) | 12.4 (14) | 0.19 |
| PVD, % (n) | 13.0 (159) | 8.2 (13) | 0.09 | 9.7 (22) | 9.7 (11) | >0.99 |
| Renal dysfunction, % (n) | 13.8 (169) | 4.4 (7) | <0.001 | 6.2 (14) | 3.5 (4) | 0.30 |
| Triple-vessel disease, % (n) | 25.5 (312) | 15.8 (25) | 0.008 | 18.1 (41) | 19.5 (22) | 0.77 |
| Prior surgery, % (n) | 13.7 (168) | 2.5 (4) | <0.001 | 3.5 (8) | 3.5 (4) | >0.99 |
| Overall LVEF <30%, % (n) | 14.8 (181) | 6.3 (10) | 0.004 | 9.7 (22) | 8.0 (9) | 0.59 |
| Logistic EuroSCORE | 10.6 (5.9–22.5) | 5.7 (3.1–10.7) | <0.001 | 7.4 (4.3–13.0) | 6.4 (3.7–11.4) | 0.29 |
| Rheumatic mitral valve disease | 16.2 (198) | 9.5 (15) | 0.03 | 13.7 (31) | 9.7 (11) | 0.29 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Age at operation (years) | 73.0 (67.0–77.6) | 66.8 (57.9–73.9) | <0.001 | 69.7 (63.6–74.9) | 69.9 (63.7–74.5) | 0.62 |
| BMI (kg/m2), % (n) | 26.9 (24.1–30.5) | 27.6 (24.6–31.2) | 0.13 | 27.6 (24.7–31.6) | 27.6 (24.6–31.2) | 0.86 |
| Female gender, % (n) | 38.8 (476) | 35.4 (56) | 0.41 | 39.8 (90) | 39.8 (45) | >0.99 |
| Angina Class IV, % (n) | 6.8 (83) | 1.9 (3) | 0.02 | 2.2 (5) | 2.7 (3) | >0.99 |
| Recent MI, % (n) | 6.3 (77) | 1.9 (3) | 0.03 | 2.2 (5) | 2.7 (3) | >0.99 |
| Current smoker, % (n) | 6.4 (79) | 3.8 (6) | 0.19 | 2.2 (5) | 3.5 (4) | 0.49 |
| Diabetes, % (n) | 21.9 (269) | 13.9 (22) | 0.02 | 16.8 (38) | 15.0 (17) | 0.68 |
| Hypercholesterolaemia, % (n) | 69.7 (855) | 58.2 (92) | 0.003 | 62.4 (141) | 64.6 (73) | 0.69 |
| Hypertension, % (n) | 58.3 (715) | 51.9 (82) | 0.12 | 54.4 (123) | 58.4 (66) | 0.49 |
| Respiratory disease, % (n) | 45.4 (556) | 38.0 (60) | 0.08 | 39.8 (90) | 42.5 (48) | 0.64 |
| Cerebrovascular disease, % (n) | 15.8 (194) | 10.1 (16) | 0.06 | 8.0 (18) | 12.4 (14) | 0.19 |
| PVD, % (n) | 13.0 (159) | 8.2 (13) | 0.09 | 9.7 (22) | 9.7 (11) | >0.99 |
| Renal dysfunction, % (n) | 13.8 (169) | 4.4 (7) | <0.001 | 6.2 (14) | 3.5 (4) | 0.30 |
| Triple-vessel disease, % (n) | 25.5 (312) | 15.8 (25) | 0.008 | 18.1 (41) | 19.5 (22) | 0.77 |
| Prior surgery, % (n) | 13.7 (168) | 2.5 (4) | <0.001 | 3.5 (8) | 3.5 (4) | >0.99 |
| Overall LVEF <30%, % (n) | 14.8 (181) | 6.3 (10) | 0.004 | 9.7 (22) | 8.0 (9) | 0.59 |
| Logistic EuroSCORE | 10.6 (5.9–22.5) | 5.7 (3.1–10.7) | <0.001 | 7.4 (4.3–13.0) | 6.4 (3.7–11.4) | 0.29 |
| Rheumatic mitral valve disease | 16.2 (198) | 9.5 (15) | 0.03 | 13.7 (31) | 9.7 (11) | 0.29 |
Continuous data shown as median (25th–75th percentile), comparisons made with Wilcoxon rank-sum tests; categorical data shown as percentage (number), comparisons made with Chi-square tests. BMI: body mass index; MI: myocardial infarction; PVD: peripheral vascular disease; LVEF: left ventricular ejection fraction.
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Age at operation (years) | 73.0 (67.0–77.6) | 66.8 (57.9–73.9) | <0.001 | 69.7 (63.6–74.9) | 69.9 (63.7–74.5) | 0.62 |
| BMI (kg/m2), % (n) | 26.9 (24.1–30.5) | 27.6 (24.6–31.2) | 0.13 | 27.6 (24.7–31.6) | 27.6 (24.6–31.2) | 0.86 |
| Female gender, % (n) | 38.8 (476) | 35.4 (56) | 0.41 | 39.8 (90) | 39.8 (45) | >0.99 |
| Angina Class IV, % (n) | 6.8 (83) | 1.9 (3) | 0.02 | 2.2 (5) | 2.7 (3) | >0.99 |
| Recent MI, % (n) | 6.3 (77) | 1.9 (3) | 0.03 | 2.2 (5) | 2.7 (3) | >0.99 |
| Current smoker, % (n) | 6.4 (79) | 3.8 (6) | 0.19 | 2.2 (5) | 3.5 (4) | 0.49 |
| Diabetes, % (n) | 21.9 (269) | 13.9 (22) | 0.02 | 16.8 (38) | 15.0 (17) | 0.68 |
| Hypercholesterolaemia, % (n) | 69.7 (855) | 58.2 (92) | 0.003 | 62.4 (141) | 64.6 (73) | 0.69 |
| Hypertension, % (n) | 58.3 (715) | 51.9 (82) | 0.12 | 54.4 (123) | 58.4 (66) | 0.49 |
| Respiratory disease, % (n) | 45.4 (556) | 38.0 (60) | 0.08 | 39.8 (90) | 42.5 (48) | 0.64 |
| Cerebrovascular disease, % (n) | 15.8 (194) | 10.1 (16) | 0.06 | 8.0 (18) | 12.4 (14) | 0.19 |
| PVD, % (n) | 13.0 (159) | 8.2 (13) | 0.09 | 9.7 (22) | 9.7 (11) | >0.99 |
| Renal dysfunction, % (n) | 13.8 (169) | 4.4 (7) | <0.001 | 6.2 (14) | 3.5 (4) | 0.30 |
| Triple-vessel disease, % (n) | 25.5 (312) | 15.8 (25) | 0.008 | 18.1 (41) | 19.5 (22) | 0.77 |
| Prior surgery, % (n) | 13.7 (168) | 2.5 (4) | <0.001 | 3.5 (8) | 3.5 (4) | >0.99 |
| Overall LVEF <30%, % (n) | 14.8 (181) | 6.3 (10) | 0.004 | 9.7 (22) | 8.0 (9) | 0.59 |
| Logistic EuroSCORE | 10.6 (5.9–22.5) | 5.7 (3.1–10.7) | <0.001 | 7.4 (4.3–13.0) | 6.4 (3.7–11.4) | 0.29 |
| Rheumatic mitral valve disease | 16.2 (198) | 9.5 (15) | 0.03 | 13.7 (31) | 9.7 (11) | 0.29 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Age at operation (years) | 73.0 (67.0–77.6) | 66.8 (57.9–73.9) | <0.001 | 69.7 (63.6–74.9) | 69.9 (63.7–74.5) | 0.62 |
| BMI (kg/m2), % (n) | 26.9 (24.1–30.5) | 27.6 (24.6–31.2) | 0.13 | 27.6 (24.7–31.6) | 27.6 (24.6–31.2) | 0.86 |
| Female gender, % (n) | 38.8 (476) | 35.4 (56) | 0.41 | 39.8 (90) | 39.8 (45) | >0.99 |
| Angina Class IV, % (n) | 6.8 (83) | 1.9 (3) | 0.02 | 2.2 (5) | 2.7 (3) | >0.99 |
| Recent MI, % (n) | 6.3 (77) | 1.9 (3) | 0.03 | 2.2 (5) | 2.7 (3) | >0.99 |
| Current smoker, % (n) | 6.4 (79) | 3.8 (6) | 0.19 | 2.2 (5) | 3.5 (4) | 0.49 |
| Diabetes, % (n) | 21.9 (269) | 13.9 (22) | 0.02 | 16.8 (38) | 15.0 (17) | 0.68 |
| Hypercholesterolaemia, % (n) | 69.7 (855) | 58.2 (92) | 0.003 | 62.4 (141) | 64.6 (73) | 0.69 |
| Hypertension, % (n) | 58.3 (715) | 51.9 (82) | 0.12 | 54.4 (123) | 58.4 (66) | 0.49 |
| Respiratory disease, % (n) | 45.4 (556) | 38.0 (60) | 0.08 | 39.8 (90) | 42.5 (48) | 0.64 |
| Cerebrovascular disease, % (n) | 15.8 (194) | 10.1 (16) | 0.06 | 8.0 (18) | 12.4 (14) | 0.19 |
| PVD, % (n) | 13.0 (159) | 8.2 (13) | 0.09 | 9.7 (22) | 9.7 (11) | >0.99 |
| Renal dysfunction, % (n) | 13.8 (169) | 4.4 (7) | <0.001 | 6.2 (14) | 3.5 (4) | 0.30 |
| Triple-vessel disease, % (n) | 25.5 (312) | 15.8 (25) | 0.008 | 18.1 (41) | 19.5 (22) | 0.77 |
| Prior surgery, % (n) | 13.7 (168) | 2.5 (4) | <0.001 | 3.5 (8) | 3.5 (4) | >0.99 |
| Overall LVEF <30%, % (n) | 14.8 (181) | 6.3 (10) | 0.004 | 9.7 (22) | 8.0 (9) | 0.59 |
| Logistic EuroSCORE | 10.6 (5.9–22.5) | 5.7 (3.1–10.7) | <0.001 | 7.4 (4.3–13.0) | 6.4 (3.7–11.4) | 0.29 |
| Rheumatic mitral valve disease | 16.2 (198) | 9.5 (15) | 0.03 | 13.7 (31) | 9.7 (11) | 0.29 |
Continuous data shown as median (25th–75th percentile), comparisons made with Wilcoxon rank-sum tests; categorical data shown as percentage (number), comparisons made with Chi-square tests. BMI: body mass index; MI: myocardial infarction; PVD: peripheral vascular disease; LVEF: left ventricular ejection fraction.
Operation and postoperative management
The main concomitant cardiac procedure with the RF ablation was mitral valve repair/replacement (MVR) ± CABG (43.7%, n = 69), followed by aortic valve replacement (AVR) ± CABG (27.2%, n = 43). Concomitant ablation and isolated CABG was performed in 16.5% (n = 26) with half of them (8.2%, n = 13) being off-pump CABG (OPCAB). Cases of double valves and tricuspid valve repair/replacements (TVR) ± MVR ± CABG comprised 10% (n = 16), and there were four (2.5%) other cardiac procedures such as myxoma and ASD closure.
After the induction of general anaesthesia a trans-oesophageal echocardiogram (TOE) probe was inserted before median sternotomy. In the on-pump cases, after the initiation of cardio-pulmonary bypass (CPB), bipolar RF ablation (Atricure, Cardiologic Limited, Thirsk, UK), with an additional pen in some cases, was performed with a cross-clamp on. In OPCAB cases, RF ablation was performed after harvesting the conduits and prior to performing the anastomosis.
We conducted the ablation with the bipolar clamp device at least twice for each line. Ablation was carried out around the pulmonary veins (PV), a connector line between PVs, around the base of the left atrial appendage, a further connector from the right PV to mitral valve and a line from the left PV to the base of the left atrial appendage. The mitral line was either completed with a bipolar pen or using the clamp. The clamp technique for the mitral line involved taking the midpoint of the line from the right inferior pulmonary vein (RIPV) to mitral annulus into the jaw of the clamp, so that the tip of the clamp contains the mitral annulus and RIPV orifice. No mitral lines were ablated in OPCAB cases. The left atrial appendage was sutured from the inside in mitral valve operations and was stitched from the outside in the rest of the cases. The process of ablation lasted between 10 and 12 min. Ventricular pacing wires were inserted in all patients and pacing employed if indicated.
Postoperatively, all patients having RF ablation were started on intravenous amiodarone 1.2 g for 24 h, followed by oral amiodarone 200 mg three times a day for 7 days, further followed by twice a day for another 7 days and once a day until they were assessed in the outpatient clinic at 6 weeks. They were all anticoagulated using warfarin prior to discharge.
In the outpatient clinic, if patients have remained in SR, amiodarone was stopped and a month later they were assessed with a 7-day Holter monitor and a trans-thoracic echocardiogram (TTE). If they remained in SR throughout the test, and had an A wave on the TTE examination, warfarin was subsequently discontinued.
Statistics
Statistical analysis was carried out using SAS for Windows Version 8.2. Continuous variables not normally distributed are shown as median with 25th and 75th percentiles. Categorical data are shown as percentages. Univariate comparisons were made by means of Wilcoxon rank-sum tests and Chi-square tests as appropriate. Deaths occurring over time were described using Kaplan–Meier survival curves [16].
To account for differences in case mix we developed a propensity score for ablation group membership [17]. The propensity for ablation group membership was determined regardless of outcome, using multivariable logistic regression analysis [18]. A full nonparsimonious model was developed that included all variables listed in Table 1. The goal was to balance patient characteristics by incorporating everything recorded that may relate to either systematic bias. We then used a macro (available at: http://www2.sas.com/proceedings/sugi29/165-29.pdf) to perform a 1:2 propensity matching for each group with preoperative AF with and without ablation. As a result, in the ablation group 113 patients (Group A) were compared with 226 patients (Group B) who did not have ablation (Table 1). In all cases a P value < 0.05 was considered significant.
RESULTS
Preoperative/operative characteristics
As shown in Table 1, before propensity matching, patients in Group A, who had ablation, were found to be significantly younger and had less co-morbidities compared with Group B, with a logistic EuroSCORE of 5.7 and 10.6 in Groups A and B, respectively. Less cases with recent history of myocardial infarction (MI), diabetes, renal impairment and poor ventricular function were ablated (P < 0.05). Co-morbidities such as respiratory conditions, previous stroke and peripheral vascular disease (PVD) were also more common in Group A compared with Group B, but the difference did not reach statistical significance. Previous cardiac surgery, emergency cases and triple vessel disease were also less common in the group who were ablated. Furthermore, less OPCAB cases were ablated. On the other hand, more MVR cases were performed in Group A (43.7 versus 26.8% in A and B).
The average bypass time was around 12 min longer with the ablation before propensity matching, and 16 min after adjusting for the patient characteristics (P < 0.001). The average cross-clamp time was 2–3 min longer in the group who had ablation with no statistical difference (P = 0.14).
Postoperative outcome
During the postoperative course, use of inotropic support, intra-aortic balloon pump (IABP) and ventilation time were similar before adjusting for the preoperative and operative patient characteristics. Other complications such as renal failure, stroke, re-exploration for bleeding and the amount of blood loss postoperatively were statistically similar between the two groups even before risk adjustment. Despite a higher level of CK-MB after the ablation, rate of postoperative MI remained the same with or without the ablation. Postoperative intensive care unit (ICU) stay, and hospital stay were also similar between the two groups (Table 2).
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Non-elective, % (n) | 19.3 (236) | 12.7 (20) | 0.045 | 12.8 (29) | 13.3 (15) | 0.91 |
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Procedure, % (n) | ||||||
| MVR ± CABG | 26.8 (329) | 43.7 (69) | <0.001 | 39.4 (89) | 37.2 (42) | 0.69 |
| AVR ± CABG | 27.6 (338) | 27.2 (43) | 0.93 | 29.2 (66) | 30.1 (34) | 0.87 |
| MVR + TVR ± CABG, % | 5.1 (63) | 6.3 (10) | 0.53 | 7.5 (17) | 7.1 (8) | 0.88 |
| MVR + AVR ± CABG, % | 8.3 (102) | 3.8 (6) | 0.046 | 2.7 (6) | 4.4 (5) | 0.52 |
| Isolated CABG | 21.5 (263) | 16.5 (26) | 0.15 | 17.3 (39) | 17.7 (20) | 0.92 |
| Off-pump CABG | 14.2 (174) | 8.2 (13) | 0.04 | 9.3 (21) | 8.9 (10) | 0.89 |
| Other | 10.7 (131) | 2.5 (4) | <0.001 | 4.0 (9) | 3.5 (4) | >0.99 |
| Complications, % (n) | ||||||
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Inotrope support | 58.0 (711) | 51.9 (82) | 0.14 | 52.2 (118) | 45.1 (51) | 0.22 |
| IABP support | 2.7 (30) | 2.9 (4) | 0.79 | 1.8 (4) | 3.5 (4) | 0.45 |
| Ventilation >48 h | 7.8 (96) | 6.3 (10) | 0.50 | 6.2 (14) | 8.9 (10) | 0.37 |
| Permanent pacemaker insertion | 3.4 (37) | 2.9 (4) | >0.99 | 4.4 (10) | 2.7 (3) | 0.56 |
| Acute renal failure | 13.2 (162) | 8.9 (14) | 0.12 | 10.2 (23) | 11.5 (13) | 0.71 |
| Surgical wound infection | 1.7 (21) | 3.2 (5) | 0.21 | 0.4 (1) | 4.4 (5) | 0.02 |
| Stroke | 3.6 (44) | 1.3 (2) | 0.13 | 3.1 (7) | 1.8 (2) | 0.72 |
| Re-exploration for bleeding | 6.8 (83) | 6.3 (10) | 0.84 | 6.6 (15) | 8.9 (10) | 0.46 |
| Blood loss in ITU (ml) | 540 (320–910) | 495 (345–890) | 0.78 | 450 (310–770) | 520 (330–980) | 0.10 |
| MI | 0.2 (3) | 0 (0) | >0.99 | 0.4 (1) | 0 (0) | >0.99 |
| CK-MB (U/l) | 17 (0–31) | 25.5 (11.5–39.5) | <0.001 | 20 (5–33) | 26 (12–41) | 0.008 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Non-elective, % (n) | 19.3 (236) | 12.7 (20) | 0.045 | 12.8 (29) | 13.3 (15) | 0.91 |
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Procedure, % (n) | ||||||
| MVR ± CABG | 26.8 (329) | 43.7 (69) | <0.001 | 39.4 (89) | 37.2 (42) | 0.69 |
| AVR ± CABG | 27.6 (338) | 27.2 (43) | 0.93 | 29.2 (66) | 30.1 (34) | 0.87 |
| MVR + TVR ± CABG, % | 5.1 (63) | 6.3 (10) | 0.53 | 7.5 (17) | 7.1 (8) | 0.88 |
| MVR + AVR ± CABG, % | 8.3 (102) | 3.8 (6) | 0.046 | 2.7 (6) | 4.4 (5) | 0.52 |
| Isolated CABG | 21.5 (263) | 16.5 (26) | 0.15 | 17.3 (39) | 17.7 (20) | 0.92 |
| Off-pump CABG | 14.2 (174) | 8.2 (13) | 0.04 | 9.3 (21) | 8.9 (10) | 0.89 |
| Other | 10.7 (131) | 2.5 (4) | <0.001 | 4.0 (9) | 3.5 (4) | >0.99 |
| Complications, % (n) | ||||||
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Inotrope support | 58.0 (711) | 51.9 (82) | 0.14 | 52.2 (118) | 45.1 (51) | 0.22 |
| IABP support | 2.7 (30) | 2.9 (4) | 0.79 | 1.8 (4) | 3.5 (4) | 0.45 |
| Ventilation >48 h | 7.8 (96) | 6.3 (10) | 0.50 | 6.2 (14) | 8.9 (10) | 0.37 |
| Permanent pacemaker insertion | 3.4 (37) | 2.9 (4) | >0.99 | 4.4 (10) | 2.7 (3) | 0.56 |
| Acute renal failure | 13.2 (162) | 8.9 (14) | 0.12 | 10.2 (23) | 11.5 (13) | 0.71 |
| Surgical wound infection | 1.7 (21) | 3.2 (5) | 0.21 | 0.4 (1) | 4.4 (5) | 0.02 |
| Stroke | 3.6 (44) | 1.3 (2) | 0.13 | 3.1 (7) | 1.8 (2) | 0.72 |
| Re-exploration for bleeding | 6.8 (83) | 6.3 (10) | 0.84 | 6.6 (15) | 8.9 (10) | 0.46 |
| Blood loss in ITU (ml) | 540 (320–910) | 495 (345–890) | 0.78 | 450 (310–770) | 520 (330–980) | 0.10 |
| MI | 0.2 (3) | 0 (0) | >0.99 | 0.4 (1) | 0 (0) | >0.99 |
| CK-MB (U/l) | 17 (0–31) | 25.5 (11.5–39.5) | <0.001 | 20 (5–33) | 26 (12–41) | 0.008 |
Continuous data shown as median (25th–75th percentile), comparisons made with Wilcoxon rank-sum tests; categorical data shown as percentage (number), comparisons made with Chi-square tests. IABP: intra-aortic balloon pump; ITU: intensive treatment unit; CK-MB: creatinine kinase.
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Non-elective, % (n) | 19.3 (236) | 12.7 (20) | 0.045 | 12.8 (29) | 13.3 (15) | 0.91 |
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Procedure, % (n) | ||||||
| MVR ± CABG | 26.8 (329) | 43.7 (69) | <0.001 | 39.4 (89) | 37.2 (42) | 0.69 |
| AVR ± CABG | 27.6 (338) | 27.2 (43) | 0.93 | 29.2 (66) | 30.1 (34) | 0.87 |
| MVR + TVR ± CABG, % | 5.1 (63) | 6.3 (10) | 0.53 | 7.5 (17) | 7.1 (8) | 0.88 |
| MVR + AVR ± CABG, % | 8.3 (102) | 3.8 (6) | 0.046 | 2.7 (6) | 4.4 (5) | 0.52 |
| Isolated CABG | 21.5 (263) | 16.5 (26) | 0.15 | 17.3 (39) | 17.7 (20) | 0.92 |
| Off-pump CABG | 14.2 (174) | 8.2 (13) | 0.04 | 9.3 (21) | 8.9 (10) | 0.89 |
| Other | 10.7 (131) | 2.5 (4) | <0.001 | 4.0 (9) | 3.5 (4) | >0.99 |
| Complications, % (n) | ||||||
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Inotrope support | 58.0 (711) | 51.9 (82) | 0.14 | 52.2 (118) | 45.1 (51) | 0.22 |
| IABP support | 2.7 (30) | 2.9 (4) | 0.79 | 1.8 (4) | 3.5 (4) | 0.45 |
| Ventilation >48 h | 7.8 (96) | 6.3 (10) | 0.50 | 6.2 (14) | 8.9 (10) | 0.37 |
| Permanent pacemaker insertion | 3.4 (37) | 2.9 (4) | >0.99 | 4.4 (10) | 2.7 (3) | 0.56 |
| Acute renal failure | 13.2 (162) | 8.9 (14) | 0.12 | 10.2 (23) | 11.5 (13) | 0.71 |
| Surgical wound infection | 1.7 (21) | 3.2 (5) | 0.21 | 0.4 (1) | 4.4 (5) | 0.02 |
| Stroke | 3.6 (44) | 1.3 (2) | 0.13 | 3.1 (7) | 1.8 (2) | 0.72 |
| Re-exploration for bleeding | 6.8 (83) | 6.3 (10) | 0.84 | 6.6 (15) | 8.9 (10) | 0.46 |
| Blood loss in ITU (ml) | 540 (320–910) | 495 (345–890) | 0.78 | 450 (310–770) | 520 (330–980) | 0.10 |
| MI | 0.2 (3) | 0 (0) | >0.99 | 0.4 (1) | 0 (0) | >0.99 |
| CK-MB (U/l) | 17 (0–31) | 25.5 (11.5–39.5) | <0.001 | 20 (5–33) | 26 (12–41) | 0.008 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| Non-elective, % (n) | 19.3 (236) | 12.7 (20) | 0.045 | 12.8 (29) | 13.3 (15) | 0.91 |
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Procedure, % (n) | ||||||
| MVR ± CABG | 26.8 (329) | 43.7 (69) | <0.001 | 39.4 (89) | 37.2 (42) | 0.69 |
| AVR ± CABG | 27.6 (338) | 27.2 (43) | 0.93 | 29.2 (66) | 30.1 (34) | 0.87 |
| MVR + TVR ± CABG, % | 5.1 (63) | 6.3 (10) | 0.53 | 7.5 (17) | 7.1 (8) | 0.88 |
| MVR + AVR ± CABG, % | 8.3 (102) | 3.8 (6) | 0.046 | 2.7 (6) | 4.4 (5) | 0.52 |
| Isolated CABG | 21.5 (263) | 16.5 (26) | 0.15 | 17.3 (39) | 17.7 (20) | 0.92 |
| Off-pump CABG | 14.2 (174) | 8.2 (13) | 0.04 | 9.3 (21) | 8.9 (10) | 0.89 |
| Other | 10.7 (131) | 2.5 (4) | <0.001 | 4.0 (9) | 3.5 (4) | >0.99 |
| Complications, % (n) | ||||||
| Bypass time | 121 (92–159) | 133 (111–165) | <0.001 | 117 (94–151) | 133 (114–169) | <0.001 |
| Cross-clamp time | 85 (63–116) | 87 (73–111) | 0.21 | 83.5 (65–108.5) | 86 (73.5–116) | 0.14 |
| Inotrope support | 58.0 (711) | 51.9 (82) | 0.14 | 52.2 (118) | 45.1 (51) | 0.22 |
| IABP support | 2.7 (30) | 2.9 (4) | 0.79 | 1.8 (4) | 3.5 (4) | 0.45 |
| Ventilation >48 h | 7.8 (96) | 6.3 (10) | 0.50 | 6.2 (14) | 8.9 (10) | 0.37 |
| Permanent pacemaker insertion | 3.4 (37) | 2.9 (4) | >0.99 | 4.4 (10) | 2.7 (3) | 0.56 |
| Acute renal failure | 13.2 (162) | 8.9 (14) | 0.12 | 10.2 (23) | 11.5 (13) | 0.71 |
| Surgical wound infection | 1.7 (21) | 3.2 (5) | 0.21 | 0.4 (1) | 4.4 (5) | 0.02 |
| Stroke | 3.6 (44) | 1.3 (2) | 0.13 | 3.1 (7) | 1.8 (2) | 0.72 |
| Re-exploration for bleeding | 6.8 (83) | 6.3 (10) | 0.84 | 6.6 (15) | 8.9 (10) | 0.46 |
| Blood loss in ITU (ml) | 540 (320–910) | 495 (345–890) | 0.78 | 450 (310–770) | 520 (330–980) | 0.10 |
| MI | 0.2 (3) | 0 (0) | >0.99 | 0.4 (1) | 0 (0) | >0.99 |
| CK-MB (U/l) | 17 (0–31) | 25.5 (11.5–39.5) | <0.001 | 20 (5–33) | 26 (12–41) | 0.008 |
Continuous data shown as median (25th–75th percentile), comparisons made with Wilcoxon rank-sum tests; categorical data shown as percentage (number), comparisons made with Chi-square tests. IABP: intra-aortic balloon pump; ITU: intensive treatment unit; CK-MB: creatinine kinase.
At the time of discharge 22 (19.5%) were still in AF and 3 (2.6%) had junctional rhythm. ECG at 6 weeks in the outpatient clinic showed that 38 (33.6%) patients were in AF and after 3 months 90% were cardioverted to SR.
Mortality rates
Before adjusting for the preoperative characteristics, in-hospital mortality was 8.2% in patients with chronic AF and no ablation compared with 3.2% in the group who had the ablation (P = 0.02). This was to be expected, as Group B had a significantly higher logistic EuroSCORE and suffered from more co-morbidities. After risk adjustment, however, this difference became insignificant (P = 0.28).
During the first year five patients (4.4%) in the ablation group had died compared with 17 (7.5%) in Group B. When the mid-term results were analysed in comparison with Kaplan–Meier survival curves, better survival rate was observed in the ablation (P = 0.069, Fig. 1), and after 5 years the difference in the mortality rate between the two groups became significant before and after propensity matching, favouring ablation at 5 years (P = 0.038, Table 3).
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| ICU LOS (days) | 2 (1–4) | 1.5 (1–3) | 0.12 | 1 (1–3) | 2 (1–4) | 0.29 |
| Postoperative LOS (days) | 9 (7–14) | 8 (6–13) | 0.09 | 8 (6–11) | 8 (6–14) | 0.55 |
| Mortality, % (n) | ||||||
| In-hospital | 8.2 (101) | 3.2 (5) | 0.02 | 7.5 (17) | 4.4 (5) | 0.28 |
| 1 year | 15.1 (185) | 7.6 (12) | 0.01 | 11.1 (25) | 8.9 (10) | 0.53 |
| 2 years | 17.5 (215) | 7.6 (12) | 0.002 | 13.7 (31) | 8.9 (10) | 0.20 |
| 3 years | 19.0 (233) | 7.6 (12) | <0.001 | 14.2 (32) | 8.9 (10) | 0.16 |
| 4 years | 20.1 (246) | 7.6 (12) | <0.001 | 15.5 (35) | 8.9 (10) | 0.09 |
| 5 years | 20.7 (25) | 7.6 (12) | <0.001 | 17.3 (39) | 8.9 (10) | 0.038 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| ICU LOS (days) | 2 (1–4) | 1.5 (1–3) | 0.12 | 1 (1–3) | 2 (1–4) | 0.29 |
| Postoperative LOS (days) | 9 (7–14) | 8 (6–13) | 0.09 | 8 (6–11) | 8 (6–14) | 0.55 |
| Mortality, % (n) | ||||||
| In-hospital | 8.2 (101) | 3.2 (5) | 0.02 | 7.5 (17) | 4.4 (5) | 0.28 |
| 1 year | 15.1 (185) | 7.6 (12) | 0.01 | 11.1 (25) | 8.9 (10) | 0.53 |
| 2 years | 17.5 (215) | 7.6 (12) | 0.002 | 13.7 (31) | 8.9 (10) | 0.20 |
| 3 years | 19.0 (233) | 7.6 (12) | <0.001 | 14.2 (32) | 8.9 (10) | 0.16 |
| 4 years | 20.1 (246) | 7.6 (12) | <0.001 | 15.5 (35) | 8.9 (10) | 0.09 |
| 5 years | 20.7 (25) | 7.6 (12) | <0.001 | 17.3 (39) | 8.9 (10) | 0.038 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| ICU LOS (days) | 2 (1–4) | 1.5 (1–3) | 0.12 | 1 (1–3) | 2 (1–4) | 0.29 |
| Postoperative LOS (days) | 9 (7–14) | 8 (6–13) | 0.09 | 8 (6–11) | 8 (6–14) | 0.55 |
| Mortality, % (n) | ||||||
| In-hospital | 8.2 (101) | 3.2 (5) | 0.02 | 7.5 (17) | 4.4 (5) | 0.28 |
| 1 year | 15.1 (185) | 7.6 (12) | 0.01 | 11.1 (25) | 8.9 (10) | 0.53 |
| 2 years | 17.5 (215) | 7.6 (12) | 0.002 | 13.7 (31) | 8.9 (10) | 0.20 |
| 3 years | 19.0 (233) | 7.6 (12) | <0.001 | 14.2 (32) | 8.9 (10) | 0.16 |
| 4 years | 20.1 (246) | 7.6 (12) | <0.001 | 15.5 (35) | 8.9 (10) | 0.09 |
| 5 years | 20.7 (25) | 7.6 (12) | <0.001 | 17.3 (39) | 8.9 (10) | 0.038 |
| . | Unmatched . | 2:1 Propensity matched . | ||||
|---|---|---|---|---|---|---|
| Pre-op AF, no ablation (n = 1226) . | Surgical AF ablation (n = 158) . | P value . | Pre-op AF, no ablation (n = 226) . | Surgical AF ablation (n = 113) . | P value . | |
| ICU LOS (days) | 2 (1–4) | 1.5 (1–3) | 0.12 | 1 (1–3) | 2 (1–4) | 0.29 |
| Postoperative LOS (days) | 9 (7–14) | 8 (6–13) | 0.09 | 8 (6–11) | 8 (6–14) | 0.55 |
| Mortality, % (n) | ||||||
| In-hospital | 8.2 (101) | 3.2 (5) | 0.02 | 7.5 (17) | 4.4 (5) | 0.28 |
| 1 year | 15.1 (185) | 7.6 (12) | 0.01 | 11.1 (25) | 8.9 (10) | 0.53 |
| 2 years | 17.5 (215) | 7.6 (12) | 0.002 | 13.7 (31) | 8.9 (10) | 0.20 |
| 3 years | 19.0 (233) | 7.6 (12) | <0.001 | 14.2 (32) | 8.9 (10) | 0.16 |
| 4 years | 20.1 (246) | 7.6 (12) | <0.001 | 15.5 (35) | 8.9 (10) | 0.09 |
| 5 years | 20.7 (25) | 7.6 (12) | <0.001 | 17.3 (39) | 8.9 (10) | 0.038 |
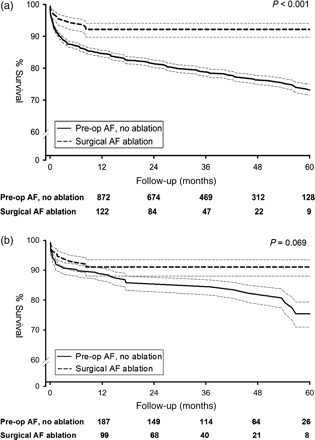
(a) Unmatched mortality and (b) matched survival curves (note y-axes begin at 60%).
COMMENTS
AF is associated with high stroke rate, increased risk of heart failure and worse survival [19]. It also negatively affects the quality of life of the individual due to related symptoms and the need for anti-coagulation. As previously shown in multivariable analyses and propensity-matched studies, preexisting AF significantly reduces survival after cardiac surgery. AF is found to be an independent risk factor after cardiac surgery [2–6, 20].
Good results have been reported with the AF surgery/ablation. RF ablation has been successful in restoring sinus rhythm in the majority of cases, with improved quality of life and an increased overall survival of the patients [8–10, 19]. Previous studies have focused on various ablative lines, freedom from AF, or have mainly compared rhythm control with rate control in improving the quality of life [21–25]. The main question still remains unanswered; does restoring sinus rhythm improve survival after cardiac surgery in patients with preexisting AF? Or does the cardiomyopathy as a result of long-standing AF negatively affect the survival, despite conversion to sinus rhythm? Data on this matter are yet to be determined.
In our centre, only 11% of patients with preexisting AF were ablated in combination with other cardiac procedures. We have been deliberately highly selective with regard to the age of the patient, atrial dimensions and the duration of AF. Similar reasons and other factors such as lack of funding, unavailability of the equipment or a surgeon's reluctance to ablate in conjunction with cardiac surgery are seen in most centres worldwide.
Despite the better results reported with small atrial size, younger patients and shorter AF duration, the recent joint guidelines on surgical ablation have recommended that all patients undergoing other cardiac surgery should be considered for AF ablation, if the risk of adding the procedure is low and a reasonable chance for success is expected [19] and age should not be considered as an exclusion criterion.
We have also demonstrated that ablation does not significantly increase cross-clamp time or postoperative complications and more importantly in our study cohort, all-cause mortality at 5 years was significantly reduced with the ablation even after propensity matching.
The main limitation of this study is that it was a retrospective observational study on a small number of patients. Due to its retrospective nature, changes in the quality of life before and after ablation could not be assessed. However, due to concomitant other cardiac operations, even in prospective studies it is not feasible to exclude the effect of other cardiac operation on the change in quality of life. Another limitation of this study is that our database has a joint section for persistent and paroxysmal AF; therefore, we could have not determined the survival difference between the two groups in this retrospective study. The duration of AF preoperatively is another factor that can affect the survival in short- and long term which is not documented in most of the cardiac surgery databases worldwide. Moreover, we have not investigated the cause of death in these patients and their cohort. But our study is unique in investigating the survival after ablation in mid-term. Large prospective multicentre randomized clinical trials are recommended to determine the effect of RF ablation on the mid- and long-term survival. Accurate registration of the ablated cases at national and international level is also recommended [19].
In conclusion, concomitant RF ablation requires a short learning curve that carries virtually no complications and improves the survival rate post cardiac surgery. All patients with AF undergoing cardiac surgery should be considered for ablation and the technique should be made available at cardiac surgery centres.
Conflict of interest: none declared.
REFERENCES
APPENDIX. CONFERENCE DISCUSSION
Dr F. Wagner (Hamburg, Germany): I think this is a very interesting and important study, because all of us are involved in doing more and more of these concomitant procedures along with our cardiac surgery, and, particularly with ablation, there is not a great deal of data really proving that what we do, even if we achieve sinus rhythm, really improves survival. Most of them are retrospective data. There is no prospective study, at least to my knowledge, that has ever proven that concomitant treatment of AF really does something for long-term survival in these patients. So, I think it is a very interesting study, since you have shown that after five years, survival differs to reach statistical significance.
Now, question number one, what was your percentage of success with establishing sinus rhythm, at the different time points? And number two, looking at those patients who died and those who survived, what was the percentage of patients in sinus rhythm at time of death versus those with ongoing AF? I think you need to separate these groups, because if patients after ablation are still in AF and die earlier, I am not sure that this has anything to do with our procedure.
Dr Attaran: In response to your first question, 19% of the patients remained in AF at the time of discharge, after 7 days, and 33% in the postoperative clinic. In answer to the second question, unfortunately we do not have the long-term follow-up of their rhythm because they go directly to the cardiologist. So, that is why it shows the importance of randomized controlled trials, to observe these patients postoperatively, short-term and long-term, and to assess whether reverting back to atrial fibrillation has any effect or not.
Dr Wagner: Okay. May I just add a quick further question. Do you know the causes of death in those patients who died? Was it cardiac-related or was it otherwise?
Dr Attaran: This data was extracted from the national death registry and we don't have their cause of death, and that is why I have written ‘all-cause mortality’. So, that needs to be analysed in detail in the future.
Dr S. Benussi (Milan, Italy): To begin with, were the patients in the unablated group and the control group you selected treated appropriately, at least as far as their left appendage is concerned? Meaning it is pretty evident that when you do ablation, everybody closes the appendage routinely, but some surgeons forget that it is even more important to close the appendage when they decide not to ablate AF.
And my second question is, did you consider the surgeon in your variables for your propensity-matched study?
Dr Attaran: No, we didn't, because this is based on our old database and three surgeons do ablations. So, this includes all of their patients, all of the patients that were done in Liverpool Heart and Chest Hospital. And your first question, did you ask about the atrial appendage?
Dr Benussi: The patients who were not ablated, the control group, did they receive appendage closure at the time of surgery? Or does not doing any ablation mean not doing anything, because that will impact on the stroke rate.
Dr Attaran: So, you mean the patients who did have the indication for the ablation but not the benefit?
Dr Benussi: No. Those who had no ablation but were nevertheless in atrial fibrillation, did they have their appendage closed?
Dr Attaran: Yes. They were all managed medically.
Dr Benussi: Even without ablation?
Dr Attaran: Yes, without ablation.
Author notes
Presented at the 25th Annual Meeting of the European Association for Cardio-Thoracic Surgery, Lisbon, Portugal, 1–5 October 2011.
- atrial fibrillation
- warfarin
- cardiac surgery procedures
- hemorrhage
- cerebrovascular accident
- ischemic stroke
- atrium
- kidney failure
- hospital mortality
- intensive care unit
- preoperative care
- survival rate
- mortality
- sinus rhythm
- persistence
- radiofrequency ablation
- ablation
- surgical outcome
- inotropic support




