-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroyuki Nakajima, Junjiro Kobayashi, Koichi Toda, Tomoyuki Fujita, Yusuke Shimahara, Yoichiro Kasahara, Soichiro Kitamura, Angiographic evaluation of flow distribution in sequential and composite arterial grafts for three vessel disease, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 4, April 2012, Pages 763–769, https://doi.org/10.1093/ejcts/ezr057
Close - Share Icon Share
Abstract
We sought to delineate the effects of the severity of target lesions and their combinations on the occurrence of competitive flow, especially in the composite Y-graft and to establish an optimal strategy for graft arrangement and patient selection.
We reviewed early and late angiograms of 2514 bypass grafts in 601 patients, who underwent off-pump coronary revascularization to three-vessel vascular regions using the internal thoracic artery (ITA) and radial artery (RA) without aortic manipulation. As a standard technique, the left anterior descending artery (LAD) was bypassed with the in situ ITA, and the left circumflex and right coronary arteries (RCA) were bypassed with the composite RA. Bypass flow was graded as antegrade, competitive or no flow.
The early patency rate was 98.1% (2466/2514), while competitive flow was detected in 6.4% (162/2514). For the LAD, the individual and sequential in situ ITA provided lower incidence of competitive flow than the composite graft (0.3% (1/298) versus 7.6% (23/303), P < 0.0001). Regarding the RA to non-LAD bypass, 86.3% (113/131) of competitive flow occurred at the distal end of the I- or Y-graft, and the cumulative patency rate was significantly lower than that of sequential proximal anastomosis (80.1 versus 56.6% at 5 years, P < 0.0001). The number of sequential anastomoses did not affect the cumulative patency rate (P = 0.09). For the composite Y-graft to three-vessel regions, the rate of antegrade flow in patients with 76–100% stenosis in both the LAD and the RCA was 95.7% (178/186), which was significantly higher than that of 78.1% (100/128) in patients with 76–100% stenosis in the LAD and 51–75% stenosis in RCA (P < 0.0001).
Sequential and composite grafting was considered reliable, exclusively in appropriately selected situations. To secure entire patency of the Y-graft to three-vessel regions, balanced bypass flow toward LAD and RCA would be crucial.
INTRODUCTION
By creation of a composite graft with the in situ internal thoracic artery (ITA) and radial artery (RA), total arterial complete revascularization can be achieved with minimal graft materials with excellent early graft patency and clinical outcomes [1, 2]. However, since flow capacity of bypass grafts entirely depends on the in situ ITA, the incidence of competitive flow is relatively high. To achieve durable completeness of revascularization, prevention of competitive flow is mandatory, because graft occlusion associated with insufficient flow occurs in the early period [3–5].
We have introduced total arterial off-pump coronary artery bypass grafting (CABG) without aortic manipulation as our standard technique. Graft patency and flow had been evaluated in the early postoperative angiography for a decade. Competitive flow usually occurs, when native coronary stenosis is not severe. In addition, we previously reported that some specific combinations of target branches affected distribution of blood flow in the composite and sequential grafts [6]. Regarding sequential grafting, the severity of stenosis in the most distal target played a definitive role in entire patency of the bypass graft [7]. Regarding Y-grafting to two targets, imbalance of severity of stenosis could be a cause of imbalance of flow distribution [6].
The objectives of this study were to examine the effects of the severity of native coronary stenosis in the target branches and their combinations on the occurrence of competitive flow, and to establish indications for the Y-graft to three-vessel territories, from the viewpoint of flow distribution.
MATERIALS AND METHODS
We reviewed the clinical and angiographic records of 615 patients in this study. Consecutive patients, who underwent total arterial off-pump coronary revascularization to three-vessel vascular regions with sequential and composite grafts using the in situ ITA and RA and postoperative angiography between April 2000 and April 2010, were included. Exclusion criteria for this study were patients who had an aortocoronary bypass, gastroepiploic artery or other graft materials, did not have a RA graft, or did not undergo postoperative angiography. Institutional approval was obtained for this retrospective observational study. The native coronary artery stenosis and the graft patency were independently evaluated by cardiologists. The severity of stenosis was calculated from the minimal luminal diameter, and the maximal severity of stenosis was recorded for all targets. In our institution, early postoperative angiography was performed routinely, except for patients with a considerable risk of catheter angiography, such as renal failure or aged >80 years. In the present study, 601 (97.7%) patients with 2514 bypass grafts underwent early postoperative angiography. It was usually performed ∼2 weeks after surgery during hospitalization. Bypass flow was graded as antegrade, competitive or no flow in the early postoperative angiography. Antegrade was defined as a situation in which the target branch was opacified antegradely in most of multi-plane ITA graft injection. Competitive flow was defined as a situation in which the target vessel was slightly opacified or not opacified from the ITA graft injection, and the bypass graft was filled clearly by retrograde flow from the native coronary injection. Late angiography was performed in 111 (18.5%) patients with 443 bypass grafts for clinical reasons. The mean follow-up period was 54 ± 31 months (Table 1).
| No. of patients | 615 |
| Age (years) | 66 ± 9 |
| Male/female | 504/111 |
| Hypertension | 344 (56%) |
| Hyperlipidaemia | 339 (55%) |
| Diabetes | 263 (43%) |
| End-diastolic volume index of LV (ml/m2) | 83.7 ± 27.8 |
| Ejection fraction of LV (%) | 48.1 ± 11.2 |
| ITA | |
| Single | |
| Y-graft | 189 |
| K-graft | 29 |
| Bilateral | 397 |
| Postoperative angiography | |
| Early | 601 patients |
| Late | 111 patients |
| Total distal anastomoses | 2572 |
| Targets per patient | 4.18 ± 0.95 |
| No. of patients | 615 |
| Age (years) | 66 ± 9 |
| Male/female | 504/111 |
| Hypertension | 344 (56%) |
| Hyperlipidaemia | 339 (55%) |
| Diabetes | 263 (43%) |
| End-diastolic volume index of LV (ml/m2) | 83.7 ± 27.8 |
| Ejection fraction of LV (%) | 48.1 ± 11.2 |
| ITA | |
| Single | |
| Y-graft | 189 |
| K-graft | 29 |
| Bilateral | 397 |
| Postoperative angiography | |
| Early | 601 patients |
| Late | 111 patients |
| Total distal anastomoses | 2572 |
| Targets per patient | 4.18 ± 0.95 |
ITA: internal thoracic artery; LV: left ventricle.
| No. of patients | 615 |
| Age (years) | 66 ± 9 |
| Male/female | 504/111 |
| Hypertension | 344 (56%) |
| Hyperlipidaemia | 339 (55%) |
| Diabetes | 263 (43%) |
| End-diastolic volume index of LV (ml/m2) | 83.7 ± 27.8 |
| Ejection fraction of LV (%) | 48.1 ± 11.2 |
| ITA | |
| Single | |
| Y-graft | 189 |
| K-graft | 29 |
| Bilateral | 397 |
| Postoperative angiography | |
| Early | 601 patients |
| Late | 111 patients |
| Total distal anastomoses | 2572 |
| Targets per patient | 4.18 ± 0.95 |
| No. of patients | 615 |
| Age (years) | 66 ± 9 |
| Male/female | 504/111 |
| Hypertension | 344 (56%) |
| Hyperlipidaemia | 339 (55%) |
| Diabetes | 263 (43%) |
| End-diastolic volume index of LV (ml/m2) | 83.7 ± 27.8 |
| Ejection fraction of LV (%) | 48.1 ± 11.2 |
| ITA | |
| Single | |
| Y-graft | 189 |
| K-graft | 29 |
| Bilateral | 397 |
| Postoperative angiography | |
| Early | 601 patients |
| Late | 111 patients |
| Total distal anastomoses | 2572 |
| Targets per patient | 4.18 ± 0.95 |
ITA: internal thoracic artery; LV: left ventricle.
Usage of the bilateral in situ ITA is our standard technique for three-vessel revascularization, as reported previously [7], whereas the single in situ ITA as the Y-graft has been utilized for the patients at considerable risk. There was no patient whose bilateral RAs were used. The Y-graft was made by side-to-side proximal anastomosis of the RA to avoid kinking of the graft [8]. In the sequential anastomosis by the side-to-side method, we usually made the diamond-shape anastomosis by longitudinal arteriotomy on the target branch and the RA to save the graft length.
Analysis plan for a composite Y-graft to three-vessel regions
We assessed the impacts of combinations of the targets in the Y-graft on graft flow and graft patency. Of 189 patients, who had the composite Y-graft of an in situ ITA and RA, 9 patients were excluded because they did not undergo early postoperative angiography. In all 180 patients, the end of the ITA was independently anastomosed to the main trunk of left anterior descending artery (LAD), and the RA was sequentially anastomosed to at least one bypass graft to each of the left circumflex artery (LCX) and right coronary artery (RCA) regions. Diagonal branch was grafted with the RA in a side-to-side fashion if necessary.
The Y-graft to three-vessel regions consists of two sequential grafts. One is the in situ ITA graft anastomosed with the RA and the LAD, and the other is the RA graft anastomosed with LCX and RCA. In our previous study on sequential grafting, when severity of stenosis in the most distal target was moderate, competitive flow commonly occurred and the patency rate was significantly low, as compared with when the distal end was anastomosed to a severely stenotic target [7]. If we applied this hypothesis to the Y-graft to three-vessel regions, the severity of stenosis in the LAD and the RCA would be crucial to achieve successful Y-grafting. Our previous study also revealed that when an occluded or near-occluded branch was anastomosed in a side-to-side fashion as the sequential proximal anastomosis, competitive flow can be provoked at the end [6]. If this hypothesis was applied to the composite Y-graft to three-vessel regions, an occluded or near-occluded LCX might promote competitive flow from the RCA and competitive flow from the LAD might be increased, when the RA was anastomosed with occluded or near-occluded LCX and RCA branches. In the present study, we, therefore, examined the severity of stenosis in the LAD and RCA branches, and their combinations. Moreover, subgroup analyses were performed according to the presence or absence of the occluded or near-occluded target at the sequential proximal anastomosis.
Statistical analysis
The continuous variables are expressed as the mean values ± standard deviation (SD). The data of two independent groups were compared by Fisher's exact probability test. Longitudinal data were estimated by the Kaplan–Meier method and the difference between groups was compared with the log-rank method. The differences in the outcomes were considered statistically significant when the P-value was <0.05.
RESULTS
In the early angiography, the graft patency rate was 98.1% (2466/2514), while competitive flow occurred in 6.4% (162/2514) (Table 2).
| Severity of stenosis in the target . | Overall . | 51–75% . | 76–100% . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics of bypass grafts . | Patent . | % . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . |
| Total | 2466 | 98.1 | 162 | 6.4 | 2514 | 134 | 12.3 | 1093 | 28 | 2.0 | 1421 |
| ITA-LAD (main target) | 596 | 99.2 | 24 | 4.0 | 601 | 17 | 6.1 | 277 | 7 | 2.2 | 324 |
| Conduit type | |||||||||||
| In-situ ITA individual | 234 | 99.6 | 0 | 0 [1] | 235 | 0 | 0 [5] | 103 | 0 | 0 [9] | 132 |
| In situ ITA sequential | 61 | 96.8 | 1 | 1.6 [2] | 63 | 1 | 3.4 [6] | 29 | 0 | 0 [10] | 34 |
| Y-graft | 264 | 99.2 | 18 | 6.8 [3] | 266 | 14 | 10.6 [7] | 132 | 4 | 3.0 [11] | 134 |
| K-graft | 37 | 100 | 5 | 13.5 [4] | 37 | 2 | 15.4 [8] | 13 | 3 | 12.5 [12] | 24 |
| RA-non-LAD (diagonal, LCX and RCA) | 1710 | 97.8 | 131 | 7.5 | 1749 | 111 | 15.1 | 736 | 20 | 2.0 | 1013 |
| Conduit type | |||||||||||
| I-graft | 919 | 97.7 | 60 | 6.4 [13] | 941 | 52 | 13.7 | 380 | 8 | 1.4 | 561 |
| Y-graft | 667 | 97.7 | 58 | 8.5 [14] | 683 | 48 | 15.7 | 306 | 10 | 2.7 | 377 |
| K-graft | 124 | 99.2 | 13 | 10.4 [15] | 125 | 11 | 22.0 | 50 | 2 | 2.7 | 75 |
| Sequential anastomoses | |||||||||||
| 1 (non-sequential) | 120 | 96.8 | 7 | 5.6 | 124 | 7 | 12.5 | 56 | 0 | 0.0 | 68 |
| 2 | 493 | 96.3 | 48 | 9.4 | 512 | 42 | 18.0 | 233 | 6 | 2.2 | 279 |
| 3 | 623 | 98.0 | 43 | 6.8 | 636 | 35 | 13.2 | 266 | 8 | 2.2 | 370 |
| 4∼ | 474 | 99.4 | 33 | 6.9 | 477 | 27 | 14.9 | 181 | 6 | 2.0 | 296 |
| Anastomotic fashion | |||||||||||
| End-to-side (graft end) | 680 | 95.9 | 113 | 15.9 [16] | 709 | 93 | 37.5 [18] | 248 | 20 | 4.3 | 461 |
| Side-to-side (sequential proximal) | 1030 | 99.0 | 18 | 1.7 [17] | 1040 | 18 | 3.7 [19] | 488 | 0 | 0.0 | 552 |
| Others | 160 | 97.6 | 7 | 4.3 | 164 | 6 | 7.5 | 80 | 1 | 1.2 | 84 |
| Severity of stenosis in the target . | Overall . | 51–75% . | 76–100% . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics of bypass grafts . | Patent . | % . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . |
| Total | 2466 | 98.1 | 162 | 6.4 | 2514 | 134 | 12.3 | 1093 | 28 | 2.0 | 1421 |
| ITA-LAD (main target) | 596 | 99.2 | 24 | 4.0 | 601 | 17 | 6.1 | 277 | 7 | 2.2 | 324 |
| Conduit type | |||||||||||
| In-situ ITA individual | 234 | 99.6 | 0 | 0 [1] | 235 | 0 | 0 [5] | 103 | 0 | 0 [9] | 132 |
| In situ ITA sequential | 61 | 96.8 | 1 | 1.6 [2] | 63 | 1 | 3.4 [6] | 29 | 0 | 0 [10] | 34 |
| Y-graft | 264 | 99.2 | 18 | 6.8 [3] | 266 | 14 | 10.6 [7] | 132 | 4 | 3.0 [11] | 134 |
| K-graft | 37 | 100 | 5 | 13.5 [4] | 37 | 2 | 15.4 [8] | 13 | 3 | 12.5 [12] | 24 |
| RA-non-LAD (diagonal, LCX and RCA) | 1710 | 97.8 | 131 | 7.5 | 1749 | 111 | 15.1 | 736 | 20 | 2.0 | 1013 |
| Conduit type | |||||||||||
| I-graft | 919 | 97.7 | 60 | 6.4 [13] | 941 | 52 | 13.7 | 380 | 8 | 1.4 | 561 |
| Y-graft | 667 | 97.7 | 58 | 8.5 [14] | 683 | 48 | 15.7 | 306 | 10 | 2.7 | 377 |
| K-graft | 124 | 99.2 | 13 | 10.4 [15] | 125 | 11 | 22.0 | 50 | 2 | 2.7 | 75 |
| Sequential anastomoses | |||||||||||
| 1 (non-sequential) | 120 | 96.8 | 7 | 5.6 | 124 | 7 | 12.5 | 56 | 0 | 0.0 | 68 |
| 2 | 493 | 96.3 | 48 | 9.4 | 512 | 42 | 18.0 | 233 | 6 | 2.2 | 279 |
| 3 | 623 | 98.0 | 43 | 6.8 | 636 | 35 | 13.2 | 266 | 8 | 2.2 | 370 |
| 4∼ | 474 | 99.4 | 33 | 6.9 | 477 | 27 | 14.9 | 181 | 6 | 2.0 | 296 |
| Anastomotic fashion | |||||||||||
| End-to-side (graft end) | 680 | 95.9 | 113 | 15.9 [16] | 709 | 93 | 37.5 [18] | 248 | 20 | 4.3 | 461 |
| Side-to-side (sequential proximal) | 1030 | 99.0 | 18 | 1.7 [17] | 1040 | 18 | 3.7 [19] | 488 | 0 | 0.0 | 552 |
| Others | 160 | 97.6 | 7 | 4.3 | 164 | 6 | 7.5 | 80 | 1 | 1.2 | 84 |
ITA: internal thoracic artery; LAD: left anterior descending artery; LCX: left circumflex artery; RA: radial artery; RCA: right coronary artery.
[1]+[2] versus [3]+[4]; P < 0.0001, [5]+[6] versus [7]+[8]; P = 0.0002, [9]+[10] versus [11]+[12]; P = 0.006, [13] versus [14]+[15]; P = 0.07, [16] versus [17]; P < 0.0001, [18] versus [19]; P < 0.0001.
| Severity of stenosis in the target . | Overall . | 51–75% . | 76–100% . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics of bypass grafts . | Patent . | % . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . |
| Total | 2466 | 98.1 | 162 | 6.4 | 2514 | 134 | 12.3 | 1093 | 28 | 2.0 | 1421 |
| ITA-LAD (main target) | 596 | 99.2 | 24 | 4.0 | 601 | 17 | 6.1 | 277 | 7 | 2.2 | 324 |
| Conduit type | |||||||||||
| In-situ ITA individual | 234 | 99.6 | 0 | 0 [1] | 235 | 0 | 0 [5] | 103 | 0 | 0 [9] | 132 |
| In situ ITA sequential | 61 | 96.8 | 1 | 1.6 [2] | 63 | 1 | 3.4 [6] | 29 | 0 | 0 [10] | 34 |
| Y-graft | 264 | 99.2 | 18 | 6.8 [3] | 266 | 14 | 10.6 [7] | 132 | 4 | 3.0 [11] | 134 |
| K-graft | 37 | 100 | 5 | 13.5 [4] | 37 | 2 | 15.4 [8] | 13 | 3 | 12.5 [12] | 24 |
| RA-non-LAD (diagonal, LCX and RCA) | 1710 | 97.8 | 131 | 7.5 | 1749 | 111 | 15.1 | 736 | 20 | 2.0 | 1013 |
| Conduit type | |||||||||||
| I-graft | 919 | 97.7 | 60 | 6.4 [13] | 941 | 52 | 13.7 | 380 | 8 | 1.4 | 561 |
| Y-graft | 667 | 97.7 | 58 | 8.5 [14] | 683 | 48 | 15.7 | 306 | 10 | 2.7 | 377 |
| K-graft | 124 | 99.2 | 13 | 10.4 [15] | 125 | 11 | 22.0 | 50 | 2 | 2.7 | 75 |
| Sequential anastomoses | |||||||||||
| 1 (non-sequential) | 120 | 96.8 | 7 | 5.6 | 124 | 7 | 12.5 | 56 | 0 | 0.0 | 68 |
| 2 | 493 | 96.3 | 48 | 9.4 | 512 | 42 | 18.0 | 233 | 6 | 2.2 | 279 |
| 3 | 623 | 98.0 | 43 | 6.8 | 636 | 35 | 13.2 | 266 | 8 | 2.2 | 370 |
| 4∼ | 474 | 99.4 | 33 | 6.9 | 477 | 27 | 14.9 | 181 | 6 | 2.0 | 296 |
| Anastomotic fashion | |||||||||||
| End-to-side (graft end) | 680 | 95.9 | 113 | 15.9 [16] | 709 | 93 | 37.5 [18] | 248 | 20 | 4.3 | 461 |
| Side-to-side (sequential proximal) | 1030 | 99.0 | 18 | 1.7 [17] | 1040 | 18 | 3.7 [19] | 488 | 0 | 0.0 | 552 |
| Others | 160 | 97.6 | 7 | 4.3 | 164 | 6 | 7.5 | 80 | 1 | 1.2 | 84 |
| Severity of stenosis in the target . | Overall . | 51–75% . | 76–100% . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics of bypass grafts . | Patent . | % . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . | Competitive flow . | % . | Number of targets . |
| Total | 2466 | 98.1 | 162 | 6.4 | 2514 | 134 | 12.3 | 1093 | 28 | 2.0 | 1421 |
| ITA-LAD (main target) | 596 | 99.2 | 24 | 4.0 | 601 | 17 | 6.1 | 277 | 7 | 2.2 | 324 |
| Conduit type | |||||||||||
| In-situ ITA individual | 234 | 99.6 | 0 | 0 [1] | 235 | 0 | 0 [5] | 103 | 0 | 0 [9] | 132 |
| In situ ITA sequential | 61 | 96.8 | 1 | 1.6 [2] | 63 | 1 | 3.4 [6] | 29 | 0 | 0 [10] | 34 |
| Y-graft | 264 | 99.2 | 18 | 6.8 [3] | 266 | 14 | 10.6 [7] | 132 | 4 | 3.0 [11] | 134 |
| K-graft | 37 | 100 | 5 | 13.5 [4] | 37 | 2 | 15.4 [8] | 13 | 3 | 12.5 [12] | 24 |
| RA-non-LAD (diagonal, LCX and RCA) | 1710 | 97.8 | 131 | 7.5 | 1749 | 111 | 15.1 | 736 | 20 | 2.0 | 1013 |
| Conduit type | |||||||||||
| I-graft | 919 | 97.7 | 60 | 6.4 [13] | 941 | 52 | 13.7 | 380 | 8 | 1.4 | 561 |
| Y-graft | 667 | 97.7 | 58 | 8.5 [14] | 683 | 48 | 15.7 | 306 | 10 | 2.7 | 377 |
| K-graft | 124 | 99.2 | 13 | 10.4 [15] | 125 | 11 | 22.0 | 50 | 2 | 2.7 | 75 |
| Sequential anastomoses | |||||||||||
| 1 (non-sequential) | 120 | 96.8 | 7 | 5.6 | 124 | 7 | 12.5 | 56 | 0 | 0.0 | 68 |
| 2 | 493 | 96.3 | 48 | 9.4 | 512 | 42 | 18.0 | 233 | 6 | 2.2 | 279 |
| 3 | 623 | 98.0 | 43 | 6.8 | 636 | 35 | 13.2 | 266 | 8 | 2.2 | 370 |
| 4∼ | 474 | 99.4 | 33 | 6.9 | 477 | 27 | 14.9 | 181 | 6 | 2.0 | 296 |
| Anastomotic fashion | |||||||||||
| End-to-side (graft end) | 680 | 95.9 | 113 | 15.9 [16] | 709 | 93 | 37.5 [18] | 248 | 20 | 4.3 | 461 |
| Side-to-side (sequential proximal) | 1030 | 99.0 | 18 | 1.7 [17] | 1040 | 18 | 3.7 [19] | 488 | 0 | 0.0 | 552 |
| Others | 160 | 97.6 | 7 | 4.3 | 164 | 6 | 7.5 | 80 | 1 | 1.2 | 84 |
ITA: internal thoracic artery; LAD: left anterior descending artery; LCX: left circumflex artery; RA: radial artery; RCA: right coronary artery.
[1]+[2] versus [3]+[4]; P < 0.0001, [5]+[6] versus [7]+[8]; P = 0.0002, [9]+[10] versus [11]+[12]; P = 0.006, [13] versus [14]+[15]; P = 0.07, [16] versus [17]; P < 0.0001, [18] versus [19]; P < 0.0001.
Regarding the ITA to LAD bypass graft, the incidence of competitive flow in the composite Y- and K-graft was significantly higher than that in the in situ ITA graft (7.6% (23/303) versus 0.3% (1/298), P < 0.0001). There was no significant difference between the cumulative graft patency rate of the in situ ITA and that of the composite graft, which included Y- and K-graft (88.3 versus 80.5% at 5 years, P = 0.35) (Fig. 1). The cumulative patency rate of the bypass grafts with competitive flow was significantly lower than that of the bypass grafts with antegrade flow (42.7 versus 93.4% at 5 years, P = 0.0001) (Fig. 2).
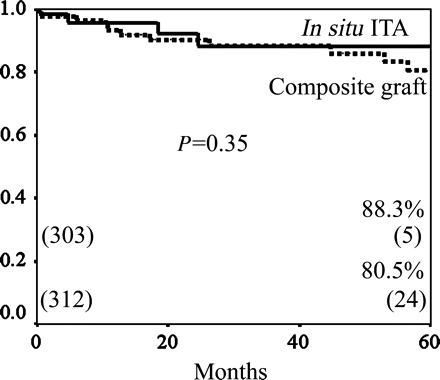
The cumulative graft patency rates of the bypass grafts to the LAD; in situ ITA versus composite Y- and K-graft. The cumulative patency rate of in situ ITA to the LAD bypass grafts was significantly higher than that of composite grafts (88.3 versus 80.5% at 5 years, P = 0.35).
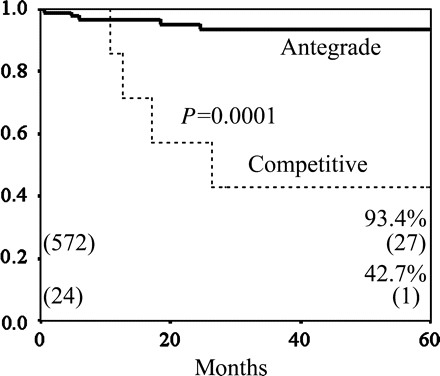
The cumulative graft patency rates of the bypass grafts to the LAD; antegrade flow versus competitive flow. The cumulative patency rate of bypass grafts with antegrade flow was significantly higher than that of bypass grafts with competitive flow (93.1 versus 42.7% at 5 years, P = 0.0001).
Regarding bypass grafts of the RA to non-LAD branches, which included diagonal branch, LCX and RCA branches, there was no significant difference in the incidence of competitive flow for the conduit type and the number of sequential anastomoses. The incidence of competitive flow in the sequential proximal was significantly lower than that in the graft distal end (1.7% (18/1040) versus 15.9% (113/709), P < 0.0001) (Table 2). There was no significant difference between the cumulative patency rates of the Y-graft and the I-graft (at 5 years, 69.3 versus 61.3%, P = 0.09), and between grafting to one or two targets and grafting to three or more targets in the sequential fashion (at 5 years, 71.0 versus 63.0%, P = 0.92) (Fig. 3). The cumulative graft patency rate of the distal end of the RA was significantly lower than that of the sequential proximal anastomoses (P < 0.0001) (Fig. 4).
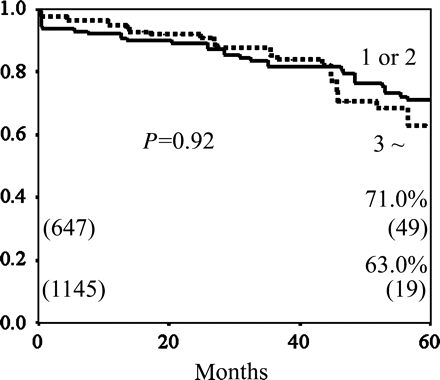
The cumulative graft patency rates of the RA to non-LAD according to the number of sequential anastomoses; 1 or 2 versus 3 or more. The cumulative patency rate of the RA with 1 or 2 sequential anastomoses was similar to that of the RA with 3 or more sequential anastomoses (70.0 versus 63.0% at 5 years, P = 0.92).
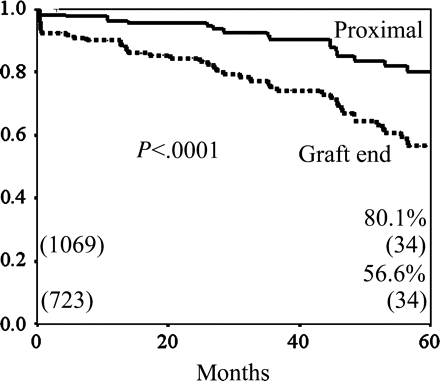
The cumulative graft patency rates of the RA to non-LAD according; the distal end versus sequential proximal anastomoses. The cumulative patency rate of the distal end of the RA was significantly lower than that of the sequential proximal anastomoses of the RA (56.6 versus 80.1% at 5 years, P < 0.0001).
Flow distribution in 180 Y-grafts to three-vessel regions
As shown in Table 3, 180 Y-grafts were divided into 9 sections, according to native coronary stenosis in the LAD and the RCA, which was the target of the distal end of the RA. The target of the distal end of the RA was the posterior descending branch in 150 patients and the atrioventricular branch in 30 patients.
| . | . | Coronary branch . | RCA . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Severity of stenosis . | 51–75% . | 76–90% . | 91–100% . | ||||||
| Coronary branch . | Severity of stenosis . | . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . |
| LAD | 51–75% | Section no. | 1 | 2 | 3 | ||||||
| No. of target | 75 | 24 | 51 | 116 | 32 | 84 | 119 | 36 | 83 | ||
| Antegrade | 65 (86.7) | 22 | 43 | 104 (89.7) | 28 | 76 | 110 (92.4) | 29 | 81 | ||
| Competitive | 10 | 2 | 8 | 11 | 4 | 7 | 6 | 6 | 0 | ||
| No flow | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 1 | 2 | ||
| 76–90% | Section no. | 4 | 5 | 6 | |||||||
| No. of target | 73 | 20 | 53 | 51 | 14 | 37 | 56 | 17 | 39 | ||
| Antegrade | 57 (78.1) | 18 | 39 | 49 (96.1) | 14 | 35 | 53 (94.6) | 17 | 36 | ||
| Competitive | 13 | 1 | 12 | 2 | 1 | 1 | 2 | 2 | 0 | ||
| No flow | 3 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 91–100% | Section no. | 7 | 8 | 9 | |||||||
| No. of target | 55 | 15 | 40 | 34 | 9 | 25 | 45 | 13 | 32 | ||
| Antegrade | 43 (78.2) | 15 | 28 | 31 (91.2) | 9 | 22 | 45 (100) | 13 | 32 | ||
| Competitive | 12 | 0 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| No flow | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | ||
| . | . | Coronary branch . | RCA . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Severity of stenosis . | 51–75% . | 76–90% . | 91–100% . | ||||||
| Coronary branch . | Severity of stenosis . | . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . |
| LAD | 51–75% | Section no. | 1 | 2 | 3 | ||||||
| No. of target | 75 | 24 | 51 | 116 | 32 | 84 | 119 | 36 | 83 | ||
| Antegrade | 65 (86.7) | 22 | 43 | 104 (89.7) | 28 | 76 | 110 (92.4) | 29 | 81 | ||
| Competitive | 10 | 2 | 8 | 11 | 4 | 7 | 6 | 6 | 0 | ||
| No flow | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 1 | 2 | ||
| 76–90% | Section no. | 4 | 5 | 6 | |||||||
| No. of target | 73 | 20 | 53 | 51 | 14 | 37 | 56 | 17 | 39 | ||
| Antegrade | 57 (78.1) | 18 | 39 | 49 (96.1) | 14 | 35 | 53 (94.6) | 17 | 36 | ||
| Competitive | 13 | 1 | 12 | 2 | 1 | 1 | 2 | 2 | 0 | ||
| No flow | 3 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 91–100% | Section no. | 7 | 8 | 9 | |||||||
| No. of target | 55 | 15 | 40 | 34 | 9 | 25 | 45 | 13 | 32 | ||
| Antegrade | 43 (78.2) | 15 | 28 | 31 (91.2) | 9 | 22 | 45 (100) | 13 | 32 | ||
| Competitive | 12 | 0 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| No flow | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | ||
ITA: internal thoracic artery; LAD: left anterior descending artery; RA: radial artery; RCA: right coronary artery.
| . | . | Coronary branch . | RCA . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Severity of stenosis . | 51–75% . | 76–90% . | 91–100% . | ||||||
| Coronary branch . | Severity of stenosis . | . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . |
| LAD | 51–75% | Section no. | 1 | 2 | 3 | ||||||
| No. of target | 75 | 24 | 51 | 116 | 32 | 84 | 119 | 36 | 83 | ||
| Antegrade | 65 (86.7) | 22 | 43 | 104 (89.7) | 28 | 76 | 110 (92.4) | 29 | 81 | ||
| Competitive | 10 | 2 | 8 | 11 | 4 | 7 | 6 | 6 | 0 | ||
| No flow | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 1 | 2 | ||
| 76–90% | Section no. | 4 | 5 | 6 | |||||||
| No. of target | 73 | 20 | 53 | 51 | 14 | 37 | 56 | 17 | 39 | ||
| Antegrade | 57 (78.1) | 18 | 39 | 49 (96.1) | 14 | 35 | 53 (94.6) | 17 | 36 | ||
| Competitive | 13 | 1 | 12 | 2 | 1 | 1 | 2 | 2 | 0 | ||
| No flow | 3 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 91–100% | Section no. | 7 | 8 | 9 | |||||||
| No. of target | 55 | 15 | 40 | 34 | 9 | 25 | 45 | 13 | 32 | ||
| Antegrade | 43 (78.2) | 15 | 28 | 31 (91.2) | 9 | 22 | 45 (100) | 13 | 32 | ||
| Competitive | 12 | 0 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| No flow | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | ||
| . | . | Coronary branch . | RCA . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | Severity of stenosis . | 51–75% . | 76–90% . | 91–100% . | ||||||
| Coronary branch . | Severity of stenosis . | . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . | Antegrade (%) . | ITA-LAD . | RA-non-LAD . |
| LAD | 51–75% | Section no. | 1 | 2 | 3 | ||||||
| No. of target | 75 | 24 | 51 | 116 | 32 | 84 | 119 | 36 | 83 | ||
| Antegrade | 65 (86.7) | 22 | 43 | 104 (89.7) | 28 | 76 | 110 (92.4) | 29 | 81 | ||
| Competitive | 10 | 2 | 8 | 11 | 4 | 7 | 6 | 6 | 0 | ||
| No flow | 0 | 0 | 0 | 1 | 0 | 1 | 3 | 1 | 2 | ||
| 76–90% | Section no. | 4 | 5 | 6 | |||||||
| No. of target | 73 | 20 | 53 | 51 | 14 | 37 | 56 | 17 | 39 | ||
| Antegrade | 57 (78.1) | 18 | 39 | 49 (96.1) | 14 | 35 | 53 (94.6) | 17 | 36 | ||
| Competitive | 13 | 1 | 12 | 2 | 1 | 1 | 2 | 2 | 0 | ||
| No flow | 3 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | ||
| 91–100% | Section no. | 7 | 8 | 9 | |||||||
| No. of target | 55 | 15 | 40 | 34 | 9 | 25 | 45 | 13 | 32 | ||
| Antegrade | 43 (78.2) | 15 | 28 | 31 (91.2) | 9 | 22 | 45 (100) | 13 | 32 | ||
| Competitive | 12 | 0 | 12 | 1 | 0 | 1 | 0 | 0 | 0 | ||
| No flow | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 0 | 0 | ||
ITA: internal thoracic artery; LAD: left anterior descending artery; RA: radial artery; RCA: right coronary artery.
The rates of antegrade flow were 96.1% in section 5, 94.6% in section 6, 91.2% in section 8 and 100% in section 9, respectively. The rate of antegrade flow in patients with 76–100% stenosis in both the LAD and the RCA, who were in sections 5, 6, 8 and 9, was 95.7% (178/186), which was significantly higher than that of 78.1% (100/128) in patients with 76–100% stenosis in the LAD and 51–75% stenosis in RCA (P < 0.0001), who were in sections 4 and 7. In keeping with the hypothesis, section 3 was divided. In 9 patients of 36 patients in the section 3, LCX had 91–100% stenosis. Their all targets of the RA were 91–100% stenosis. In 5 (55.6%) of these 9 patients, competitive flow was found in the ITA-LAD bypass. In the remaining, 27 patients of section 3, the rate of antegrade flow was 97.7% (86/88), being comparable with that of the sections 5, 6, 8 and 9 (P = 0.51).
These sections were categorized into 3 groups. Group I consisted of sections 5, 6, 8, 9 and the 27 patients of section 3, who had 51–90% stenotic LCX. Group I included 80 patients with 274 targets. Group II consisted of 191 targets in 56 patients of sections 1 and 2. Group III consisted of sections 4, 7, and the 9 patients of section 3, who had 91–100% stenotic LCX. In group III, there were 159 targets in 44 patients.
The rate of antegrade flow in group I was significantly higher than that in group II (96.4 (264/274) versus 88.5% (169/191), p = 0.001). The rate of antegrade flow in group II was significantly higher than that in group III (88.5 (169/191) versus 78.0% (124/159), p = 0.009).
The rate of competitive flow in group I was significantly lower than that in group II (2.6 (7/274) versus 11.0% (21/191), p = 0.0002). The rate of competitive flow in group III was 18.2% (29/159), being higher than in group II, but did not reach statistical significance (P = 0.07).
The cumulative graft patency rates at 5 years were 94.6% in group I, 75.4% in group II and 53.3% in group III (P = 0.0003; group I versus group III, P = 0.005; group II versus group III, P = 0.40; group I versus group II) (Fig. 5).
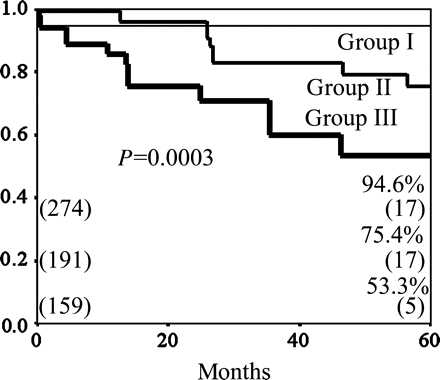
The cumulative graft patency rates according to the three groups. The cumulative patency rates at 5 years were 94.6% in the group I, 75.4% in the group II and 53.3% in the group III (P = 0.0003; group I versus group III, P = 0.005; group II versus group III, P = 0.40; group I versus group II).
DISCUSSION
Previous reports described that the in situ ITA had adaptability to flow demand and abundant flow capacity. The amount of bypass flow in the in situ ITA increased remarkably after several months, and became comparable with the aorto-coronary saphenous vein graft via growth of the ITA and reduction in the vascular resistance in the myocardium [9, 10]. The creation of the Y-graft also significantly increased the flow capacity of the in situ ITA [11]. In addition, as Kim et al. reported, when graft patency could be achieved for 5 years after CABG, the beneficial effect of the Y-graft, which was made with the bilateral ITA, on the myocardium was similar to that of bilateral in situ ITA [12]. However, in spite of these papers supporting flow capacity of the in situ ITA graft, it must be true that composite and sequential grafting have increased risk of competitive flow.
It has been widely accepted that patients who have calcified ascending aorta can be suitable candidates for aorta no-touch sequential and composite grafting [13]. In addition, when a patient has limitation of the graft materials, limitation of approach, such as redo cases, advanced age [14] or other procedural risks, the composite Y-graft can be a useful option. Recently, left thoracotomy combined with technique of the Y-graft has been reported as an alternative approach for multivessel revascularization [15]. However, indications or contraindications for this technique have not been fully discussed.
In the present study, we attempted to evaluate combinations of three targets and two graft materials by distribution of bypass flow. Based on the results of this study, we would suggest classification of the expectancy of long-term patency of composite Y-grafting to three-vessel vascular regions. Patients in group I, who have 76–100% stenosis both in the LAD and the RCA or those who have 51–75% stenosis in the LAD, 91–100% stenosis in RCA and 51–90% stenosis in LCX, would be the most suitable candidates for the composite Y-graft, in terms of durable patency of the grafts. Patients in group II, who have 51–75% stenosis in the LAD and 51–90% stenosis in RCA, were considered less favorable, when compared with group I. However, it would not be clear whether competitive flow in group II was due to the Y-graft configuration or simply moderate stenosis of the target vessels. On the contrary, we presume that the high incidence of competitive flow in Group III would be attributed to the Y-graft configuration itself. When the Y-graft was connected with moderately stenotic LAD, in which intracoronary pressure was high, and near-occluded or occluded RCA and LCX, in which the intracoronary pressure was considerably low, driving pressure from the ITA to the LAD will be spoiled and competitive flow can be highly predicted. As previously reported [16], the increase in blood flow in the proximal portion of ITA increases the pressure gradient between the origin of the ITA and the anastomotic site with the RA. For such patients, the blood source of the bypass graft to the LAD should be separated from the bypass graft to LCX and RCA regions. In patients who have 76–100% in the LAD and 51–75% stenosis in RCA, competitive flow frequently occurred at the distal end of the RA from RCA. For such patients, the aorto-coronary bypass to RCA may be reasonable [17, 18]. Since longer bypass graft has higher resistance by itself, and consequently, can provide only lower intraluminal pressure at the end, severe stenosis is required for sufficient antegrade flow to RCA, which is anatomically distant from the origin of the ITA.
This study has some limitations. First, this study was retrospective, non-randomized and based on the single-center experience. Second, the flow demand or peripheral vascular resistance was not concerned. Third, even with statistical significance, the number of patients and the number of late angiography and graft occlusion may not be enough to achieve adequate outcomes after statistical analyses to conclude this issue. In the present study, no adjustment for multiplicity could be done. In addition, patients who underwent late angiography would be biased, because it was performed, when graft occlusion or progression of the native coronary lesion was suspected by stress test or computed tomography. However, early angiography is the only way to identify competitive flow, and has been performed routinely in our institution. Moreover, the limitation of angiographic assessment for moderate stenosis may be a critical issue. Recently, a FAME study has demonstrated the uncertainty of angiographic visual assessment for the moderately stenotic lesions [19]. In addition, Hamilos et al. reported a discrepancy in the assessment of the functional significance between reviewers [20, 21]. In our experience, the severity of stenosis in LCX and diagonal branch was less influential, when compared with RCA. Improvement of accuracy in the preoperative assessment of moderate lesions will contribute to appropriate surgical decision-making including necessity of the bypass graft, and establishment of consensus in surgical strategy.
Composite and sequential grafting was considered useful and reliable, exclusively in appropriately selected situations. In creation of bypass grafts, durable completeness of revascularization and function of the ITA to the LAD bypass should not be compromised. We would suggest that the balanced bypass flow toward the LAD and the RCA is noticeably important to achieve an entire patency of the Y-graft for three-vessel regions. This study is not conclusive. Interactions between the target coronary branches and bypass grafts on the flow distribution and graft patency may be the next concerns.
Conflict of interest: none declared.




