-
PDF
- Split View
-
Views
-
Cite
Cite
Hiroo Takayama, Yoshifumi Naka, Susheel K. Kodali, Julie A. Vincent, Linda J. Addonizio, Ulrich P. Jorde, Mathew R. Williams, A novel approach to percutaneous right-ventricular mechanical support, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 2, February 2012, Pages 423–426, https://doi.org/10.1016/j.ejcts.2011.05.041
Close - Share Icon Share
Abstract
We report our initial experience with our newly developed percutaneous right-ventricular assist device (VAD) with CentriMag (perc CM-RVAD).
A flexible outflow cannula placed from the right internal jugular vein to the pulmonary artery and an inflow cannula placed from the femoral vein to the right atrium constituted the perc CM-RVAD. When needed, biventricular support was provided with left VAD (LVAD), either with a percutaneous LVAD placed through axillary or femoral artery access or with a fully implantable LVAD.
Between January 2009 and June 2010, all of the attempted patients successfully received perc CM-RVAD (n = 8). Mean blood pressure, heart rate, and central venous pressure showed a trend toward improvement after the perc CM-RVAD, with less inotrope/vasopressor requirement. Mixed venous oxygen saturation (SvO2) increased significantly from 64 ± 20 Torr to 78 ± 6 Torr (P < 0.01). The percutaneous VADs were explanted after myocardial recovery in seven patients; however, in three of these, perc CM-RVAD was used as a temporary bridge to other devices. One patient was bridged to a surgical biventricular assist device (BiVAD) and transferred back to the referring hospital on support. One death occurred due to multiple-organ failure 8 days after explantation of the RVAD with recovery.
Perc CM-RVAD was feasible and provided hemodynamic improvement.
INTRODUCTION
In the urgent or emergent setting, extracorporeal ventricular assist devices (VADs) are used due to the ease of implantation and the flexibility of configuration [1,2]. Recent advance in technology now allows insertion of a VAD percutaneously, which might be more advantageous in these patients. Here, we describe our initial experience in percutaneous insertion of right-ventricular assist device (RVAD) using CentriMag (Levitronix, LLC., Waltham, MA, USA).
MATERIALS AND METHODS
This study was approved by our Institutional Review Board.
This was a retrospective study of patients who underwent percutaneous CentriMag RVAD (perc CM-RVAD) placement at our institution between January 2009 and June 2010. Patients were identified from the Columbia VAD database, and their charts were reviewed for demographics, perioperative hemodynamic parameters, and postoperative outcomes. Inotropic score was defined as follows: low (score 1): epinephrine, dobutamine, or dopamine 1–4 µg kg−1 min−1, milrinone 0.1–0.24 µg kg−1 min−1; moderate (score 2): epinephrine, dobutamine, or dopamine 5–9 µg kg−1 min−1, milrinone 0.25–0.49 µg kg−1 min−1; high (score 3); epi-nephrine, dobutamine, or dopamine more than 9 µg kg−1 min−1, milrinone more than 0.49 µg kg−1 min−1.
Student's t-test was performed to compare the variables before and after the perc CM-RVAD.
Data are shown as mean ± SD.
Insertion technique
The operations were performed in our hybrid operating room or catheterization laboratory. A transesophageal echocardiogram was placed. Two venous access sites for the perc CM-RVAD and one arterial access site for the LVAD, if indicated, were determined, and an arterial-pressure- monitoring line and a venous line were placed in the remaining access sites. When an LVAD was necessary at the time of the perc CM-RVAD, an Impella LP (Abiomed, Danvers, MA, USA) was inserted percutaneously before the perc CM-RVAD. The Impella LP LVAD was inserted through either a femoral artery or an axillary artery. Our general preference is to use an axillary artery whenever feasible for better stabilization as well as patient comfort. A total of 5000 units of heparin were given intravenously, and an 8-mm Dacron graft was sewn in an end-to-side fashion to the axillary artery. The Impella LP LVAD was placed through the graft under guidance of fluoroscopy and transesophageal echocardiogram.
For the perc CM-RVAD, a guidewire was placed with a Seldinger technique through a femoral vein with its tip in the right atrium. A Swan—Ganz catheter was placed through an internal jugular vein and was advanced to the right pulmonary artery under fluoroscopic guidance. The catheter was exchanged to a stiff guidewire (Meier guidewire, Boston Scientific, Natick, MA, USA) with the tip remaining in the right pulmonary artery. A BioMedicus femoral venous cannula (Medtronic, Minneapolis, MN, USA) was introduced as the outflow of the RVAD over the guidewire with its tip at the distal main pulmonary artery. The guidewire in the femoral vein was exchanged to another BioMedicus cannula with its tip in the right atrium as the inflow of the RVAD (Fig. 1). The cannulae were connected to the CentriMag circuit and the RVAD was actuated with the LVAD, if present.
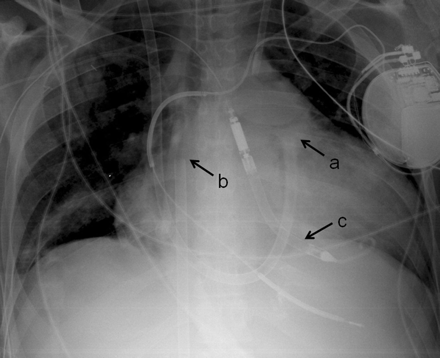
Percutaneous CentriMag right-ventricular assist device with Impella LP 5.0 left ventricular assist device. (a) Tip of outflow cannula in distal main pulmonary artery, (b) tip of inflow cannula in the right atrium, (c) Impella LP 5.0 left ventricular assist device placed tough the right axillary artery.
RESULTS
Eight patients underwent a perc CM-RVAD placement during the study period (Table 1). The average age of the patients was 37 years (range: 11–78 years). The indication for RVAD was acute rejection of the transplanted heart in two patients, post-cardiotomy shock in two, acute RV infarction in one, RV failure after implantable LVAD in one, acute myocarditis in one, and exacerbation of congestive heart failure in one.
| . | Age sex . | Indication . | LVAD . | Outcome . |
|---|---|---|---|---|
| 1 | 18F | Transplant heart acute rejection | Impella LP 2.5 | Explanted after myocardial recovery |
| 2 | 16F | Acute myocarditis | Impella LP 2.5 | Bridged to ECMO with ultimate myocardial recovery |
| 3 | 59M | Post-cardiotomy shock | — | Died due to MOF |
| 4 | 38M | HIV-associated heart failure | Impella LP 5.0 | Bridged to surgical BiVAD, discharged to OSF with BiVAD |
| 5 | 11F | Transplant heart acute rejection | Impella LP 2.5 | Explanted myocardial recovery |
| 6 | 47F | Post-cardiotomy shock | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
| 7 | 25F | RV failure s/p HM2 LVAD | HM2 | Explanted after recovery of RV |
| 8 | 78M | RV infarction | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
| . | Age sex . | Indication . | LVAD . | Outcome . |
|---|---|---|---|---|
| 1 | 18F | Transplant heart acute rejection | Impella LP 2.5 | Explanted after myocardial recovery |
| 2 | 16F | Acute myocarditis | Impella LP 2.5 | Bridged to ECMO with ultimate myocardial recovery |
| 3 | 59M | Post-cardiotomy shock | — | Died due to MOF |
| 4 | 38M | HIV-associated heart failure | Impella LP 5.0 | Bridged to surgical BiVAD, discharged to OSF with BiVAD |
| 5 | 11F | Transplant heart acute rejection | Impella LP 2.5 | Explanted myocardial recovery |
| 6 | 47F | Post-cardiotomy shock | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
| 7 | 25F | RV failure s/p HM2 LVAD | HM2 | Explanted after recovery of RV |
| 8 | 78M | RV infarction | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
BiVAD: biventricular assist device; ECMO: extra-corporeal membrane oxygenation; HIV: human immunodeficiency virus; LVAD: left ventricular assist device; MOF: multiple-organ failure; OSF: outside facility; RV: right ventricle.
| . | Age sex . | Indication . | LVAD . | Outcome . |
|---|---|---|---|---|
| 1 | 18F | Transplant heart acute rejection | Impella LP 2.5 | Explanted after myocardial recovery |
| 2 | 16F | Acute myocarditis | Impella LP 2.5 | Bridged to ECMO with ultimate myocardial recovery |
| 3 | 59M | Post-cardiotomy shock | — | Died due to MOF |
| 4 | 38M | HIV-associated heart failure | Impella LP 5.0 | Bridged to surgical BiVAD, discharged to OSF with BiVAD |
| 5 | 11F | Transplant heart acute rejection | Impella LP 2.5 | Explanted myocardial recovery |
| 6 | 47F | Post-cardiotomy shock | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
| 7 | 25F | RV failure s/p HM2 LVAD | HM2 | Explanted after recovery of RV |
| 8 | 78M | RV infarction | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
| . | Age sex . | Indication . | LVAD . | Outcome . |
|---|---|---|---|---|
| 1 | 18F | Transplant heart acute rejection | Impella LP 2.5 | Explanted after myocardial recovery |
| 2 | 16F | Acute myocarditis | Impella LP 2.5 | Bridged to ECMO with ultimate myocardial recovery |
| 3 | 59M | Post-cardiotomy shock | — | Died due to MOF |
| 4 | 38M | HIV-associated heart failure | Impella LP 5.0 | Bridged to surgical BiVAD, discharged to OSF with BiVAD |
| 5 | 11F | Transplant heart acute rejection | Impella LP 2.5 | Explanted myocardial recovery |
| 6 | 47F | Post-cardiotomy shock | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
| 7 | 25F | RV failure s/p HM2 LVAD | HM2 | Explanted after recovery of RV |
| 8 | 78M | RV infarction | — | Bridged to surgical BiVAD with ultimate myocardial recovery |
BiVAD: biventricular assist device; ECMO: extra-corporeal membrane oxygenation; HIV: human immunodeficiency virus; LVAD: left ventricular assist device; MOF: multiple-organ failure; OSF: outside facility; RV: right ventricle.
Prior to the RVAD placement, five patients were on intra-aortic balloon pump support, three were intubated, three were on inhaled nitric oxide, and two were on continuous venovenous hemodialysis.
Insertion was successful in all patients with no technical complications. Five patients also required device support of the LV including the one who already had an implantable LVAD placed. One patient underwent the operation without endotracheal intubation. Immediate hemodynamic improvement was observed, as shown in Table 2. Mean blood pressure, heart rate, and central venous pressure showed a trend toward improvement after the perc CM-RVAD. Mixed venous oxygen saturation (SvO2) increased significantly from 64 ± 20 Torr to 78 ± 6 Torr (P < 0.01). Inotropic and vasopressor requirements also became less, although this was not statistically significant.
Hemodynamic change before and after the percutaneous CentriMag right-ventricular assist device
| . | Pre-VAD . | Post-VAD . | P . |
|---|---|---|---|
| Mean BP (mmHg) | 72 ± 11 | 79 ± 12 | ns |
| (ppm) | 127 ± 31 | 118 ± 19 | ns |
| CVP (mmHg) | 21 ± 5 | 11 ± 7 | ns |
| SvO2 (Torr) | 64 ± 20 | 78 ± 6 | <0.01 |
| Inotrope score * | 3 (3–4) | 3 (1–4) | ns |
| # of pressors * | 1 (0–2) | 0 (0–2) | ns |
| . | Pre-VAD . | Post-VAD . | P . |
|---|---|---|---|
| Mean BP (mmHg) | 72 ± 11 | 79 ± 12 | ns |
| (ppm) | 127 ± 31 | 118 ± 19 | ns |
| CVP (mmHg) | 21 ± 5 | 11 ± 7 | ns |
| SvO2 (Torr) | 64 ± 20 | 78 ± 6 | <0.01 |
| Inotrope score * | 3 (3–4) | 3 (1–4) | ns |
| # of pressors * | 1 (0–2) | 0 (0–2) | ns |
BP: blood pressure; CVP: central venous pressure, heart rate; VAD: ventricular assist device. The values are expressed as mean ± standard deviation except for the values indicated with *, which are expressed as median (range).
Hemodynamic change before and after the percutaneous CentriMag right-ventricular assist device
| . | Pre-VAD . | Post-VAD . | P . |
|---|---|---|---|
| Mean BP (mmHg) | 72 ± 11 | 79 ± 12 | ns |
| (ppm) | 127 ± 31 | 118 ± 19 | ns |
| CVP (mmHg) | 21 ± 5 | 11 ± 7 | ns |
| SvO2 (Torr) | 64 ± 20 | 78 ± 6 | <0.01 |
| Inotrope score * | 3 (3–4) | 3 (1–4) | ns |
| # of pressors * | 1 (0–2) | 0 (0–2) | ns |
| . | Pre-VAD . | Post-VAD . | P . |
|---|---|---|---|
| Mean BP (mmHg) | 72 ± 11 | 79 ± 12 | ns |
| (ppm) | 127 ± 31 | 118 ± 19 | ns |
| CVP (mmHg) | 21 ± 5 | 11 ± 7 | ns |
| SvO2 (Torr) | 64 ± 20 | 78 ± 6 | <0.01 |
| Inotrope score * | 3 (3–4) | 3 (1–4) | ns |
| # of pressors * | 1 (0–2) | 0 (0–2) | ns |
BP: blood pressure; CVP: central venous pressure, heart rate; VAD: ventricular assist device. The values are expressed as mean ± standard deviation except for the values indicated with *, which are expressed as median (range).
During the support with perc CM-RVAD, one patient developed significant hemolysis requiring exchange to surgical biventricular assist device (BiVAD), and one patient had displacement of the Impella LP requiring repositioning at the bedside under transthoracic echocardiogram. Myocardial recovery with perc CM-RVAD support was observed in three patients, who underwent explantation on day #4, 8, and 9 of the support. Exchange to other mechanical circulatory support device (MCSD) was required in four patients, surgical BiVAD in three and extracorporeal membrane oxygenation (ECMO) in one. This was due to sustained end-organ dysfunction despite perc CM-RVAD support. Postoperatively, four patients required continuous venovenous hemofiltration and dialysis, and, on average, the liver function tests did not improve significantly. The end-organ function of those who did not improve with the perc VAD did recover after exchanging perc VAD to other MCSD. Among the four patients who underwent the exchange operation, three had myocardial recovery and the MCSD was explanted. Overall, seven out of eight patients had myocardial recovery. One was discharged to the referring hospital with the surgical BiVAD.
One death occurred in a patient who underwent emergent placement of perc CM-RVAD for RV failure on postoperative day #13, after an aortic hemiarch and root replacement with coronary artery bypass grafting. The perc CM-RVAD was removed after 12 days of support with myocardial recovery, but the patient eventually succumbed to multiple-organ failure on day #8 after explant of the RVAD.
DISCUSSION
Technological advance has now enabled percutaneous insertion of LVAD, such as Impella LP and TandemHeart (CardiacAssist, Pittsburgh, PA, USA) [3–5]. Although the current main use of these percutaneous LVADs is to support the circulation during high-risk percutaneous coronary interventions, these devices are ideal for patients with acute transient hemodynamic compromise, such as allograft rejection and myocarditis. The use of these devices for this indication will continue to grow. Importantly, cardiogenic shock patients frequently present with biventricular failure and compromised end-organ function. Fitzpatrick et al. demonstrated that planned institution of BiVAD resulted in improved outcomes in such patients [6]. With increased use of percutaneous LVAD for patients with cardiogenic shock, the demand for percutaneous RVAD will increase.
The other indication for percutaneous RVAD is to temporarily support the failing RV after an implantable LVAD. RV failure occurs in 20–40% of LVAD recipients, and it is a cause of poor outcomes [7–9]. For severe RV failure unresponsive to medical management, RVAD support is indicated. This usually is to provide temporary support with the goal of ultimate recovery of RV function. The patient is taken back to the operating room and undergoes re-opening the sternotomy and placement of an RVAD. Once the RV is recovered, the patient needs to go back to the operating room again with a repeat sternotomy for explantation of the RVAD. The use of percutaneous RVAD allows less invasive insertion and explantation of the RVAD. With the increased use of implantable LVAD for bridge to transplantation and for destination therapy, percutaneous RVAD placement might have significant impact on improving patient care.
The last indication is for patients with isolated RV failure, although this entity is uncommon. Moazami et al. reported their experience of RVAD use for isolated RV failure in 30 patients [10]. RV failure developed in patients after coronary artery bypass surgery either alone or combined with valve surgery (12 patients), valvular surgery (five), ascending aortic replacement (six), heart transplantation (three), and pulmonary endarterectomy (four). As is the case with an implantable LVAD, the goal of RVAD support in these circumstances is myocardial recovery.
In the present study, four out of eight perc RVAD patients required exchange of the device to either surgical BiVAD or venoarterial ECMO (VA ECMO) to completely restore other end-organ function. All of these patients had eventual recovery of the end-organ function, indicating that VA ECMO and surgical BiVAD provide better circulatory support than our perc CM-RVAD in those patients. This might be regarded as a limitation of the present device. VA ECMO and surgical BiVAD can produce much higher flow, which might have contributed to the improvement in blood flow to the end organs and better decompression of the venous system, whereas the flow of perc CM-RVAD can be limited by the output from the LV. In our cases, the output from the LV was somewhat compromised, as two of the isolated RVAD cases were post-cardiotomy patients and four of five BiVAD cases had Impella LP to support the LV. Neither post-cardiotomy LV nor Impella LP can generate the same amount of flow as VA ECMO or surgical BiVAD can. In fact, one patient who received perc CM-RVAD subsequent to the implantable LVAD operation had rapid improvement in hemodynamics and end-organ functions, with excellent flow through both VADs (around 3 l min−1 m−2 of flow, which was over 6 l min−1). The two patients who had perc CM-RVAD for allograft rejection were smaller in size and had excellent end-organ recovery, probably because the flow delivered was adequate for their smaller body surface area. Six patients ultimately had myocardial recovery, and one patient was discharged with surgical BiVAD, leaving only one death in this morbid cohort of patients.
This technique is widely applicable. Candidates for this technique only need to have feasible anatomy as well as to tolerate anticoagulation for the insertion as well as the maintenance although this does not have to be stringent. Concern may be raised regarding the right-sided valves, which may be deformed due to the large-bore outflow cannula. Comparison of the echocardiogram before and after the placement showed worsened tricuspid regurgitation in one case and improved regurgitation in two. There was no pulmonary regurgitation. More experience is needed to address this concern.
In summary, perc CM-RVAD was feasible in patients with acute heart failure and provided hemodynamic improvement. Accumulating clinical experience is warranted.
Conflict of interest: none declared.
REFERENCES
- myocardium
- hemodynamics
- vasoconstrictor agents
- patient referral
- pulmonary artery
- right atrium
- heart rate
- ventricular assist device
- axilla
- femoral artery
- femoral vein
- objective (goal)
- heart ventricle
- surgical procedures, operative
- central venous pressure, measurement of
- central venous pressure
- multiple organ dysfunction syndrome
- inotropic agents
- mixed venous oxygen saturation measurement
- mean arterial pressure
- medical devices
- mmhg
- right internal jugular vein
- pulmonary embolism rule-out criteria




