-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Czerny, Sybilla Stöhr, Thierry Aymard, Gottfried H. Sodeck, Marek Ehrlich, Tomasz Dziodzio, Andrzej Juraszek, Thierry Carrel, Effect on false-lumen status of a combined vascular and endovascular approach for the treatment of acute type A aortic dissection, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 2, February 2012, Pages 409–413, https://doi.org/10.1016/j.ejcts.2011.05.063
Close - Share Icon Share
Abstract
The aim of the study is to evaluate midterm results with regard to false-lumen status of a combined vascular and endovascular approach for the treatment of acute type A aortic dissection.
We performed ascending/hemiarch replacement during hypothermic circulatory arrest with additional open implantation of the Djumbodis Dissection System (non-self-expanding bare metal stent) to readapt the dissected layers in the arch and the proximal descending aorta in a consecutive series of 15 patients (mean age 61 years, 20% female) suffering from acute type A aortic dissections. The primary end point was the status of the false lumen at the level of the stent.
We observed three in-hospital deaths (20%). Complete thrombosis of the false lumen was observed in one patient (8%). In 25% of patients, partial thrombosis of the false lumen was observed. The remaining patients had continuing antegrade perfusion. Surgical conversion during a mean follow-up of 37 months was required in two patients (16%) due to continuing enlargement of the distal arch and the proximal descending aorta. No late deaths were observed.
Additional implantation of the Djumbodis Dissection System to readapt the dissected layers in the arch and the proximal descending aorta does not seem to have additive value as an adjunct to standard ascending/hemiarch replacement with regard to closure of the false lumen in the arch and the proximal descending aorta. The most limiting factor seems to be the non-self-expanding capability of the device.
INTRODUCTION
Conventional repair of acute type A aortic dissection is tear oriented [1, 2]. As such, replacement of proximal segments, including the concavity of the aortic arch remains sufficient for the acute situation in the majority of patients. As experience grows, the awareness of late dilatation in primarily untreated segments requiring secondary surgical intervention in downstream segments increases [3, 4]. Consequently, approaches offering one-stop treatment of the entire thoracic aorta are warranted. Several different conceptual approaches have been introduced recently [5–7].
The aim of this study was to evaluate midterm results with regard to false-lumen status of a combined vascular and endovascular approach for the treatment of acute type A aortic dissection.
PATIENTS AND METHODS
Patients
We performed ascending/hemiarch replacement during hypothermic circulatory arrest with additional open implantation of the Djumbodis Dissection System (non-self-expanding bare metal stent) to readapt the dissected layers in the arch and the proximal descending aorta in a consecutive series of 15 patients (mean age 61 years, 20% female) suffering from acute type A aortic dissections. Patient demographics are shown in Table 1.
| . | N overall = 15 . |
|---|---|
| Demographics and etiology of dissection | |
| Age, mean (SD) | 61 (13) |
| Female, n (%) | 3 (20%) |
| Iatrogenic etiology, n (%) | 2 (13%) |
| CT-morphology of dissection | |
| Supraaortic involvement, n (%) | 11 (73%) |
| Aortic arch diameter, total (TL) | 35 (16) |
| Descending aortic diameter, total (TL) | 26 (10) |
| Thoracoabdominal junction diameter, total (TL) | 30 (12) |
| Surgery and in-hospital outcome | |
| ECC in min, mean (SD) | 122 (9) |
| Aortic cross clamp time in min, mean (SD) | 77 (30%) |
| Circulatory arrest time in min, mean (SD) | 24 (11) |
| In-hospital death, n (%) | 3 (20%) |
| . | N overall = 15 . |
|---|---|
| Demographics and etiology of dissection | |
| Age, mean (SD) | 61 (13) |
| Female, n (%) | 3 (20%) |
| Iatrogenic etiology, n (%) | 2 (13%) |
| CT-morphology of dissection | |
| Supraaortic involvement, n (%) | 11 (73%) |
| Aortic arch diameter, total (TL) | 35 (16) |
| Descending aortic diameter, total (TL) | 26 (10) |
| Thoracoabdominal junction diameter, total (TL) | 30 (12) |
| Surgery and in-hospital outcome | |
| ECC in min, mean (SD) | 122 (9) |
| Aortic cross clamp time in min, mean (SD) | 77 (30%) |
| Circulatory arrest time in min, mean (SD) | 24 (11) |
| In-hospital death, n (%) | 3 (20%) |
Diameters in mm. ‘Total’ reflects total aortic diameter, ‘TL’ reflects true lumen diameter. Unless otherwise indicated, data are number (percentage).
| . | N overall = 15 . |
|---|---|
| Demographics and etiology of dissection | |
| Age, mean (SD) | 61 (13) |
| Female, n (%) | 3 (20%) |
| Iatrogenic etiology, n (%) | 2 (13%) |
| CT-morphology of dissection | |
| Supraaortic involvement, n (%) | 11 (73%) |
| Aortic arch diameter, total (TL) | 35 (16) |
| Descending aortic diameter, total (TL) | 26 (10) |
| Thoracoabdominal junction diameter, total (TL) | 30 (12) |
| Surgery and in-hospital outcome | |
| ECC in min, mean (SD) | 122 (9) |
| Aortic cross clamp time in min, mean (SD) | 77 (30%) |
| Circulatory arrest time in min, mean (SD) | 24 (11) |
| In-hospital death, n (%) | 3 (20%) |
| . | N overall = 15 . |
|---|---|
| Demographics and etiology of dissection | |
| Age, mean (SD) | 61 (13) |
| Female, n (%) | 3 (20%) |
| Iatrogenic etiology, n (%) | 2 (13%) |
| CT-morphology of dissection | |
| Supraaortic involvement, n (%) | 11 (73%) |
| Aortic arch diameter, total (TL) | 35 (16) |
| Descending aortic diameter, total (TL) | 26 (10) |
| Thoracoabdominal junction diameter, total (TL) | 30 (12) |
| Surgery and in-hospital outcome | |
| ECC in min, mean (SD) | 122 (9) |
| Aortic cross clamp time in min, mean (SD) | 77 (30%) |
| Circulatory arrest time in min, mean (SD) | 24 (11) |
| In-hospital death, n (%) | 3 (20%) |
Diameters in mm. ‘Total’ reflects total aortic diameter, ‘TL’ reflects true lumen diameter. Unless otherwise indicated, data are number (percentage).
Djumbodis Dissection System
The Djumbodis prosthesis (Saint Come-Chirurgie, Marseille, France), approved in Europe for the treatment of type A aortic dissection in combination with conventional surgery, is an uncovered, non-self-expandable stent made of biocompatible 316 L stainless steel, available in five different lengths of 4, 9, 14, 19, and 24 cm. Prostheses longer than 4 cm are made of 4 cm elements separated from each other by joints of 1 cm. Each prosthesis is mounted on a balloon catheter and can be deployed under a period of hypothermic circulatory arrest along the open aortic arch or descending aorta, by inflating a highly compliant latex balloon. Imaging guidance by echocardiography or fluoroscopy is subjected to the individually treating physician. Stent diameter reaches 4.5 cm at maximal balloon inflation [8]. The proximal end of the stent is located 2 cm distal to the hemiarch anastomosis. In other words, according to the individual situation, the struts are located in proximity to the offspring of the supraaortic branches. Due to the loose mesh of the stent, no impairment of supraaortic perfusion may occur. Furthermore, bending of a strut is feasible, if appropriate.
End points
The primary end point was the status of the false lumen at the level of the stent. Secondary end points were survival as well as the need for re-intervention in downstream aortic segments.
Data collection and follow-up protocol
Data were prospectively collected. Patients were subjected to a strict follow-up protocol that requires a contrast spiral computed tomography (CT) scan and clinical as well as laboratory evaluations at 3, 6, and 12 months after surgery and then annually thereafter. True- and false-lumen diameter measurements are taken and reported from the mid-level of the stent. In this particular setting, we have also considered other levels, proximal and distal end of the stent. However, as diameters corresponded, we focus on mid-level diameters.
Statistical methods
After Kolmogorov—Smirnov testing, normally distributed continuous data are presented as mean ± standard deviation. Categoric variables are presented as absolute and relative frequencies. Overall survival and freedom from secondary interventions were calculated according to the method of Kaplan and Meier. All calculations were performed with Statistical Package for Social Sciences (SPSS) 19.0 software for MacOSX (IBM Inc, Somers, NY, USA).
RESULTS
Preoperative morphology
All patients had DeBakey type I aortic dissection. In 73% of patients, at least one supraaortic branch was affected. Median diameters of the thoracic aortic segments are shown in Table 1.
Clinical results
Perioperative mortality was 20%. One patient died due to neurologic injury and two patients died due to the sequelae of preoperative malperfusion. Intra-operative parameters are shown in Table 1.
False-lumen status and lumen-diameter changes
Complete thrombosis of the false lumen was observed in one patient (8%). In 25% of patients, partial thrombosis of the false lumen was observed. The remaining patients had continuing antegrade perfusion. There was no change with regard to true- and false-lumen diameter changes as well as total aortic diameter before and after surgery as well as during follow-up (Table 2).
| . | N overall = 12 . |
|---|---|
| Postoperative follow-up | |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 27 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 30 (12) |
| Long-term outcome | |
| Follow-up in months, mean (SD) | 37 (20) |
| False-lumen partial thrombosis, n (%) | 3 (25%) |
| False-lumen complete thrombosis, n (%) | 1 (8%) |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 37 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 38 (12) |
| Surgical conversion, n (%) | 2 (17%) |
| Late death, n (%) | 0 (0%) |
| . | N overall = 12 . |
|---|---|
| Postoperative follow-up | |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 27 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 30 (12) |
| Long-term outcome | |
| Follow-up in months, mean (SD) | 37 (20) |
| False-lumen partial thrombosis, n (%) | 3 (25%) |
| False-lumen complete thrombosis, n (%) | 1 (8%) |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 37 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 38 (12) |
| Surgical conversion, n (%) | 2 (17%) |
| Late death, n (%) | 0 (0%) |
Diameters in mm. ‘Total’ reflects total aortic diameter, ‘TL’ reflects true lumen diameter. Unless otherwise indicated, data are number (percentage).
| . | N overall = 12 . |
|---|---|
| Postoperative follow-up | |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 27 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 30 (12) |
| Long-term outcome | |
| Follow-up in months, mean (SD) | 37 (20) |
| False-lumen partial thrombosis, n (%) | 3 (25%) |
| False-lumen complete thrombosis, n (%) | 1 (8%) |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 37 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 38 (12) |
| Surgical conversion, n (%) | 2 (17%) |
| Late death, n (%) | 0 (0%) |
| . | N overall = 12 . |
|---|---|
| Postoperative follow-up | |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 27 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 30 (12) |
| Long-term outcome | |
| Follow-up in months, mean (SD) | 37 (20) |
| False-lumen partial thrombosis, n (%) | 3 (25%) |
| False-lumen complete thrombosis, n (%) | 1 (8%) |
| Aortic arch diameter, mean (SD) | 32 (17) |
| Descending aortic diameter, mean (SD) | 37 (10) |
| Thoracoabdominal junction diameter, mean (SD) | 38 (12) |
| Surgical conversion, n (%) | 2 (17%) |
| Late death, n (%) | 0 (0%) |
Diameters in mm. ‘Total’ reflects total aortic diameter, ‘TL’ reflects true lumen diameter. Unless otherwise indicated, data are number (percentage).
Follow-up and need for re-interventions
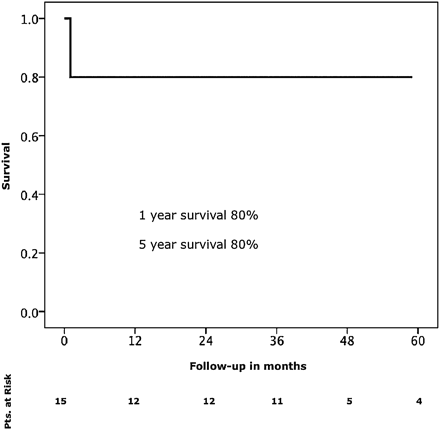
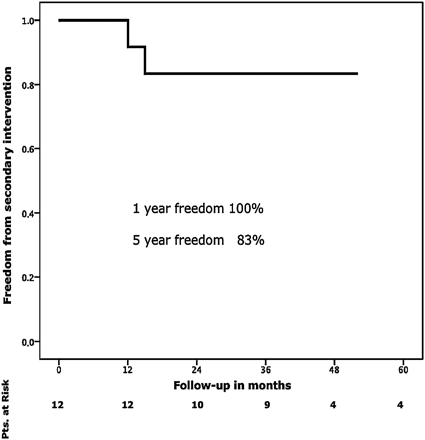
Mean follow-up was 37 months. Surgical conversion during follow-up was required in two patients (16%) due to continuing enlargement of the distal arch and the proximal descending aorta. One- and 5-year survival rates were 80% (Fig. 1). Freedom from re-intervention was 100% at 1 year and 83% at 5 years, respectively (Fig. 2).
COMMENT
Additional implantation of the Djumbodis Dissection System to readapt the dissected layers in the arch and the proximal descending aorta does not seem to have additive value as an adjunct to standard ascending/hemiarch replacement with regard to closure of the false lumen in the arch and the proximal descending aorta. The most limiting factor seems to be the non-self-expanding capability of the device.
Perioperative mortality in this series was 20%. This fact does not deserve intensive discussion as it is in line with recent literature and the reasons for death are a surrogate of the initial extent and aggressiveness of the underlying pathology [9].
Several approaches of varying efficacy to promote false-lumen thrombosis within the entire thoracic aorta to prevent late aneurysm formation and thus re-intervention have been promoted recently [4, 7, 8]. Our group started nearly a decade ago with open implantation of short stent grafts into the proximal descending aorta during hypothermic circulatory arrest (HCA) [3]. However, efficacy was limited and we learned that a circular suture line at the transition of the distal aortic arch and the proximal descending aorta was necessary to prevent type Ia endoleak formation [11]. As a consequence, a combined vascular and endovascular prosthesis (Evita open, JOTEC, Hechingen, Germany) was developed to solve this problem. By this approach, results tremendously improved [12, 13].
The Djumbodis system follows a different philosophy. The idea of this device is to readapt the dissecting membrane to the adventitial layer in direct continuity to the ascending/hemiarch prosthesis to promote false-lumen thrombosis [8, 10, 14]. As such, the concept shows similarities to the Provisional Extension to Induce Complete Attachment After Stent-Graft Placement in Type B Aortic Dissection (PETTICOAT) approach in acute type B aortic dissection [15].
However, this effect was reached only in casuistics. The main limitation of this device seems to be its non-self-expanding capability. Total diameter changes of the aortic arch and the proximal descending aorta — besides the native healthy ascending aorta — are more pronounced than total diameter changes of any other vascular bed due to their high content of elastic fibers. Thereby, the device is not able to adapt to the systolic and diastolic wall-motion changes during the cardiac cycle. Despite the fact that the device is molded with a Latex balloon to fit to the diastolic diameter of the aortic arch or the proximal descending aorta, this effect is not sustained after resuming pulsatile perfusion or even post-pump physiologic motion patterns of the affected aortic segments. As such, the device fulfils a function of a classical Palmaz stent known from peripheral interventions. Figs. 3 and 4 depict this classical situation. Affection of the supraaortic branches may further contribute to our findings, as dissective involvement of the supraaortic branches is also known to be associated with less efficacy of stent grafts regarding false-lumen thrombosis [3]. Finally, the edges of the device are sharp and may thereby lead to membrane rupture distal to the stent, as shown in Fig. 5. This mechanism may also be observed proximally, eventually leading to free rupture which was most likely observed in other series [10,14]. Membrane rupture has also been identified as a mechanism of failure in thoracic endovascular aortic repair (TEVAR) of chronic type B aortic dissections [16].
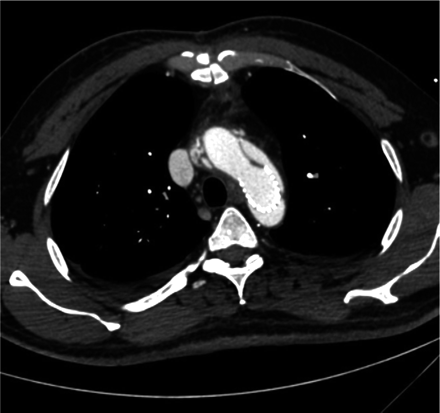
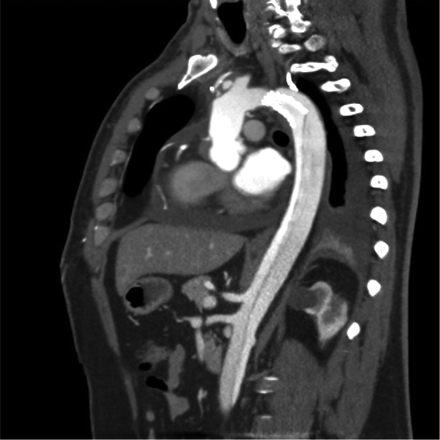
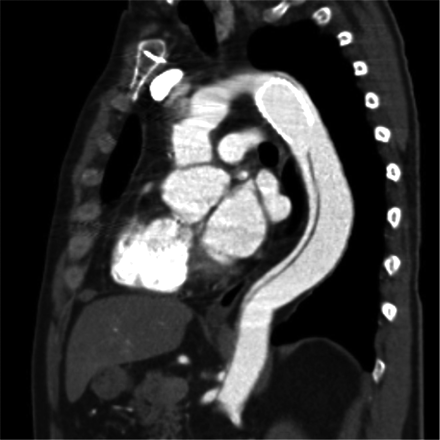
Membrane rupture at the distal end of the stent-sagittal view.
Limitations and value of the study
It is needless to say that the patient number is extremely small. However, we do feel that it is important to state that the effect of this system is a very limited one and that it may even be harmful. As such, we are convinced that the non-proof of the principle can even be convincingly stated by such a small series.
Summarizing, additional implantation of the Djumbodis Dissection System to readapt the dissected layers in the arch and the proximal descending aorta does not seem to have additive value as an adjunct to standard ascending/hemiarch replacement with regard to closure of the false lumen in the arch and the proximal descending aorta. The most limiting factor seems to be the non-self-expanding capability of the device.
Conflict of interest: none declared.




