-
PDF
- Split View
-
Views
-
Cite
Cite
Renato Franco, Giuseppe Pirozzi, Stefania Scala, Monica Cantile, Giosuè Scognamiglio, Rosalba Camerlingo, Gerardo Botti, Gaetano Rocco, CXCL12-binding receptors expression in non-small cell lung cancer relates to tumoral microvascular density and CXCR4 positive circulating tumoral cells in lung draining venous blood, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 2, February 2012, Pages 368–375, https://doi.org/10.1016/j.ejcts.2011.05.009
Close - Share Icon Share
Abstract
Lung cancer is the main cause of cancer-related death in Western countries. Despite early diagnosis, approximately 40% of patients have undergone surgical resection for localized non-small cell lung cancer relapse within 24 months after surgery. Current prognostic criteria for patients with non-small cell lung cancer are gradually enriched by the discovery of critical biological markers in surgical samples to better stratify patients with high risk for recurrent and metastatic disease after surgical manipulation. In fact, specific biological features are needed to drive metastasis development and, among these chemokine receptors, when activated, seem to play a relevant role, promoting both neovessels formation and tumoral cell migration.
To this purpose, blood samples from the closed stumps of the pulmonary veins were drawn immediately after major pulmonary surgery in 45 patients with resectable non-small cell lung cancer to evaluate the expression of chemokine CXCL12 receptor, CXCR4, in circulating tumor cells. In addition, primary tumor sections have been used to assess microvascular density (MVD) and vessels invasion and build prognostic tissue micro-array to investigate the expression of CXCL12 receptors CXCR4 and CXCR7.
Cells positive for cytokeratins from tumor draining pulmonary venous blood were detectable in 11 cases (23.9%). In 8 out of 11 cases, CK positive cells coexpressed CXCR4. Moreover, in tumoral tissue high CXCR4 expression was significantly associated to high mMVD (p = 0.046), high CXCR7 expression (p = 0.001), adenocarcinoma histotype (p = 0.023), and to the presence of circulating tumoral cells in pulmonary veins (p = 0.001). Finally, vessel invasions relate to high MVD.
In conclusion, the results of our study underline the significant potential role of CXCL12 receptors in determining both vessel formation and tumoral cell migration to blood stream, favoring metastasis development.
INTRODUCTION
Surgery represents the main therapeutic method aimed to cure early stage non-small cell lung cancer (NSCLC). Nevertheless, 5-year survival rates in lung cancer are reported to range between 60% and 80%. It has been long recognized that most deaths are related to neoplastic hematogenous spread and the attendant metastatic process [1].
The metastatic process is strictly related to the biological ability of cancer cells to enter the blood stream and to proliferate away from the primary site in a favorable background. Thus, prerequisites of metastasis are development of neoplastic vessels and the acquisition of specific biological characteristics by cancer cells, promoting both vessel invasion and diffusion to blood stream and then to sites outside the lung. In this setting, microvascular density (MVD), as directly expression of neoangogenesis, and neoplastic vessel invasion have been proposed as a marker of risk progression in many tumors, particularly in lung cancer [2–4]. In this view, the presence of circulating tumoral cells (CTCs) could represent the direct expression of the ability of neoplastic cells to spread into the blood stream, being the most critical factor for the unfavorable clinical outcome of patients with NSCLC, but they could not be used in routine staging procedures at the time of primary surgery, because of the lack of handily methodology [1]. As occult tumor cells are detectable in pulmonary venous blood prior to any surgical manipulation, the passage into the bloodstream cannot be explained by lung manipulation but by the acquisition by neoplastic clones of new molecular profiles that ensure the adhesions of cancer cells to the endothelium of the microcirculation [5].
The molecular basis of the metastatic process from blood vessel invasion to the growth to distant sites is poorly understood because it involves multiple steps with a high degree of complexity. Recent data indicate that chemokine and chemokine receptors could play a critical role for tumoral angiogenesis, cancer cell attachment to the endothelial cells on microvasculature, and diffusion outside primary site of tumors.
Different types of cancers express varying combinations of CTC and CXC chemokine motifs (CXC) chemokine receptors but the chemokine receptor CXCR4 – the predominant stromal cell-derived factor-1 SDF-1/CXCL12 receptor – appears to be expressed by the majority of cancer types [6]. In particular, CXCR4 and SDF-1/CXCL12 regulate migration and metastasis of NSCLC [7]. In fact, CXCR4 expression in NSCLC tissues with or without regional invasion, lymph node metastasis, or distant metastasis appears low in normal lung tissues but high in malignant tumors with clinical metastasis [8]. Finally, it was demonstrated that CXCR4 expression was markedly increased on peripheral blood circulating pan-cytokeratin+ cells of patients with NSCLC, as compared to normal control subjects [9].
Moreover, CXCR4 promotes angiogenesis through Vascular Endothelial Growth Factor (VEGF) upregulation [10–12]. Interaction between the CXCR4/CXCL12 axis and VEGF expression has been widely demonstrated in tumors. In fact in colon cancer cell lines, SW620, Lovo, and HT29 cells, CXCL12/CXCR4 interaction triggers VEGF production, while inhibition of CXCR4/CXCL12 axis by AMD3100 reduces VEGF [11]. Finally, recently it has been demonstrated that CXCR4 expression strongly upregulates VEGF expression in prostatic cell lines PC-3 [13].
In this setting, CXCL12 was shown to bind with high affinity besides the classical CXCR4 to the orphan receptor CXCR7/ RDC1 which has a critical role in tumor angiogenesis and in promoting growth of breast and lung cancer [14].
Accordingly, the quantitative assessment of angiogenesis by MVD in lung tumors, blood vessel invasion, the detection of such CTC, and the characterization of their metastatic potential mediated by chemokine receptors may contribute in the refinement of the diagnostic and therapeutic pathways of lung cancer patients [4]. Thus, in the present study we have selected patients undergoing lobectomy or pneumonectomy for resectable NSCLC. Upon completion of pulmonary resection, the pulmonary venous blood was sampled from the tumor draining pulmonary vein stumps. In the same series of patients, we evaluated MVD and vessel invasion; we also performed a prognostic lung TMA to study CXCR4 and CXCR7 expression. The aim of this study was to investigate the existing relationship between MVD, vessel invasion, CXCR4 and CXCR7 expression in primary tumors, and the metastatic potential of circulating tumor cells (CTCs) to establish if the combination of these parameters could represent a new diagnostic and prognostic tool for NSCLC.
MATERIALS AND METHODS
Patients and follow-up
This was a prospective study on 45 consecutive patients undergoing resection for primary NSCLC. In all cases, preoperative staging, including routine chest and upper abdomen CT and PET scanning, had demonstrated resectable tumors (T1–T4) without evidence of distant metastasis (M1) or contralateral or supraclavicular lymph node involvement (N3). In general, a lobectomy or pneumonectomy with systematic mediastinal lymphadenectomy was performed. Immediately after completion of the major anatomical pulmonary resection, the tumor-draining pulmonary vein stumps were punctured to obtain blood samples.
Follow-up studies included physical examination, chest X-ray, and blood tests at a 3-month interval and an additional thoracic chest and abdomen (CT) scan, along with flexible bronchoscopy at a 6-month interval. Close follow-up was documented by contacting family practitioners with questionnaires concerning local relapse, distant metastasis, and death. If possible, recurrent disease was investigated at our institution and the patient was admitted for subsequent therapy.
Ethics committee approval was given in all instances (IRB approval number 556).
Demographics
Patient characteristics are shown in Table 1. Male patients were 31 and female patients 14, with median age at the time of surgery of 74.2 years (range 48–81). The median follow-up duration was 16.3 months (range 1–26). Pathological stage I was recorded in 32 patients, stage II in 8 patients, and stage III in 5 patients. Relapses were recorded in 11 cases at a mean occurring time of 14 months. Death was observed in six cases. At the end of follow-up, 32 patients were alive without disease and 7 patients were alive with disease. Follow-up studies included physical examination, chest X-ray, and blood tests performed at 3-month intervals, and additional chest and upper abdomen CT scans and flexible bronchoscopy at 6-month intervals. Close follow-up was performed at our institution and the patient was admitted for subsequent therapy in the case of relapse.
| . | Patients (45) . | CTC presence . | CTC absence . | MVD high . | MVD low . | Invasion presence . | Invasion absence . |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <60 | 15 (33.3%) | 5 (11.1%) | 10 (22.2%) | 6 (13.3%) | 9 (19.9%) | 6 (13.3%) | 9 (20%) |
| >60 | 30 (66.7%) | 6 (13.3%) | 24 (53.3%) | 11 (24.4%) | 19 (42.2%) | 9 (20%) | 21 (44.5%) |
| Gender | |||||||
| Men | 31 (68.9%) | 9 (20%) | 22 (48.9%) | 12 (26.6%) | 19 (42.2%) | 11 (24.4%) | 20 (46.7%) |
| Women | 14 (31.1%) | 2 (4.4%) | 12 (26.7%) | 5 (11.1%) | 9 (19.9%) | 4 (8,8%) | 10 (22.2%) |
| Stage | |||||||
| I | 32 (71.1%) | 5 (11.1%) | 27 (60%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| II and III | 13 (28.6%) | 6 (13.3%) | 7 (15.5%) | 5 (11.1%) | 8 (17.8%) | 5 (11.1%) | 8 (17.8%) |
| Status | |||||||
| Alive | 32 (71.1%) | 7 (15.5%) | 25 (55.5%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| Alive with disease | 7 (15.6%) | 3 (6.7%) | 4 (8.9%) | 2 (4.4%) | 5 (11.1%) | 2 (4.44%) | 4 (8.8%) |
| Death for tumor | 4 (8.9%) | 1 (2.2%) | 3 (6.7%) | 3 (6.68%) | 1 (2.2%) | 3 (6.6%) | 2 (4.4%) |
| Death for other causes | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) |
| Relapse | 11 (24.4%) | 4 (8.9%) | 7 (15.5%) | 5 (11.09%) | 6 (13.3%) | 5 (11.9%) | 6 (13.3%) |
| Istotype | |||||||
| Adenocarcinoma | 17 (37.8%) | 2 (4.4%) | 14 (31.1%) | 8 (17.8%) | 9 (20.03%) | 6 (13.3%) | 11 (24.4%) |
| Other types | 28 (62.2%) | 9 (20%) | 19 (42.2%) | 9 (20%) | 19 (42.2%) | 10 (22.2%) | 18 (40%) |
| . | Patients (45) . | CTC presence . | CTC absence . | MVD high . | MVD low . | Invasion presence . | Invasion absence . |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <60 | 15 (33.3%) | 5 (11.1%) | 10 (22.2%) | 6 (13.3%) | 9 (19.9%) | 6 (13.3%) | 9 (20%) |
| >60 | 30 (66.7%) | 6 (13.3%) | 24 (53.3%) | 11 (24.4%) | 19 (42.2%) | 9 (20%) | 21 (44.5%) |
| Gender | |||||||
| Men | 31 (68.9%) | 9 (20%) | 22 (48.9%) | 12 (26.6%) | 19 (42.2%) | 11 (24.4%) | 20 (46.7%) |
| Women | 14 (31.1%) | 2 (4.4%) | 12 (26.7%) | 5 (11.1%) | 9 (19.9%) | 4 (8,8%) | 10 (22.2%) |
| Stage | |||||||
| I | 32 (71.1%) | 5 (11.1%) | 27 (60%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| II and III | 13 (28.6%) | 6 (13.3%) | 7 (15.5%) | 5 (11.1%) | 8 (17.8%) | 5 (11.1%) | 8 (17.8%) |
| Status | |||||||
| Alive | 32 (71.1%) | 7 (15.5%) | 25 (55.5%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| Alive with disease | 7 (15.6%) | 3 (6.7%) | 4 (8.9%) | 2 (4.4%) | 5 (11.1%) | 2 (4.44%) | 4 (8.8%) |
| Death for tumor | 4 (8.9%) | 1 (2.2%) | 3 (6.7%) | 3 (6.68%) | 1 (2.2%) | 3 (6.6%) | 2 (4.4%) |
| Death for other causes | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) |
| Relapse | 11 (24.4%) | 4 (8.9%) | 7 (15.5%) | 5 (11.09%) | 6 (13.3%) | 5 (11.9%) | 6 (13.3%) |
| Istotype | |||||||
| Adenocarcinoma | 17 (37.8%) | 2 (4.4%) | 14 (31.1%) | 8 (17.8%) | 9 (20.03%) | 6 (13.3%) | 11 (24.4%) |
| Other types | 28 (62.2%) | 9 (20%) | 19 (42.2%) | 9 (20%) | 19 (42.2%) | 10 (22.2%) | 18 (40%) |
| . | Patients (45) . | CTC presence . | CTC absence . | MVD high . | MVD low . | Invasion presence . | Invasion absence . |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <60 | 15 (33.3%) | 5 (11.1%) | 10 (22.2%) | 6 (13.3%) | 9 (19.9%) | 6 (13.3%) | 9 (20%) |
| >60 | 30 (66.7%) | 6 (13.3%) | 24 (53.3%) | 11 (24.4%) | 19 (42.2%) | 9 (20%) | 21 (44.5%) |
| Gender | |||||||
| Men | 31 (68.9%) | 9 (20%) | 22 (48.9%) | 12 (26.6%) | 19 (42.2%) | 11 (24.4%) | 20 (46.7%) |
| Women | 14 (31.1%) | 2 (4.4%) | 12 (26.7%) | 5 (11.1%) | 9 (19.9%) | 4 (8,8%) | 10 (22.2%) |
| Stage | |||||||
| I | 32 (71.1%) | 5 (11.1%) | 27 (60%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| II and III | 13 (28.6%) | 6 (13.3%) | 7 (15.5%) | 5 (11.1%) | 8 (17.8%) | 5 (11.1%) | 8 (17.8%) |
| Status | |||||||
| Alive | 32 (71.1%) | 7 (15.5%) | 25 (55.5%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| Alive with disease | 7 (15.6%) | 3 (6.7%) | 4 (8.9%) | 2 (4.4%) | 5 (11.1%) | 2 (4.44%) | 4 (8.8%) |
| Death for tumor | 4 (8.9%) | 1 (2.2%) | 3 (6.7%) | 3 (6.68%) | 1 (2.2%) | 3 (6.6%) | 2 (4.4%) |
| Death for other causes | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) |
| Relapse | 11 (24.4%) | 4 (8.9%) | 7 (15.5%) | 5 (11.09%) | 6 (13.3%) | 5 (11.9%) | 6 (13.3%) |
| Istotype | |||||||
| Adenocarcinoma | 17 (37.8%) | 2 (4.4%) | 14 (31.1%) | 8 (17.8%) | 9 (20.03%) | 6 (13.3%) | 11 (24.4%) |
| Other types | 28 (62.2%) | 9 (20%) | 19 (42.2%) | 9 (20%) | 19 (42.2%) | 10 (22.2%) | 18 (40%) |
| . | Patients (45) . | CTC presence . | CTC absence . | MVD high . | MVD low . | Invasion presence . | Invasion absence . |
|---|---|---|---|---|---|---|---|
| Age | |||||||
| <60 | 15 (33.3%) | 5 (11.1%) | 10 (22.2%) | 6 (13.3%) | 9 (19.9%) | 6 (13.3%) | 9 (20%) |
| >60 | 30 (66.7%) | 6 (13.3%) | 24 (53.3%) | 11 (24.4%) | 19 (42.2%) | 9 (20%) | 21 (44.5%) |
| Gender | |||||||
| Men | 31 (68.9%) | 9 (20%) | 22 (48.9%) | 12 (26.6%) | 19 (42.2%) | 11 (24.4%) | 20 (46.7%) |
| Women | 14 (31.1%) | 2 (4.4%) | 12 (26.7%) | 5 (11.1%) | 9 (19.9%) | 4 (8,8%) | 10 (22.2%) |
| Stage | |||||||
| I | 32 (71.1%) | 5 (11.1%) | 27 (60%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| II and III | 13 (28.6%) | 6 (13.3%) | 7 (15.5%) | 5 (11.1%) | 8 (17.8%) | 5 (11.1%) | 8 (17.8%) |
| Status | |||||||
| Alive | 32 (71.1%) | 7 (15.5%) | 25 (55.5%) | 12 (26.6%) | 20 (44.4%) | 10 (22.2%) | 22 (48.9%) |
| Alive with disease | 7 (15.6%) | 3 (6.7%) | 4 (8.9%) | 2 (4.4%) | 5 (11.1%) | 2 (4.44%) | 4 (8.8%) |
| Death for tumor | 4 (8.9%) | 1 (2.2%) | 3 (6.7%) | 3 (6.68%) | 1 (2.2%) | 3 (6.6%) | 2 (4.4%) |
| Death for other causes | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) | 0 (0%) | 2 (4.4%) |
| Relapse | 11 (24.4%) | 4 (8.9%) | 7 (15.5%) | 5 (11.09%) | 6 (13.3%) | 5 (11.9%) | 6 (13.3%) |
| Istotype | |||||||
| Adenocarcinoma | 17 (37.8%) | 2 (4.4%) | 14 (31.1%) | 8 (17.8%) | 9 (20.03%) | 6 (13.3%) | 11 (24.4%) |
| Other types | 28 (62.2%) | 9 (20%) | 19 (42.2%) | 9 (20%) | 19 (42.2%) | 10 (22.2%) | 18 (40%) |
Isolation of mononuclear cells from pulmonary venous blood
The tumor-draining pulmonary vein was punctured subsequent to lung resection [5]. As a rule, the veins, in our anatomical dissections, are ligated first. With the exception of the right upper lobectomy, all lobectomies are carried out by ligating the arterial branches after the vein. In the right upper lobectomy, after the vein, the right upper lobe bronchus may be divided before all the arterial branches. Pneumonectomies are always done by dividing the veins first. Ten milliliters of pulmonary venous blood were drawn into a sterile syringe. The obtained pulmonary venous blood, yielding between 5 × 106 and 6 × 107 (mean 2.5 × 107) mononuclear cells, was centrifuged for 10 min at 1600 rpm. The interface layer contained mononuclear cells that were collected, treated with lysing solution to eliminate erythrocytes, fixed with CytolitTM solution, and then processed according to the Thin Prep2000TM method (Cytyc Co., Marlborough, MA, USA).
Immunocytochemistry on CTC
Double staining of thin prep slides has been performed to identify cells coexpressing CK and CXCR4. Antigen retrieval has been performed on all slides obtained through Thin Prep2000TM method. The first step of antigen retrieval lasted for 30 min at approximately 100 °C with a citrate-based buffer (Leica bond epitope retrieval solution, pH 5.9–6.1). CK 8/18/19 (CKpan) mouse monoclonal antibody (clone A45-B/B3, 1:100 dilution; Miltenyi Biothec GmbH, Friedrich-Ebert-Straße 68, 51429 Bergisch Gladbach, Germany) was applied and incubated for 60 min, followed by a wash step to remove any excess antibody. The antibody was visualized using the peroxidase detection system (RE7120-K, Novocastra, Newcastle, UK) and diaminobenzidine (DAB) as contrasting chromogen.
Antibody denaturation (DNS001H, 1 part A with 2 part B, Biocare Medical, USA) was then performed for 5 min to ensure that the first primary antibody was completely inactivated before applying the second antibody. Thus, CXCR4 rabbit policlonal antibody (ab2047, 1:450 dilution; Abcam Cambridge) was subsequently applied as a second primary antibody and was incubated for 30 min. CXCR4 was visualized using the MACH 2 mouse Ap-polymer (MALP521H, Biocare Medical, USA) for 30 min and incubated with Vulcan fast red for 15 min (FR805H, Biocare Medical, USA).
Slides were counterstained with haematoxylin, washed in Dawn soap, rinsed in water, and coverslipped in Aqueous mounting medium (S3025, dako cytomation, via Real Carpinteria, CA, USA). For each single case, cells only expressing panCK (brown membrane) and cells coexpressing panCK (brown membrane) and CXCR4 (brown membrane and red cytoplasm/membrane) have been counted and recorded.
Histology of primary tumors and vessel invasion
Histology from of all surgical samples has been reviewed according to World Health Organization (WHO) classification. In particular, it has been defined pathological stage, hystotype, and, when applicable, cancer grading. Moreover, vessel invasion has been assessed for each case, evaluating all tumoral sections stained with haematoxylin and eosin. In particular, vessel invasion was determined as described previously by the identification of intravascular tumor clots in the lumen of blood or lymphatic vessels, which were often covered by endothelial cells [2].
Microvascular density
Microvasculature density was determined according to standard procedures [15] and assessed with anti-CD31 antibody (clone JC70, 1:100, Ventana Medical System, Arizona, USA) on the most representative tumor section. All immunostaining techniques have been performed in paraffin-embedded representative tissue sections where a previous step of heat-induced antigen retrieval technique for all antibodies had been carried out. Accordingly, before incubation with the primary antibody, slides were heated in a pressure cooker for 3 min in a solution of 0.01 mol Γ1 sodium citrate. After incubation with the antibody, immunodetection was performed with biotinylated anti-mouse immunoglobulins, followed by peroxidase-labeled streptavidin (LSAB-DAKO, Glostrup, Denmark) with DAB chromogen as substrate.
Eye appraisal of immunohistochemicaily stained micro-vessels and microvessel counting was used for angiogenesis assessment.
For eye appraisal, sections were scanned at low power (×40 and ×100); afterward, they were scanned at ×250 to group cases in low vascular growth (LVG) and high vascular growth (HVG) categories [15]. The areas of the highest vascularization were chosen at low power (×100) and microvessel counting followed on three chosen ×250 fields of the highest density. The sum of the vessel counts was computed in these two fields. Vessels with a clearly defined lumen or well-defined linear vessel shape were taken into account for microvessel counting, whereas the ones characterized by single endothelial cells were excluded.
CD31 staining has been used to further support the identification of vessel invasion because CD31 positive endothelial cells normally cover intravessel tumor clots [2].
All sections and controls were reviewed by two experienced pathologists (RF/GB). Discrepancies between two pathologists from the same case were resolved in a joint analysis of the cases.
TMA building
Tissue micro-array (TMA) was built using the most representative areas from each single case. All tumors and controls were reviewed by two experienced pathologists (RF/GB). Discrepancies between two pathologists from the same case were resolved in a joint analysis of the cases. Tissue cylinders with a diameter of 0.6 mm were punched from morphologically representative tissue areas of each donor tissue block and brought into one recipient paraffin block (3 cm × 2.5 cm) using a semi-automated tissue arrayer (Galileo TMA).
CXCR4 and CXCR7 immunostaining
Immunohistochemistry was performed for chemokine receptors CXCR4 and CXCR7. Primary antibodies included anti-human CXCR4 and anti-human CXCR7 (ab38089, 1:600 dilution; Abcam Cambridge). Antigen retrieval was performed by microwave pretreatment in 0.01 M citrate buffer for 10 min. Sections were incubated with mouse anti-rabbit or goat anti-mouse secondary immunoglobulin (IgG) biotiny-lated secondary antibody for 30 min. Immunoreactivity was visualized by means of avidin–biotin–peroxydase complex kit reagents (Novocastra, Newcastle, UK) as the chromogenic substrate. Finally, sections were weakly counterstained with haematoxylin and mounted. Hodgkin's lymphoma (CXCR4) and kidney (CXCR7) were used as positive controls. Irrelevant rabbit or mouse IgG antibodies were applied to negative control.
For CXCR4 and CXCR7, cytoplasmatic and membranous staining was considered. Tissues were scored semi-quantitatively by evaluating the proportion of positive tumor cells over the total number of tumor cells (percentage of positive tumor cells per tissue microarray punch). Negative, low expression cases and high expression cases were recorded when neoplastic cells expressing chemokine receptor were between 0% and 10%, lower than 30% and higher than 30%, respectively.
Statistical analysis
The primary outcome variables were overall survival (OS) and disease-free survival (DFS). Survival curves were estimated using the Kaplan–Meier method and differences among groups were analyzed using the log-rank test. OS was calculated as the time from the date of surgical resection to death of any cause or until the date of the last follow-up at which point data were censored. DFS was defined as the time between surgical resection until signs of disease progression, death from any cause and last radiological assessment. Data on survivors were censored at the last follow-up. We used non-parametric tests to compare independent groups of numerical data (Mann–Whitney test) and categorical data (χ2-test and Fisher's exact test). The correlations among numerical variables were assessed by Spearman's rank correlation analysis. Multivariate analyses, with backward variable selection, were conducted using the Cox's proportional-hazard regression model. Variables at the 0.10 level in univariate analysis were included in the multivariate model. The level of significance was defined as p < 0.05. All the statistical analyses were carried out using the Statistical Package for Social Science 8.0 software (SPSS Inc., Chicago, IL, USA).
RESULTS
Cytokeratins and CXCR4 expression in CTC in draining venous blood
Cells positive for Cytokeratins were detectable in 11 cases (23.9%) from tumor draining pulmonary venous blood (Fig. 1A). Cell number positive for cytokeratins ranged from 2 to 10; 8 out of 11 cases coexpressed CXCR4 (Fig. 1B).
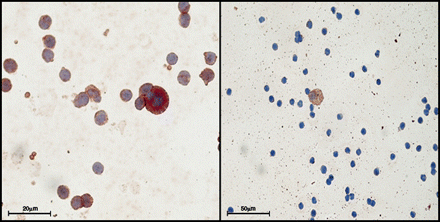
(A) 63 × Double staining for CK and CXCR4 show coexpression of both antigens in an single CTC; (B) 40× a single cell with brown staining for CK and negative staining for CXCR4; (C) graph with relative proportion of cases with CTC expressing CK and with CTC expressing only CK.
CXCR4, CXCR7, vessel invasion, and MVD in primary lung tumors
CXCR4 positivity was detected in 33 NSLC patients (73.3%). In particular, it was low in 14 samples (31.1%) and high in 19 samples (42.2%) (Table 2).
| . | Patients (45) . | CTC presence . | CTC absence . | p-Value . |
|---|---|---|---|---|
| CXCR4 histo | ||||
| Absent CXCR4 | 12 (26.6%) | 3 (6.7%) | 9 (20%) | 0.001 |
| CXCR4 low + | 14 (31.1%) | 4 (8.9%) | 10 (22.2%) | |
| CXCR4 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | |
| CXCR7 histo | ||||
| Absent CXCR7 | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | 0.000 |
| CXCR7 low + | 7 (15.5%) | 3 (6.7%) | 4 (8.9%) | |
| CXCR7 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) |
| . | Patients (45) . | CTC presence . | CTC absence . | p-Value . |
|---|---|---|---|---|
| CXCR4 histo | ||||
| Absent CXCR4 | 12 (26.6%) | 3 (6.7%) | 9 (20%) | 0.001 |
| CXCR4 low + | 14 (31.1%) | 4 (8.9%) | 10 (22.2%) | |
| CXCR4 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | |
| CXCR7 histo | ||||
| Absent CXCR7 | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | 0.000 |
| CXCR7 low + | 7 (15.5%) | 3 (6.7%) | 4 (8.9%) | |
| CXCR7 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) |
| . | Patients (45) . | CTC presence . | CTC absence . | p-Value . |
|---|---|---|---|---|
| CXCR4 histo | ||||
| Absent CXCR4 | 12 (26.6%) | 3 (6.7%) | 9 (20%) | 0.001 |
| CXCR4 low + | 14 (31.1%) | 4 (8.9%) | 10 (22.2%) | |
| CXCR4 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | |
| CXCR7 histo | ||||
| Absent CXCR7 | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | 0.000 |
| CXCR7 low + | 7 (15.5%) | 3 (6.7%) | 4 (8.9%) | |
| CXCR7 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) |
| . | Patients (45) . | CTC presence . | CTC absence . | p-Value . |
|---|---|---|---|---|
| CXCR4 histo | ||||
| Absent CXCR4 | 12 (26.6%) | 3 (6.7%) | 9 (20%) | 0.001 |
| CXCR4 low + | 14 (31.1%) | 4 (8.9%) | 10 (22.2%) | |
| CXCR4 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | |
| CXCR7 histo | ||||
| Absent CXCR7 | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) | 0.000 |
| CXCR7 low + | 7 (15.5%) | 3 (6.7%) | 4 (8.9%) | |
| CXCR7 high + | 19 (42.2%) | 4 (8.9%) | 15 (33.3%) |
Conversely, CXCR7 positivity was detected in 26 NSCLC patients (57.7%), namely high in 19 samples (42.2%) and low in 7 samples (15.5%) (Table 3).
| . | CXCR4 + . | CXCR4- . | p . |
|---|---|---|---|
| CXCR7 + | 26 | 0 | 0.001 |
| CXCR7− | 7 | 12 | |
| Istotype | |||
| Adenocarcinoma | 14 | 2 | 0.023 |
| Squamous | 18 | 7 | |
| Large cells | 1 | 2 | |
| Neuroendocrine | 0 | 1 | |
| High MVD | 13 | 4 | 0.046 |
| Low MVD | 20 | 8 | |
| N + | 5 | 1 | 0.07 |
| N − | 28 | 11 |
| . | CXCR4 + . | CXCR4- . | p . |
|---|---|---|---|
| CXCR7 + | 26 | 0 | 0.001 |
| CXCR7− | 7 | 12 | |
| Istotype | |||
| Adenocarcinoma | 14 | 2 | 0.023 |
| Squamous | 18 | 7 | |
| Large cells | 1 | 2 | |
| Neuroendocrine | 0 | 1 | |
| High MVD | 13 | 4 | 0.046 |
| Low MVD | 20 | 8 | |
| N + | 5 | 1 | 0.07 |
| N − | 28 | 11 |
| . | CXCR4 + . | CXCR4- . | p . |
|---|---|---|---|
| CXCR7 + | 26 | 0 | 0.001 |
| CXCR7− | 7 | 12 | |
| Istotype | |||
| Adenocarcinoma | 14 | 2 | 0.023 |
| Squamous | 18 | 7 | |
| Large cells | 1 | 2 | |
| Neuroendocrine | 0 | 1 | |
| High MVD | 13 | 4 | 0.046 |
| Low MVD | 20 | 8 | |
| N + | 5 | 1 | 0.07 |
| N − | 28 | 11 |
| . | CXCR4 + . | CXCR4- . | p . |
|---|---|---|---|
| CXCR7 + | 26 | 0 | 0.001 |
| CXCR7− | 7 | 12 | |
| Istotype | |||
| Adenocarcinoma | 14 | 2 | 0.023 |
| Squamous | 18 | 7 | |
| Large cells | 1 | 2 | |
| Neuroendocrine | 0 | 1 | |
| High MVD | 13 | 4 | 0.046 |
| Low MVD | 20 | 8 | |
| N + | 5 | 1 | 0.07 |
| N − | 28 | 11 |
Vessel invasion in tumor section has been observed in 15 of 44 samples (34%).
The evaluation of MVD through CD31 expression has been categorized as LVG in 28 samples (62.2%) and HVG in 17 NSCLC patients (37.8%).
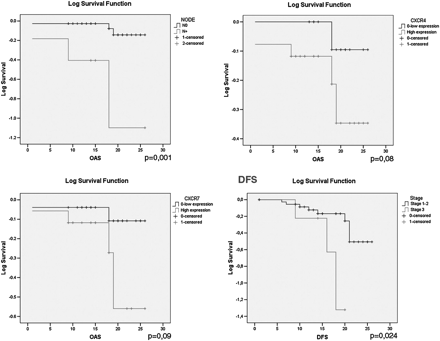
High CXCR4 expression was significantly associated to high MVD (p = 0.046) (Fig. 2), high CXCR7 expression (p = 0.001) (Fig. 3), adenocarcinoma histotype (p = 0.023) CTC (Fig. 4), and presence of CXCR4+ (p = 0.001).
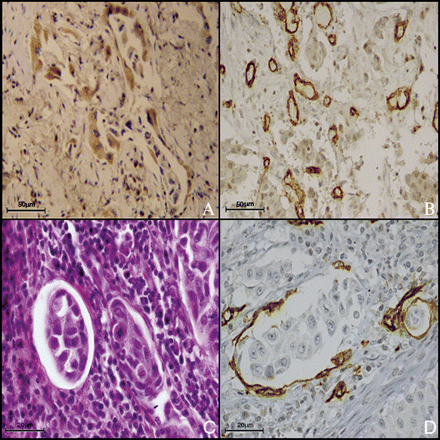
A case of adenocarcinoma with high expression of CXCR4 (A, 40×) and high MVD (B, 20×). Neoplastic clot in a vessel (C, H&E40×) and neoplastic clot covered in endothelial cell in a vessel (D, CD31 immunostaining, 63×).
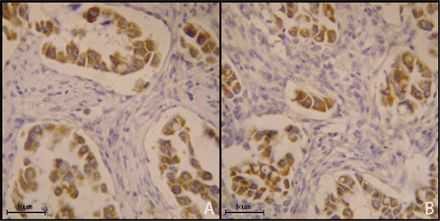
A case of adenocarcinoma expressing CXCR4 (A, 40×) and CXCR7 (B, 40×).
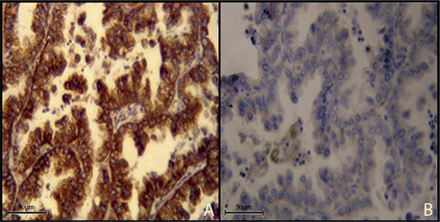
Adenocarcinoma with high expression (A, 40×) and low expression of CXCR4 (B, 40×).
Peritumoral vessel invasion significantly relates to MVD (p = 0.009).
DFS relates with tumor stage and OS with node metastasis
DFS significantly correlates with tumor stage (p = 0.024), while OS was significantly associated to node metastasis (p = 0.014). Moreover, we observed a trend of association between OS and CXCR4 expression (p = 0.08) and CXCR7 (p = 0.09) in tumor tissue (Fig. 5).
DISCUSSION
Currently, lung cancer staging reflects patient's prognosis. However, the knowledge of tumor biological features could improve prognostic stratification of patients.
Tumor angiogenesis is an essential requirement for the development, progression, and metastasis of malignant tumors. Thus, immunohistochemical characterization of MVD, a direct measurement of tumoral neoangiogenesis, represents an independent prognostic factor in several cancer types, including lung cancer [15]. In a tumoral tissue enriched in microvessel, the first step of metastasis development is represented by the biological acquirement by tumoral cells of vessel invasion. Vessel invasion simply identified in histological section was related to prognosis in stage IA NSCLC [4]. Thus, the molecular characterization of tumor cells in the peripheral blood could be expression of neoplastic cells’ ability to metastasis development, and its detection has gained considerable attention over recent years [5]. Conversely, the clinical relevance of analysis of pulmonary venous blood collected during surgical removal of primary lesions, which may represent the first source of hematogenous cancer cell dissemination, was not clearly investigated.
In this article, to identify new molecular markers and new technical approaches to better stratify patients at risk for recurrent and metastatic disease, we have prospectively included patients with NSCLC undergoing major pulmonary resection to evaluate biological status of CXCL12 receptors in CTC. Moreover, tumor sections have been studied to assess MVD and vessel invasion; from surgical samples of the same patients, we have built lung prognostic TMA to assess the expression of CXCL12 receptors.
Our data showed levels of CK positive CTCs in pulmonary venous blood draining primary lung cancer sites super-imposable to other reports using our same detection method [5]. Thus, 23.9% of patients in our series have dissemination of neoplastic cells after lung manipulation. Previously, prognostic relevance of disseminated cancer cells in NSCLC has been demonstrated in bone marrow and regional lymph nodes [16]. Blood dissemination is a complex phenomenon that generally requires specific ability by neoplastic cells but seems to be independent from lung manipulation during surgery, as demonstrated by the relative low percentage of neoplastic cells found in pulmonary venous blood. Moreover, similar percentage of CTC positive cases has been reported when venous blood samples have been obtained before lung manipulation [5]. Our data are in contrast with the ones from other studies that underline that surgical manipulation of lung increases tumor cell dissemination in blood stream, albeit the ligation of pulmonary vein before the ligation of the pulmonary artery, as in our cases, may partly reduce intraoperative tumoral cell release [17,18].
Thus, biological features more than mechanical effects seem to be determinant for blood stream invasion. In this context, our analysis of CTC expressing CXCR4 suggests that this CXCL12 receptor could drive blood dissemination, albeit probably other factors are needed to develop metastasis.
Several studies have clearly demonstrated the importance of chemokine receptors’ expression in primary tumors and metastasis to specific organs. In particular, the chemokine CXCL12 receptor, CXCR4, is able to promote the growth of primary tumors and progression to metastatic disease [19]. The activation of CXCR4–CXCL12 axis in neoplastic cells plays specific roles in proliferation, survival, and metastatization toward selected organs [7]. The molecular changes induced by CXCR4 activation provide the neoplastic cells with a greater ability to migrate and invade, while constitutive release of CXCL12 by stromal cells from sites such as lymph nodes, bone marrow, and liver can drive CXCR4 positive circulating neoplastic cells to organ selective metastasis of cancer stem cells [20]. Currently, there is evidence that CXCL12 may signal through another chemokine receptor, CXCR7 (also known as RDC1). In this setting, CXCR7 is reported to bind both chemokines CXCL11 (interferon-inducible T-cell chemoattractant, also known as I-TAC) and CXCL12, thereby having a key function in promoting tumor development and progression [21]. The role of the CXCL12 receptors has also been documented in lung cancer development. In fact, the use in vivo of monoclonal antibodies acting on the CXCL12–CXCR4 axis seems to reduce NSCLC metastatic dissemination to several organs, including adrenal glands, liver, lung, brain, and bone marrow [22]. Moreover, patients with CXCR4 high expression in primary NSCLC, particularly in adenocarcinoma, as we observed, seem to have lower metastatic potential than patients with lower expression [8]. All these findings suggest that CXCL12 receptors may be a critical determinant for the metastatic potential of NSLC, supporting the idea that circulating cells that express CXCR4 can correlate with worse prognosis and survival of patients with NSLC. Moreover, our data show that cases with CXCR4 positive CTC also correspond to cases with high CXCR4 and CXCR7 expression in tumor tissues. The coexpression of the two CXCL12 chemokine receptors was recently investigated in renal cell carcinoma (RCC) where high CXCR4 and high CXCR7 expression were able to predict shorter disease-free survival [23].
Finally, CXCR4 can also promote tumor vascularization and act as a survival or growth factor [11,12]. Our data confirm this observation, as the expression of CXCL12 receptors in tumor tissue is statistical associated to high MVD. MVD has a debated prognostic role in NSCLC. The first study correlating microvessel density with prognosis in NSCLC was published by Macchiarini et al. [24]. Although most reports on MVD have confirmed these interesting data, there is still a lack of consensus which has prevented the routine application of these parameters for risk stratification [25].
In conclusion, our data emphasize that CXCL12 receptors demonstrate a strict association with MVD, directly related to tumoral neoangiogenesis. Moreover, CXCR4 is quite constantly expressed by CTC in draining venous blood after surgical manipulation, suggesting its possible role in metastasis development. Our results can be ascribed to a variety of data that attach to the receptors of chemokines a major role in the formation of metastases. Thus, targeted disruption of the CXCL12/CXCR4 axis may be investigated in future clinical trials, through specific inhibitors, to hopefully affect the clinical course of NSCLC.
Conflict of interest: none declared.
REFERENCES
Author notes
Equal contributors.
Co-senior authors.




