-
PDF
- Split View
-
Views
-
Cite
Cite
Douglas J. Minnich, Ayesha S. Bryant, David H. Ashley, Robert J. Cerfolio, Inflow and outflow occlusion technique of the pulmonary artery and veins for the technically difficult left upper lobectomy, European Journal of Cardio-Thoracic Surgery, Volume 41, Issue 2, February 2012, Pages 353–356, https://doi.org/10.1016/j.ejcts.2011.05.036
Close - Share Icon Share
Abstract
Our objective is to assess the safety of a surgical technique applied to the difficult left upper lobectomy. The inflow-outflow occlusion technique features: dividing the superior pulmonary vein first, then proximal control by clamping the main pulmonary artery (PA), and then distal control by clamping the inferior pulmonary vein.
A retrospective cohort study of a prospective database was carried out. Patients who underwent left upper lobectomy and required clamping of the vessels were compared to those that did not.
Between January 1999 and March 2010,1796 lobectomies were performed and 360 (23%) of these were left upper lobectomies. Of these, 84 (23%) required the inflow-outflow occlusion technique. There were 70 (83%) men (median age 65 years). Fifty-one patients (61%) required resection of the PA and 33 did not. Heparin was not used in the last 17 patients. These 84 patients were compared to the remaining 276 patients who underwent standard left upper lobectomy. Although the median operative time was longer (150 vs 105 min, p < 0.001) and the median blood loss was greater (120 vs 87 ml, p = 0.03) for the inflow-outflow technique, there were no significant differences in hospital length of stay, morbidity, or mortality between the two groups.
In our experience, clamping of the inferior pulmonary vein instead of the distal PA achieves safe distal vascular control. It affords greater PA mobility and assessment of the tumor and easier PA repair. This technique can be used even when PA resection is not required.
INTRODUCTION
The incidence and mortality of lung cancer remain the number one malignancy in the United States with the cornerstone of therapy for early stage disease being lobectomy [1,2]. While small or peripheral lesions can be straightforward operations, the size and location of the primary tumor as well as nodal disease can present technical challenges. With the anatomy of the left pulmonary artery (PA), the dissection of its proximal branches with a central tumor can be difficult. In dealing with this problem, techniques have been described for obtaining proximal and distal arterial control or applying a side-biting, or Satinsky-type clamp [3]. Rendina et al. have previously reported a technique for left-sided pulmonary arterial resection and reconstruction that involves proximal control at the left main PA and distal control at the left inferior pulmonary vein [4–7]. At our institution, this technique was adopted for both pulmonary arterial resection and reconstruction as well as a technique of handling a difficult dissection of the PA during a left upper lobectomy. In 2007, we reported our experience with pulmonary arterial resection and reconstruction during operation for lung cancer [8]. In this article, we describe our experience using this technique of pulmonary arterial control for the safe dissection of the PA on difficult left upper lobectomies. The technical aspects of the procedure are described and a comparison is made between the patients undergoing this technique and those undergoing standard left upper lobectomy.
MATERIALS AND METHODS
All patients who underwent left upper lobectomy at our institution from January 1999 through March 2010 were
included in this retrospective cohort study of a prospectively collected database. This study was approved by the University of Alabama at Birmingham's Institutional Review Board (UABIRB approval number X100416024). Patients were consented for inclusion in the prospective database used for this study; however, individual patient consent was waived for this particular study. Morbidity was defined using the Society of Thoracic Surgeons database version 2.8. Major morbidity was defined as any morbidity that resulted in either delay of discharge and/or transfer to a critical care unit. Operative mortality was defined as death prior to discharge for the admission during which the lobectomy was performed or within 30 days after surgery due to any cause.
The study includes all patients that underwent a left upper lobectomy during the specified time period. For those patients with a technically difficult resection, with proximity to or involvement of the left PA, the inflow and outflow occlusion technique for the left PA and inferior PA vein was used. ‘Technically difficult’ was defined as the presence of central tumor or adenopathy that interferes with the visualization and safe dissection of the pulmonary arterial branches. During the initial portion of the dissection, the superior pulmonary vein was divided. The left main PA was circumferentially mobilized and proximal control was obtained with a vascular clamp. The inferior pulmonary vein was dissected free and distal control was likewise obtained with a vascular clamp. A single dose of heparin was given early in our experience, but no heparin (other than subcutaneous for deep venous thrombosis prophylaxis) was used in recent patients. With the left main PA and inferior pulmonary vein occluded, the left upper lobectomy was completed. Pulmonary arterial branches were tied if possible, but most required sharp division with a knife. The left upper lobe bronchus was often also divided with a knife due to the tumor size preventing the safe placement of a stapler. This allowed the large left upper lobe tumor to be removed, thus opening up the operative field to afford easy sewing of the branches of the PA using 4/0 or 5/0 prolene (Ethicon, Johnson & Johnson, Somerville, NJ, USA).
Data were collected using Excel (Microsoft, Seattle, WA, USA) and imported into SAS v. 9.1 (SAS Institute, Cary, NC, USA). Descriptive statistics were used to estimate the frequency and medians of the study variables. Differences between the two groups were assessed with the use of two-sided Fisher's exact tests or chi-square tests for categorical variables and independent sample Student's t-tests for continuous variables.
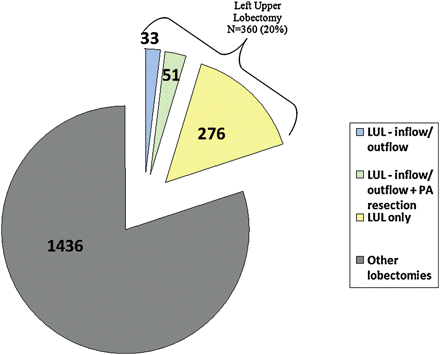
Fig. 1. Lobectomies performed between January 1999 and March 2010.
RESULTS
The study period included patients having operations from January 1999 through March 2010. During this time, 1796 patients underwent lobectomy in the Thoracic Surgery division of the University of Alabama at Birmingham. Fig. 1 shows that 360 (23%) of these resections were left upper lobectomies. Eighty-four (23%) of the 360 left upper lobectomies were defined as ‘technically difficult procedures’ that used the inflow-outflow occlusion technique.
Eighty-four patients underwent left upper lobectomy with inflow-outflow occlusion technique. A total of 276 patients underwent standard left upper lobectomy and served as the control group. As shown in Table 1, there was a higher percentage of male patients in the inflow-outflow group (p < 0.001). Otherwise, there were no significant differences in median age, race, comorbidities, or number of patients who received neo-adjuvant therapy between the two groups. Table 2 depicts the postoperative outcomes. The difference in overall morbidity neared, but did not achieve, a statistically significant difference (20% compared to 32%, p = 0.08) favoring those patients in the inflow-outflow occlusion group. There were statistically significant differences in the median operative time (150 min in the inflow-outflow occlusion group vs 105 min in the control group, p < 0.001) and median blood loss (120 ml in the inflow- outflow occlusion group vs 87 ml in the control group, p = 0.03). There were two operative mortalities in the inflow-outflow occlusion group. The first patient had a postoperative myocardial infarction and suffered a pulseless electrical activity (PEA) arrest from which he was unable to be resuscitated on postoperative day 4. The second patient had an aspiration event on postoperative day 1 while eating. His voice was normal that morning without hoarseness. This was followed by respiratory arrest and asystole. He had manifestations of an anoxic brain injury and care was subsequently withdrawn. There were five operative mortalities in the control group. Two patients expired from multisystem organ failure and sepsis. Care was withdrawn from one patient who was made ‘do not resuscitate’ after reintubation and prolonged ventilation secondary to respiratory distress. The remaining two patients expired due to causes unrelated to cancer treatment or surgery following discharge (one on postoperative day 14 and the other on postoperative day 22).
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Gender | |||
| Male | 70 (83%) | 160 (58%) | <0.001 |
| Female | 14 (17%) | 116 (42%) | |
| Age (years) ± SD median (range) | 65 ± 24 (34–80) | 64 ± 17 (23–83) | 0.96 |
| Race | |||
| White (Caucasian) | 72 (86%) | 223 (81%) | 0.65 |
| Black | 14 (14%) | 53 (19%) | |
| Hypertension | 58 (69%) | 157 (57%) | 0.06 |
| Coronary artery disease | 29 (35%) | 72 (26%) | 0.17 |
| Diabetes | 7 (8%) | 19 (7%) | 0.84 |
| Neo-adjuvant treatment | 22 (26%) | 57 (21%) | 0.37 |
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Gender | |||
| Male | 70 (83%) | 160 (58%) | <0.001 |
| Female | 14 (17%) | 116 (42%) | |
| Age (years) ± SD median (range) | 65 ± 24 (34–80) | 64 ± 17 (23–83) | 0.96 |
| Race | |||
| White (Caucasian) | 72 (86%) | 223 (81%) | 0.65 |
| Black | 14 (14%) | 53 (19%) | |
| Hypertension | 58 (69%) | 157 (57%) | 0.06 |
| Coronary artery disease | 29 (35%) | 72 (26%) | 0.17 |
| Diabetes | 7 (8%) | 19 (7%) | 0.84 |
| Neo-adjuvant treatment | 22 (26%) | 57 (21%) | 0.37 |
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Gender | |||
| Male | 70 (83%) | 160 (58%) | <0.001 |
| Female | 14 (17%) | 116 (42%) | |
| Age (years) ± SD median (range) | 65 ± 24 (34–80) | 64 ± 17 (23–83) | 0.96 |
| Race | |||
| White (Caucasian) | 72 (86%) | 223 (81%) | 0.65 |
| Black | 14 (14%) | 53 (19%) | |
| Hypertension | 58 (69%) | 157 (57%) | 0.06 |
| Coronary artery disease | 29 (35%) | 72 (26%) | 0.17 |
| Diabetes | 7 (8%) | 19 (7%) | 0.84 |
| Neo-adjuvant treatment | 22 (26%) | 57 (21%) | 0.37 |
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Gender | |||
| Male | 70 (83%) | 160 (58%) | <0.001 |
| Female | 14 (17%) | 116 (42%) | |
| Age (years) ± SD median (range) | 65 ± 24 (34–80) | 64 ± 17 (23–83) | 0.96 |
| Race | |||
| White (Caucasian) | 72 (86%) | 223 (81%) | 0.65 |
| Black | 14 (14%) | 53 (19%) | |
| Hypertension | 58 (69%) | 157 (57%) | 0.06 |
| Coronary artery disease | 29 (35%) | 72 (26%) | 0.17 |
| Diabetes | 7 (8%) | 19 (7%) | 0.84 |
| Neo-adjuvant treatment | 22 (26%) | 57 (21%) | 0.37 |
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Length of stay (days) ± SD (range) | 4.5 ± 7 (3–32) | 4.0 ± 10 (2–43) | 0.85 |
| Operative time (min) ± SD (range) | 150 ± 18 (107–195) | 105 ± 15 (90–176) | <0.001 |
| Intraoperative blood loss (ml) ± SD (range) | 120 ± 23 (75–600) | 87 ± 21 (58–500) | 0.03 |
| Intraoperative blood or platelet transfusion | 1 (1%) | 3 (1%) | 0.78 |
| Morbidity | 18 (20%) | 89 (32%) | 0.08 |
| Major morbidity | 9 (11%) | 37 (13%) | 0.64 |
| Operative mortality | 2 (2.4%) | 5 (1.8%) | 0.91 |
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Length of stay (days) ± SD (range) | 4.5 ± 7 (3–32) | 4.0 ± 10 (2–43) | 0.85 |
| Operative time (min) ± SD (range) | 150 ± 18 (107–195) | 105 ± 15 (90–176) | <0.001 |
| Intraoperative blood loss (ml) ± SD (range) | 120 ± 23 (75–600) | 87 ± 21 (58–500) | 0.03 |
| Intraoperative blood or platelet transfusion | 1 (1%) | 3 (1%) | 0.78 |
| Morbidity | 18 (20%) | 89 (32%) | 0.08 |
| Major morbidity | 9 (11%) | 37 (13%) | 0.64 |
| Operative mortality | 2 (2.4%) | 5 (1.8%) | 0.91 |
All continuous variables are presented in medians ± standard deviation (SD).
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Length of stay (days) ± SD (range) | 4.5 ± 7 (3–32) | 4.0 ± 10 (2–43) | 0.85 |
| Operative time (min) ± SD (range) | 150 ± 18 (107–195) | 105 ± 15 (90–176) | <0.001 |
| Intraoperative blood loss (ml) ± SD (range) | 120 ± 23 (75–600) | 87 ± 21 (58–500) | 0.03 |
| Intraoperative blood or platelet transfusion | 1 (1%) | 3 (1%) | 0.78 |
| Morbidity | 18 (20%) | 89 (32%) | 0.08 |
| Major morbidity | 9 (11%) | 37 (13%) | 0.64 |
| Operative mortality | 2 (2.4%) | 5 (1.8%) | 0.91 |
| . | Left upper lobectomy inflow group (N = 84) . | Left upper lobectomy no inflow (N = 276) . | p-value . |
|---|---|---|---|
| Length of stay (days) ± SD (range) | 4.5 ± 7 (3–32) | 4.0 ± 10 (2–43) | 0.85 |
| Operative time (min) ± SD (range) | 150 ± 18 (107–195) | 105 ± 15 (90–176) | <0.001 |
| Intraoperative blood loss (ml) ± SD (range) | 120 ± 23 (75–600) | 87 ± 21 (58–500) | 0.03 |
| Intraoperative blood or platelet transfusion | 1 (1%) | 3 (1%) | 0.78 |
| Morbidity | 18 (20%) | 89 (32%) | 0.08 |
| Major morbidity | 9 (11%) | 37 (13%) | 0.64 |
| Operative mortality | 2 (2.4%) | 5 (1.8%) | 0.91 |
All continuous variables are presented in medians ± standard deviation (SD).
DISCUSSION
The conduct of operations and the associated technical challenges are common discussions among groups of surgeons of any specialty. One such topic among thoracic surgeons is the management of the PA on a difficult left upper lobectomy. The techniques of obtaining proximal and distal control of the PA as well as application of a side-biting clamp have been described in textbooks of thoracic surgery [9–11]. Dr Erino Rendina, in multiple publications on sleeve resections, has described the inflow-outflowocclusion technique with clamping of the left main PA, and the inferior pulmonary vein after the superior pulmonary vein has already been divided [4–7]. Although we have modified this approach to some extent by using Satinsky vascular clamps, whose handles face in opposite directions and have avoided heparin, we have basically implemented his technique. This approach provides excellent exposure of the PA for further dissection and, if needed, resection and reconstruction. We previously reported our experience with this technique for vascular control during pulmonary arterial resection and reconstruction [8]. This article describes the use of the inflow-outflow occlusion technique for technically difficult left upper lobectomies. To our knowledge, there has not yet been a publication outlining the results of this technique for this indication and its comparison to standard lobectomy.
Technical descriptions of left upper lobectomies caution on the perils of traction injury to the first pulmonary arterial branch, the anterior-apical branch, and exposure of this portion of the artery if injured [11]. This can occur when an inexperienced assistant pulls too firmly on a large left upper lobe mass which risks tearing of the PA branches. Another situation that is not uncommon is a central tumor or adenopathy that is in close proximity to the PA and does not afford adequate visualization for safe dissection. These cases can sometimes require sharp division of the arterial branch almost flush with the PA followed by primary or patch closure. This is not the same case as a formal resection and reconstruction that is performed to obtain a negative oncologic margin. Rather, this is a technique for completing a lobectomy when characteristics of the mass or adenopathy limit the ability to obtain sufficient length of the arterial branch to safely apply a stapler or ligature. Performance of this technique requires a method of obtaining vascular control. Potential options include a side-biting clamp on the PA, clamps on the proximal and distal PA, balloon catheters, and the inflow-outflow occlusion technique.
While each of these methods of vascular control has been safely used, we believe there are many distinct advantages to the inflow-outflow occlusion technique as a method to safely complete the operation with negative margins and preserve the left lower lobe. With separation of the vascular clamps (left main PA and inferior pulmonary vein) and orienting them in opposite directions, there is wider exposure of the PA. This produces less distortion of the anatomy and allows more precise arterial dissection and closure. If a PA resection is required, it provides an easy way to assess the length of the PA needed and the tension on an end-to-end anastomosis because there is not a distal PA clamp tethering the distal end. It eliminates concerns over movement of the side-biting clamp on the artery or balloon catheters interfering with operative exposure or suture placement. A technical point to keep in mind when using this technique is to maintain proper orientation of the PA such that the lumen of the remaining vessel is not compromised during division of the first arterial branch.
For any of these techniques of vascular control to be adopted, it needs to be safe for the patient. During the inflow-outflow occlusion technique, there is a period of time when there is no pulmonary blood flow to the left lower lobe and thus concern that there may be clot that develops in the remaining pulmonary arterial tree on the left side. Therefore, a comparison was made to patients undergoing left upper lobectomy that did not have any period of vascular occlusion. This was chosen as the comparison group to account for all aspects of postoperative care and to demonstrate that the inflow-outflow patients do as well as those without any period of vascular occlusion. The only statistically significant differences were greater median operative time (150 vs 105 min) and greater median blood loss (120 vs 87 ml) in the inflow-outflow occlusion group. There was no difference in hospital length of stay, morbidity, or mortality.
One question that arises is whether to administer intravenous heparin prior to vascular occlusion. The original descriptions of this technique by Dr Rendina did include a single dose of heparin prior to application of the clamps on the left PA and inferior pulmonary vein and heparin postoperatively. Early in our experience with this technique, a similar dose of heparin was given as in the original descriptions. The first 67 patients received a single dose of 5000 units heparin intravenously prior to the application of vascular clamps. However, after several operations, we reduced and then eliminated the dose of heparin used and we never adopted heparin postoperatively. The last 17 patients received no intravenous heparin. All 84 patients received postoperative subcutaneous heparin. There were no thromboembolic events in any of the 84 patients. There was one clinically significant bleeding complication requiring reoperation from one of the patients that received intravenous heparin intraoperatively. The bleeding was from the subcarinal space related to the lymph node dissection. After the discontinuation of intraoperative intravenous heparin, we studied the first five patients in the immediate postoperative period with CT scans with contrast and found no clot. Since that time, we have evolved away from administering heparin and now do not routinely use it prior to vascular occlusion. There has been no significant data in the literature to guide decision making on this issue, but in our experience no untoward effects have been observed since this change in practice.
The strengths of this study include a large prospective database from which these patients were drawn. All patients were treated at a single institution and were compared to a control group to assess the safety of this operative technique. The limitations of this study include retrospective study design, nonrandomized treatment protocol, and the relative rarity of this clinical problem. During 10 years at a high volume center, only 84 patients underwent the inflow-outflow occlusion technique as a component of a left upper lobectomy. In addition, there was some variation in treatment protocol during the study period with regard to heparin administration as previously discussed.
In conclusion, the technically difficult or challenging left upper lobectomy can require a method of vascular control for safe dissection of the PA. Inflow occlusion of the left main PA and outflow occlusion of the inferior pulmonary vein (which serves as distal PA control) represent a safe option. This method provides vascular control and excellent exposure for completion of the resection. Our data have shown no difference in morbidity or mortality as compared to patients undergoing standard left upper lobectomy.
Funding
D.J.M. has a financial relationship with superDimension (speaker). A.S.B. and D.H.A. have no industry financial relationships to disclose.
Conflict of interest: R.J.C. has the following industry financial relationships: E plus health care - speaker, Ethicon - speaker/consultant, Neomend - consultant, Millicore - speaker/consultant, Medela - speaker/consultant, Deknatel - speaker, Closure/Johnson & Johnson - consultant, OSI Pharmaceuticals - speaker, Atrium - consultant/speaker, Intuitive-consultant/speaker, Oncotech - speaker, Covidien - speaker, Precision - consultant/speaker, Caris speaker.
REFERENCES
Author notes
Data presented at the Southern Thoracic Surgical Annual Meeting; Orlando, FL, USA (November, 2010).




