-
PDF
- Split View
-
Views
-
Cite
Cite
Anna Maria Ciccone, Federico Venuta, Antonio D’Andrilli, Claudio Andreetti, Mohsen Ibrahim, Tiziano De Giacomo, Domenico Massullo, Erino Angelo Rendina, Long-term patency of the stapled bovine pericardial conduit for replacement of the superior vena cava, European Journal of Cardio-Thoracic Surgery, Volume 40, Issue 6, December 2011, Pages 1487–1491, https://doi.org/10.1016/j.ejcts.2011.03.008
Close - Share Icon Share
Abstract
Objective: Artificial prosthesis of the superior vena cava (SVC) may occlude with time. For this reason, we proposed in 2003 the use of a biological material (bovine pericardium) and devised an original technique to construct the prosthetic conduit. We hereby report the long-term results in 15 patients. Methods: The SVC prosthetic conduit is realized by wrapping a bovine pericardial leaflet around a 5 or 10 cm3 syringe and stapling it on the side by a 60–80 linear stapler. This procedure is carried out intra-operatively after the size of the patient’s SVC has been ascertained; the conduit is then cut to the appropriate length. We have employed this technique in 15 patients with lung (eight) or mediastinal (seven) tumors; after a minimum follow-up of 1 year, all patients underwent computed tomographic-volume rendering (CT-VR) studies of the SVC. Results: Technically, the stapled pericardial conduit has several advantages: (1) it is simple and expeditious; (2) it allows an even and regular suture line, which cannot be achieved by hand suturing; (3)‘one size fits all’: with one single pericardial leaflet, conduits of all sizes can be realized; this is important for an operation which is performed only few times per year; (4) patency is granted by the intrinsic rigidity of the pericardium and staple line, without the need for any reinforcement; (5) different calibers at the two extremities can be obtained by simply placing the stapler obliquely; and (6) the staple line is excellent for the orientation of the conduit while suturing. In our patients, SVC clamping time ranged between 18 and 50 min (mean 29 min); one patient needed cardiopulmonary bypass. Intra-operative anticoagulation (1.500–2.500 units of heparin) was continued postoperatively subcutaneously for 7 days and then shifted to oral anticoagulation for 6 months. One patient died postoperatively of heart failure (mortality 6%). One to 5 years after surgery, CT-VR showed full patency of the pericardial conduit, no clots or thrombus formation, and absence of collateral venous circulation in all 14 patients. One- and 5-year survival was 93% and 73%, respectively (Kaplan–Meier). Conclusions: The stapled bovine pericardial conduit is a simple, expeditious, and economic solution to SVC replacement, and offers reliable long-term patency without permanent anticoagulation.
1 Introduction
For many years, malignant invasion of the superior vena cava (SVC) was considered as a contraindication for any type of resection. Klassen et al. reported the first SVC bypass in 1951 [1]. In the 1970s and 1980s, animal research and clinical trials confirmed the feasibility of operative replacement of the SVC for malignant diseases [2]. Since that time, several publications have largely proven that SVC resection and patch or prosthetic reconstruction is a feasible and safe technique allowing radical surgical treatment in selected patients [3–12]. Extended operations, including resection and reconstruction of the SVC, represent a major technical challenge, especially because of the potential detrimental effect of clamping a patent vessel [13]. In the past two decades, a variety of techniques and materials, such as biologic (autologous or heterologous) and synthetic materials, have been proposed for repairing low-pressure vessels after oncological resection [14–18]. However, the type of vessel reconstruction is still debated and the search for improved technical devices is currently active, considering also that synthetic prosthesis may occlude with time.
Whichever method is used, the overall goals are to relieve symptoms, minimize the risk of complications, and ensure long-term patency of the SVC. We previously described [19] an original technique to replace the SVC by a stapled pericardial conduit and we hereby report the long-term results of the stapled bovine pericardial conduit technique.
2 Materials and methods
The specific objective of the study was to evaluate the long-term results of SVC resection and reconstruction with the stapled pericardial conduit for treatment of malignant pulmonary or mediastinal disease, both in terms of patency of the prosthetic replacement and survival. We obtained approval of the Ethical Committee for the study. All patients underwent thorough work-up and staging to determine tumor extension including contrast-enhanced thoracic and abdominal computed tomography (CT), brain CT or magnetic resonance imaging (MRI), bone scintigraphy if indicated, and when available beginning in 2005, F-18 fluorodeoxyglucose (FDG) whole-body positron emission tomography (PET) in patients for whom specific preoperative diagnosis was not obtained. Preoperative assessment also included clinical examination, evaluation of operability using respiratory function tests and perfusion lung scintigraphy in patients for whom doubt regarding function resectability was present, and bronchoscopy and mediastinoscopy to assess the nodal status. All lung cancer patients included in the study, some after induction therapy, presented N0/N1 preoperative clinical status. Intra-operative data included access, duration of SVC clamping, associated lung resection, and intra-operative adverse events. After surgery, all patients received contrast-enhanced thoracic CT scan before discharge and before each follow-up visit, as it was also performed to determine local recurrence of disease. Metastatic survey was performed with contrast total body CT scan, bone scan, and PET. Postoperative and follow-up data comprised postoperative deaths, morbidity, status of the patient at last follow-up, and patency of SVC graft at the last control. After a minimum follow-up of 1 year, all patients underwent computed tomographic-volume rendering (CT-VR) studies of the SVC.
2.1 Intra-operative management
In the case of lung tumors, the surgical approach used was muscle-sparing lateral thoracotomy in the fifth intercostal space, with the harvesting of the intercostal muscle in case of planned associated bronchoplastic or carinal resection, as described in our previous publication [20]. In the case of mediastinal tumors, median sternotomy were used.
After complete isolation and distal and proximal clamping of the SVC, the caval segment infiltrated by the tumor was resected and vascular continuity was restored by interposition of a heterologous pericardial prosthetic tube (Peri-Guard® Repair Patch 8 × 14 cm). Intravenous sodium heparin (0.3–0.5 mg kg−1) was administered before clamping. Temporary interruption of the flow during prosthetic replacement with proximal and distal caval clamps and the consequent acute SVC syndrome and the expected drop in arterial pressure were managed with intravascular fluid expansion, lower-extremity venous access, hyperventilation to reduce vasogenic cerebral edema, reverse Trendelenburg position, and vasoactive agents to elevate cerebral perfusion pressure. We previously devised and employed an original technique for the construction of a pericardial conduit [19] to be used for SVC replacement. The bovine pericardial leaflet is trimmed to a rectangular shape of the resected caval segment length, wrapped around a syringe to obtain the appropriate diameter, and sutured longitudinally by a 60–80 linear reloadable stapler (GIA – gastrointestinal anastomosis stapler with 3.8-mm titanium staples). (Fig. 1 ) A 5- or 10-ml syringe (depending on the diameter of the resected vessel) was used to calibrate the graft diameter. The construction of the conduit is carried out intra-operatively after the size of the patient’s SVC has been ascertained. The distal anastomosis was performed first, with a 5/0 polypropylene running suture. The proximal anastomosis was subsequently performed always on the proximal SVC stump. The need for suturing the prosthesis directly on the right atrial appendage never occurred. Intra-operative heparinization was not reversed by protamine after declamping. Low-weight heparin (100 U kg−1 day−1) was administered subcutaneously after the operation until discharge. Until 2004, low-weight heparin was then shifted to oral anticoagulation after 1 month and continued until 6 months postoperatively. Since 2005, we shifted from low-weight heparin to oral antiaggregant agents (ticlopidine) after 7–30 days from the operation and continued until 6 months postoperatively (Fig. 2 ).
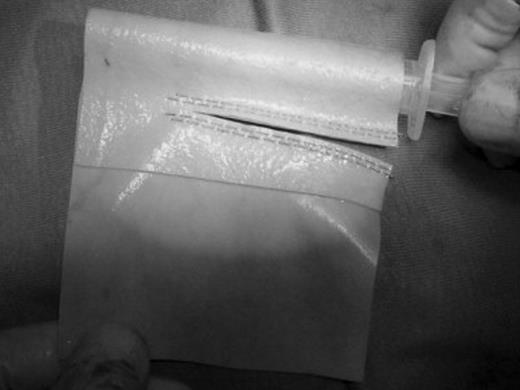
Construction of the pericardial tube. Longitudinal suture is performed by applying the linear reloadable stapler to the leaflet wrapped around a syringe.
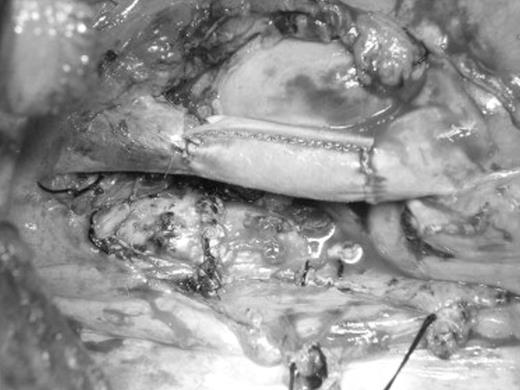
Intraoperative view of SVC reconstruction with bovine pericardial conduit after sleeve pneumonectomy through a right lateral thoracotomy.
2.2 Statistical analysis
Postoperative mortality was defined as any death occurring during hospitalization or within 30 days after surgery. Morbidity was analyzed to assess whether age, sex, histology, duration of SVC clamping time, preoperative chemotherapy, surgical access, and the use of extracorporeal circulation had an impact on postoperative risk; comparisons were performed by univariate analysis (corrected χ2 test). Survival curves were obtained by the Kaplan–Meier method.
3 Results
Between January 2003 and April 2009 in our department, 15 patients underwent SVC resection and reconstruction with the prosthetic pericardial conduit for treatment of malignant pulmonary or mediastinal disease. There were 10 males and five females. The mean age was 56 ± 12 years (range: 42–76 years). All cases of SVC invasion were malignant, including eight cases (53%) of lung cancer (one leiomyosarcoma, four squamous cell carcinomas, and three adenocarcinomas) and seven cases (46%) of mediastinal tumors (five thymomas, one SVC sarcoma, and one mediastinal invasion by recurrence of thyroid carcinoma). Four patients had SVC syndrome before the operation. Six patients (40%) received preoperative induction treatment. Staging mediastinoscopy was performed in two of eight patients (25%) with lung cancer. Ipsilateral N2 disease was confirmed in two patients and treated with induction chemotherapy. Both patients were included for surgery after induction chemotherapy because of the disappearance of the mediastinal lymphoadenopathy at CT and PET scans. The other six patients with lung cancer were clinically N0 (n = 4) or N1 (n = 2).
Pulmonary resections included two carinal pneumonectomies, three right-upper-sleeve lobectomies, and three standard-right-upper lobectomies. Adjuvant chemotherapy was administered to all patients but one, who underwent radioisotope therapy for recurrence of thyroid carcinoma.
All prosthetic replacements were performed using the cross-clamping technique, except one (6%) in which cardiopulmonary bypass was used to remove an invasive SVC sarcoma.
SVC clamping time ranged between 18 and 50 min (mean 29 min). A caval-to-caval shunt was never used. Complete resection (R0) was achieved in all cases. There were no intra-operative deaths.
We observed one (6%) major postoperative complication for heart failure. Minor (non-life-threatening) postoperative complications developed in two (13%) patients (one chest wall hematoma and one atrial fibrillation). There were no intra-operative or postoperative complications related to the reconstructive procedures. We did not observe late complications. Postoperative in-hospital stay ranged from 5 to 13 days (mean: 7.2 days). Postoperative mortality was 6% (n = 1) due to heart failure in a 76-year-old patient preoperatively treated with six cycles of induction chemotherapy for T4 lung cancer. Three patients died between 2 and 5 years after surgery as a result of metastatic disease. Postoperative morbidity was negatively affected by older age (>60-year-old vs ≪60-year-old) and by epidermoidal histology (vs non-epidermoidal) showing a statistically significant difference, whereas, postoperative morbidity was not negatively affected by preoperative chemotherapy, gender, type of surgical approach (thoracothomy vs sternotomy), clamping time, and intra-operative bypass. Eleven patients are still alive: 10 disease free and one is alive with local recurrence at 35 months from the operation. All patients were evaluated postoperatively with CT-VR at time points ranging from 1 to 5 years after surgery; the examinations showed a perfectly patent caval graft, neither clots nor graft thrombus formation, and absence of collateral venous circulation in all 14 patients. (Fig. 3 ) Mean follow-up was 46 months (range: 6–70 months). One- and 5-year overall survival was 93% and 73%, respectively.
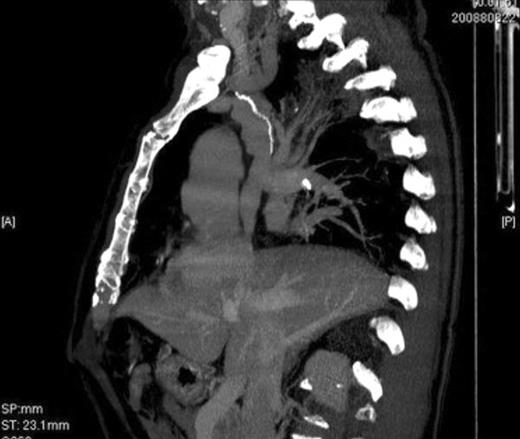
One-year postoperative computed tomography image with injection of contrast material showing excellent alignment and patency of the stapled SVC prosthesis.
4 Discussion
The optimal strategy for SVC reconstruction remains controversial [16–18]. Among the synthetic materials employed for graft reconstruction of the SVC (Dacron, polytetrafluoroethylene (PTFE), and Gore-Tex), the PTFE is the option of choice. Ringed PTFE grafts have been reported to remain patent for several years [5], and some authors believe that the PTFE prosthesis has the advantage of ‘rings’, which avoid graft collapse when the central venous pressure becomes negative [21]; according to others [22], the rigidity of the reconstruction does not allow compression due to surrounding structures at closure (sternum) and is not modified when mediastinal fibrosis due to surgical scars or radiotherapy occurs. Furthermore, shortly after its implantation, it becomes re-epitheliarized with autogenous epithelial cells in humans, has low risk of infection, less platelet deposition, and less thrombogenicity of the flow surface in comparison with Dacron grafts. Other conduit options include tubularized bovine pericardium [12,19], spiral saphenous vein graft and, more recently, cryopreserved arterial allograft [23]. The main problems of PTFE replacement are the need for long-term anticoagulation therapy and thrombosis. Biologic materials have achieved large acceptance in the reconstructive procedures of low-pressure thoracic great vessels after oncologic resection, as also previously reported in our experience [15,24,25]. Autologous and heterologous pericardium represent an ideal material for both patch and prosthetic reconstruction of the SVC. However, when an SVC replacement is required, bovine pericardium is preferred because, unfortunately, the availability of autologous pericardium is almost always insufficient to create a long SVC conduit. Autologous pericardium has a number of advantages (biocompatibility, adequate resistance, cost free, and availability), but it is difficult to handle. The available detoxified bovine pericardial flaps have strongly improved the graft biocompatibility, providing heterologous tissue advantages comparable to those of autologous tissue. The pericardial conduit has lower risks of infection and thrombosis if compared with synthetic materials and does not require long-term anticoagulation. Moreover, bovine pericardium shows further favorable features with respect to fresh tissue. It has even and stiffer edges that make it easier to suture the graft to the vascular wall, and exhibits reduced elasticity, which makes it easier to trim the pericardial graft to the appropriate size, thus not prolonging surgical duration. We previously described an original technique for the construction of a biologic conduit [15]; and, afterwards, we tried to optimize this technique by performing the longitudinal suture with a linear reloadable stapler [19]. The mechanical stapler enables a quicker and easier procedure for the bovine pericardial conduit construction and confers a more regular shape to the vascular graft; it allows an even suture line, which cannot be achieved by hand suturing, and its adaptation to the caval stumps is facilitated. Furthermore, concerning technical aspects, it is possible to realize conduits of all sizes with one single pericardial leaflet in the operating room stock; this is important for an operation, which is performed only few times per year. Moreover, if any discrepancy exists between the proximal and the distal caval stumps in terms of difference in the internal diameter, it is possible to obtain two different calibers at the two extremities of the graft simply placing the stapler obliquely. The staple line is excellent for the orientation of the conduit while suturing. The distal anastomosis is performed first, with a 5/0 polypropylene suture, starting from the posterior aspect of the prosthesis. The proximal anastomosis is subsequently performed with the same technique and the mechanical suture line helps to keep the graft properly positioned and aligned. Furthermore, the intrinsic rigidity of the pericardium and staple line grant patency of the graft without the need for any reinforcement. It must be emphasized that the long-term mortality in our patients was due to oncological reasons, not related to the SVC reconstruction, which actually offered reliable long-term patency.
An optimal duration of the anticoagulant therapy has not been established [5]. Ticlopidine was prescribed to all our patients since 2005 at discharge from the hospital or after chest tube removal, with an antiaggregant time of 6 months and no need of permanent anticoagulation. After an initial experience with postoperative anticoagulation continued for 6 months, since 2005 we have started using only antiaggregant therapy at discharge after the postoperative administration of low-dose heparin.
In our series, all prosthetic replacements were performed using the cross-clamping technique, except one (6%) in which cardiopulmonary bypass was used to remove an invasive SVC sarcoma. We believe that SVC resection is feasible without cardiopulmonary support in all patients, if selection is appropriate. Therefore, we avoid the use of cardiopulmonary bypass during SVC replacement and minimize the hemodynamic derangement by several pharmacologic solutions, trying to keep venous clamp times within 30 min. Short clamping times and dedicated pharmacologic support allowed us to limit hemodynamic derangements without observing any intra-operative remarkable hemodynamic impairment. In fact, it has been previously demonstrated that key issues for intra-operative management during cross-clamping technique are heparinization, fluid intake, the use of vasoactive agents to maintain an adequate mean arterial pressure and, above all, a short clamping time, reporting poor tolerance in humans when SVC clamping time exceeded 45 min [13].
In the present series, a caval-to-caval shunt was never used. From the surgical point of view, the placement of intraluminal or extraluminal shunts (between the brachiocephalic vein or the internal jugular vein and the right atrium) may reduce the effects of clamping. However, thrombosis of the shunt may occur; furthermore, these devices occupy space in the operative field determining increased difficulties for the vascular sutures.
5 Conclusion
We believe that the replacement of the SVC by a stapled bovine pericardial conduit is an excellent solution in selected cases, as it is a simple, expeditious, and economic solution. Furthermore, it offers reliable long-term patency without permanent anticoagulation.
Presented at the 24th Annual Meeting of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 11–15, 2010.
Appendix A Conference discussion
Dr P. Dartevelle (LePlessis-Robinson, France): I totally agree with your interest in performing this kind of extended surgery, for which we have long-term survival. With truncular replacement of the superior vena cava, even with PTFE grafts, patency is evident after 5 or 10 years. You never have occlusion of a truncular replacement by a PTFE graft if the replacement is truncular. The only occlusion I observed was with an indwelling catheter inside the graft, which was from the oncologist. But when you perform replacement of the superior vena cava with an anastomosis on the brachiocephalic vein, you may have some occlusion when the collateral circulation is well established. For the first question, I would like to ask you how many replacements of the superior vena cava were performed with an anastomosis on only one brachiocephalic vein? My second question is why do you prefer bovine pericardium rather than PTFE? PTFE is easier to use.
Dr Ciccone: Regarding the first question, none of these reconstructive procedures was performed with an anastomosis on the brachiocephalic vein. All of the cases included in this study were only SVC trunk replacement. As for the second question, actually I don’t have the correct answer. Our preference is for bovine pericardium, essentially for a couple of reasons beyond the ones already mentioned. First of all, it’s less expensive, and secondly, you can have different sizes of grafts using the same pericardial leaflet, and so you don’t need to have different sizes of PTFE prostheses. Another important reason is that with bovine pericardium, we don’t need permanent anticoagulation. In fact, all of our patients after SVC reconstruction shift from low-weight heparin, which is initially administered subcutaneously, to anti-aggregant therapy at discharge, and they continue this therapy for 6 months.
Dr M. Dusmet (London, United Kingdom): Erino, is there anything you would like to add to why you prefer bovine pericardium over PTFE?
Dr E. Rendina (Rome, Italy): Thank you for asking me, but Dr Ciccone knows this very well. The bovine pericardial tube is very easy to use, it’s very easy to orientate, it is economic, because, as she said, you only need one leaflet to construct all different kinds of pericardial conduits, and, most important, it doesn’t require permanent anticoagulation. These are essentially the reasons why we prefer to use this over PTFE, which remains an excellent material for replacement of the superior vena cava.
Dr R. Schmid (Bern, Switzerland): I have one question. When we replace, for example, the pericardium with a pericardial patch, it shrinks when you go back after a while into the thoracic cavity. I wonder if you have long-term results on these prostheses with respect to shrinking of the diameter?
Dr Ciccone: You mean if the graft kinks after surgery?
Dr Schmid: Maybe the patients don’t survive that long, but maybe after 3 years or 4 years, is there a narrowing of the graft on CT scan?
Dr Ciccone: No. All of the patients have a caliber of 100%.
Dr Schmid: After what time?
Dr Ciccone: Between 1 and 5 years after surgery. In particular, in the case shown on this slide, the patient is alive after 4 years. But actually he had a very good oncological pattern. The pathologist found only one metastatic lymph node out of 29, and it was a regional lymph node.
Acknowledgment
We wish to thank Dr Elisabetta Grigioni for editorial revision and data management.




