-
PDF
- Split View
-
Views
-
Cite
Cite
Xuming Mo, Weisong Zuo, Zhifei Ma, Kaihong Wu, Jian Sun, Wei Peng, Jirong Qi, Jinyang Ding, Hybrid procedure with cardiopulmonary bypass for muscular ventricular septal defects in children, European Journal of Cardio-Thoracic Surgery, Volume 40, Issue 5, November 2011, Pages 1203–1206, https://doi.org/10.1016/j.ejcts.2011.02.015
Close - Share Icon Share
Abstract
Objective: To summarize the technique and clinical experience of the hybrid procedure with cardiopulmonary bypass in children with muscular ventricular septal defect (mVSD). Methods: From January 2006 to June 2010, 45 cases of mVSD underwent hybrid procedures with cardiopulmonary bypass (CPB) under the guidance of transesophageal echocardiography. mVSDs were closed with devices under direct vision in the 45 cases. Fourteen patients had another lesion that required surgical repair. Large membranous VSDs were closed with a pericardial patch after the initiation of CPB in 38 cases. Results: Out of the 45 cases, 42 had only one occluder and three had two occluders. The size of the device for mVSD closure ranged from 3 to 8 mm. All cases recovered smoothly after treatment without residual shunting, aortic or mitral valve regurgitation, or restriction of surrounding structures. All the children survived the operation with no late deaths during the follow-up. Conclusion: The hybrid procedure is safe and effective for the closure of congenital heart defects in children.
1 Introduction
Muscular ventricular septal defects (mVSDs) constitute a major form of congenital heart disease. As postoperative morbidity and mortality rates remain high, mVSD repair is also a challenging procedure for surgeons [1]. Therefore, the recently developed hybrid technique constitutes a new type of treatment modality for mVSD repair [2]. In this article, we summarize our technique and clinical experience with regard to the use of the hybrid procedure for the repair of mVSDs in 45 children with the assistance of cardiopulmonary bypass (CPB) under the guidance of transesophageal echocardiography (TEE).
2 Materials and methods
2.1 Clinical data
Between January 2006 and June 2010, the device closure of an mVSD was attempted in 45 children (20 males and 25 females) ranging in age from 52 days to 12 years (mean ± SD: 2.1 ± 2.5 years). Their weight ranged from 3 to 30 kg (mean ± SD: 11.9 ± 7.7 kg). Most of the children in this series were highly susceptible to respiratory tract infections.
The hybrid approach was used in all patients with CPB under the guidance of TEE. The manufacturer of the device is Shanghai Shape Memory Alloy Co., Ltd. The diameter of mVSDs ranged from 2 to 7 mm, and the number of individual mVSDs was from 1 to 7 under TEE. Preoperatively, 26 patients had increased rates of pulmonary blood flow and three patients had sternal recession, while 19 patients had left axis deviation on electrocardiography (ECG). Out of 45 patients, seven patients had a single mVSD, and 38 had multiple mVSDs. The quantity of the mVSDs in the right ventricular side was as follows: single (n = 7), two (n = 24), three (n = 8), four (n = 5), and seven (n = 1). The location of VSDs was as follows (Table 1 ): of the 45 patients, 14 had other congenital heart defects, including transposition of great arteries (TGA; n = 2), tetralogy of Fallot (TOF; n = 3), pulmonary artery stenosis (PS; n = 4), patent ductus arteriosus (PDA; n = 3), and atrial septal defects (ASD; n = 2) (Table 2 ). On the whole, 22 of the patients in our cohort were combined with pulmonary hypertension.


2.2 Operative procedure
A standard midline sternotomy was performed in all the patients; the sternum was then braced and the pericardium was incised. Extracorporeal circulation was then established and crystal cardioplegic solution was injected at the root of the aorta when the aorta was clamped. A slit was made in the right atrium and the mVSDs were explored with the use of a slender divining rod via the tricuspid valve. If the passage of the divining rod into the left ventricle was affirmed, the guide wire was inserted through the spatium intermusculare. Of all patients, 38 children had comparatively large perimembraneous VSDs through which the guide wire could be threaded through into the left ventricle. The other seven children did not have perimembraneous VSDs, and the guide wire was threaded out through the bicuspid valve with discission of the atrial septum earlier in the procedure. Then, under TEE, the arterial hemostasis sheath was placed into the left ventricular cavity over the guide wire via the mVSD and the guide wire within the sheath was withdrawn; the mVSD device was then positioned. When the device was advanced through the sheath into the left ventricle, the left ventricular disk was deployed and adhered to the wall tightly after the whole sheath was withdrawn. The waist and right ventricular disk were then deployed. For the seal to be firm, the device could be secured onto the right ventricular wall twice with a 4/0 clout needle. In the case of single mVSD, in this series, the aorta was opened when releasing the left ventricular gas. After the procedures that were undertaken, while multiple VSDs were present, the big perimembrane VSD would be repaired with pericardial patches from the patient using a continuous suture.
Patients with other congenital heart anomalies had the other defects repaired under the same anesthetic; one patient with TGA underwent an mVSD device closure before the Switch procedure, three patients with PDA underwent ligation before CPB was established, and the patients with TOF, ASD, and PS also had their anomalies repaired before the hybrid procedure. Following the recovery of myocardial contractility and the establishment of a sinus rhythm, the satisfactory position of the device was confirmed by TEE. Residual shunts, dysfunction of the atrioventricular valve and chordae tendineae, and tertiary atrioventricular block were evaluated and treated; usually if a satisfactory recovery could not be established, the device was replaced again. However, no such complication occurred in our series.
2.3 Statistical analysis
All quantitative data are presented as means ± SD unless otherwise stated. Numerical variables of cardiac function based on clinical and echocardiographic data between groups of patients were compared using two-tailed t-test or the Mann–Whitney U test if not normally distributed. The relationship of the recovery of patients and the duration of CPB was analyzed using the Mann–Whitney U test. Continuous variables were analyzed using the independent Student’s t-test. A significant difference was found when probability values were smaller than 0.05.
3 Results
All of the 45 hybrid procedures undertaken in this series were successful and no deaths occurred in this cohort. A total of 48 devices were placed in 45 patients. Single devices were implanted in 42 children (device size ranged from 3 to 8 mm) and two devices were implanted in three children (device size ranged from 4 to 7 mm). The average time on CPB was 58 ± 20.7 min (mean ± SD), while aortic crossclamp time was 35 ± 14.8 min (mean ± SD). The time on mechanical ventilation postoperatively ranged from 2 h to 6 days. With regard to the 13 infants aged less than 8 months, there was a significant difference in cardiac function in the early postoperative period compared to the older children. However, they recovered with the use of supportive treatment, for example, using vasoactive agents, cardiocinetic agents, diuresis, and mechanical ventilation. Postoperatively, enteric-coated aspirin was given at a dose of 5 mg kg−1 day−1 for a period of 3–6 months as usual. No major complications were encountered during the operation under TEE. The average duration of hospitalization was 10 days. All patients attended follow-up at 1 week, 1 month, 3 months, 6 months, 1 year, and 2 years post-procedure; during this period, there was no instance of migration of any of the devices, residual shunt, aortic regurgitation, atrioventricular valve dysfunction, tertiary conduction block, arrhythmia, or death. The representative echocardiogram of the patients before, during, and after operation is shown in Figs. 1–3 .
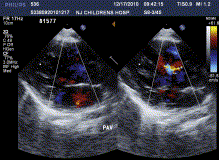
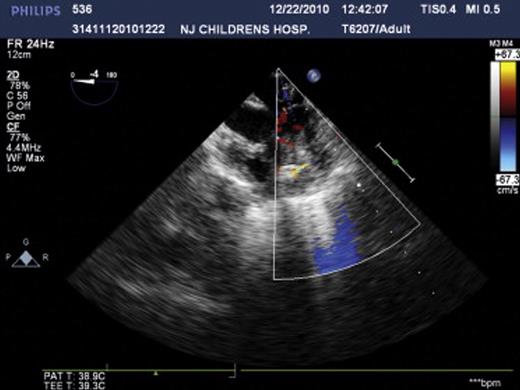
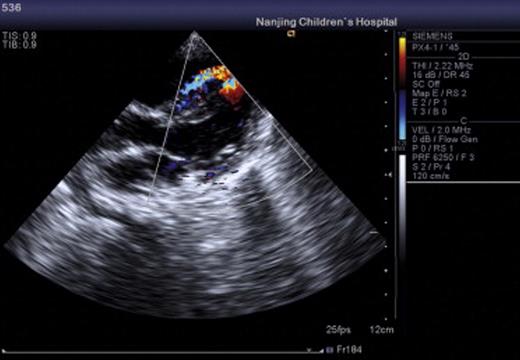
4 Discussion
The incidence of mVSDs constitutes 5–20% of all VSDs, and there is a particularly high incidence in infants [3]. Because of the typical location of mVSD, the success of interventional therapy remains low and the incidence of residual shunt after operation reaches up to 50% [3]. As the recovery of patients is significantly correlated with the duration of CPB, a shorter duration of CPB can increase the surgical survival rate, decrease surgical trauma, shorten the overall operation time, and increase the surgical therapeutic effect [4]. Meanwhile, the mortality of patients who have mVSDs combined with other cardiac defects increases obviously, especially in the case of complex congenital heart disease. Therefore, treatment of mVSD has always been challenging for clinicians, and the recently developed hybrid procedure has introduced a significant new treatment option with positive clinical effects. The mVSDs can be present not only as a single entity, but also as multiple holes, that may resemble a ‘Swiss cheese’. They can also exist concomitantly with perimembranous VSDs. The mVSDs characteristically have muscle mass around the edge of defects, variable defect locations, thickened muscle bundles stretched across defects, and multiple holes can be present in both the left and right ventricle. Patients with mVSDs easily develop pulmonary hypertension and cardiac insufficiency because of the consequent left-to-right shunt. Closing mVSDs surgically is relatively difficult because of their variable and complex anatomical structure. It is often better to leave a residual shunt, which can influence cardiac function, especially in infants, rather than carrying out a procedure with a significant risk of death. In spite of the increase of surgical technique and postoperative care, it is also challenging to expose and repair the defects. Although the possible surgical approaches are many, the most common include entering through the right atrium, right ventricle, and left ventricle. However, major disadvantages exist for each approach. The right atrial incision affects the compliance of the right ventricle and movement of the ventricular septum, which causes low cardiac output; if the incision is made through the apex of the right ventricle, it is easy to damage the anterior papillary muscle and acute marginal ramus of the coronary arteries; furthermore, an incision of the apex of the left ventricle carries a high risk of causing low cardiac output. In addition, it is difficult to expose an adequate surgical field via the transatrial approach, and this approach causes a long duration of repair and a high incidence of residual shunting. The transventricular approach can result in severe low cardiac output, arrhythmias, and apical aneurysms. Therefore, the incidence of complications and mortality remains high despite numerous approaches for the repair of mVSDs, and to date there is no agreement as to the optimal surgical intervention.
Although the surgical treatment of mVSD is controversial, percutaneous interventional therapies also have their disadvantages. It is not a suitable intervention for small mVSDs or in patients younger than 2 years of age. In the case of mVSDs where thickened muscle bundles stretch over the defect, it is difficult to deploy the device. Otherwise, it is easy to injure peripheral blood vessels, valves, and chordae tendineae, leading to tricuspid valve regurgitation, perforation of the ventricle, and hemodynamic imbalance. With regard to patients with multiple mVSDs that are associated with other cardiac abnomalies, who would not survive for more than 2 years without any treatment, percutaneous intervention would add no survival benefit.
Recently, the hybrid procedure has been shown to be an appropriate intervention for any type of mVSD [5]. We have demonstrated in this present study that the treatment of mVSDs by hybrid procedure under CPB is feasible and safe. The indication to close these VSDs is that: the patients have mVSDs which is especially located at trabecular, and the size of mVSD is between 2 and 8 mm. However, the procedure is new, the indication is variable with the surgical skill improving. The advantages of this procedure include: (1) in our study group, all patients suffer from mVSD with other cardiac defect or failing in implanting device in beating heart; however, the hybrid procedure can reduce the time of CPB as the procedure is carried out under direct vision; (2) the safety of therapy is increased, as the devices are placed under direct vision and can be adjusted until they are at the most appropriate location with the best fit; (3) the devices can be locked directly onto the right septal wall; (4) the whole operation is performed under TEE, which enables the precise location of devices, as well as indicates when replacements are necessary; (5) this procedure results in a low incidence of residual shunt, and, in our series, no instance of residual shunt occurred, compared to reported incidences as high as 50% by the surgical approach [6]; (6) the hybrid procedure offers the option of an mVSD repair for infants with low birth weights who cannot tolerate a long anesthetic and there is a consequent reduction in their mortality; in our series, the lowest weight of patients was 3 kg and the hybrid procedure was successful; (7) in contrast to the interventional therapy approaches, patients can avoid the associated complications due to damage to the tunica intima of the traversed blood vessels and consequent hemodynamic balance. The hybrid approach does not use X-ray, and the long-term harmful consequences from large dose of radiation are also avoided; and finally, (8) the technique is simple to perform, while the outcome is satisfactory and the recovery of the patients is quick.
The choice of device used in the hybrid procedure depends on the size of the mVSD. The device size is from 3 to 8 mm. As the width of device border is 2 mm and the size/defect size ratio is about 1:1, this implies that the size of devices is equal to that of the mVSDs or larger than the size of mVSDs, but not larger than 1 mm. In the case of multiple mVSDs, it is standard practice to choose one or two devices that are larger than the mVSDs. The disks of the devices can press against the septum and close the smaller mVSDs. In this manner, multiple closures are avoided and it is economical, but a residual shunt is more likely to occur postoperatively.
The length of the CPB time plays an important role in the success of cardiac surgery, in terms of the complication rate and duration of recovery. The hybrid procedure shortens this time significantly and the curative effects are predominant. In our series, in one patient who had an mVSD in combination with dextro-transposition of the great arteries (D-TGA), the length of the hybrid procedure was only 2 min compared with 20 min by traditional surgical repair, and no residual shunt was left. The use of the hybrid procedure is not limited by the age, weight or blood vessel caliber, and anatomy of patients. It combines a surgical and interventional approach and enables the shortening of the duration of both, particularly in low birth-weight infants who cannot tolerate a prolonged duration of surgery and CPB.
5 Conclusion
The reasonable application of the hybrid procedure for mVSDs in children is feasible and safe. It will generally be adopted in clinical situations and we anticipate that it will be used increasingly as cardiologists and surgeons become more aware of its potential.
References
Author notes
This work was supported by a grant from the ‘333’ Project Foundation of Jiangsu Province, People’s Republic of China (No. 2009016).




