-
PDF
- Split View
-
Views
-
Cite
Cite
Naomichi Uchida, Akira Katayama, Kentaro Tamura, Miwa Sutoh, Masatsugu Kuraoka, Naoki Murao, Hiroshi Ishihara, Long-term results of the frozen elephant trunk technique for extended aortic arch disease, European Journal of Cardio-Thoracic Surgery, Volume 37, Issue 6, June 2010, Pages 1338–1345, https://doi.org/10.1016/j.ejcts.2010.01.007
Close - Share Icon Share
Abstract
Objectives: This study describes the long-term safety and effectiveness of extended aortic arch replacement with the frozen elephant trunk technique from our 12 years of experience. Methods: Between September 1997 and September 2008, 156 patients (mean age 67.9 years) with different pathologies from the aortic arch to the extended descending aorta in 100 dissections (acute A/acute B/chronic B = 66/26/8) and 56 thoracic arteriosclerotic aneurysms (TAAs) had the frozen elephant technique performed upon them. During moderate hypothermic circulation with selective cerebral perfusion, the stent graft was inserted through the transected proximal aortic arch with trans-oesophageal echo guidance. Results: Six patients (3.2%) (acute A/acute B/chronic B/TAA = 3/2/0/1) died in hospital. Postoperative morbidity induced four (2.6%) strokes (acute A/acute B/chronic B/TAA = 2/0/0/2) and three (2.0%) spinal injuries (paraplegia in two and transient paraparesis in one) (acute A/acute B/chronic B/TAA = 0/0/1/2). In the long-term follow-up (mean 63.3 ± 39.2 months, maximum 144 months) 16 patients died. The survival rate was 99.3%, 86.5% and 74.9% at 1, 5 and 10 years, respectively. An additional operation was performed in 15 (9.4%) (ascending aorta/aortic root/descending aorta/abdominal aorta = 1/2/5/7) including three stent-graft-related events (2.1%), and the additional repair proved successful. A follow-up computed tomography (CT) image was available for 96.0% (143/149) of patients who survived longer than 12 months. The size of false lumen or aneurysm increased in four patients, was unchanged in 20 patients (14.0%), shrank in 66 (46.2%) and was completely obliterated in 55 (37.1%). Conclusions: The frozen elephant technique could be an attractive treatment for extended aortic arch disease to the extended descending aorta for acute aortic dissection as well as arteriosclerotic aneurysm.
Patients with extensive aortic aneurysms involving the ascending aorta, aortic arch and the descending aorta are still considered to be a challenge for many cardiovascular surgeons. The elephant trunk technique was initially introduced by Borst and co-workers [1] in 1983 as the preparation to a planned two-stage approach of the descending repair for treatment of extended aortic aneurysms. Kato and co-workers [2] also introduced the open stent-grafting technique in 1994. This technique, which has been described subsequently as frozen elephant trunk (FET) technique in some articles [3–7], involves conventional replacement of the ascending aorta with a four-branched graft and stent grafting into the descending aorta through the opened aortic arch. We aim to describe the long-term safety and effectiveness of extended aortic arch replacement with the FET technique from our 12 years of experience.
1 Patients and methods
1.1 Patients
The use of endovascular stent grafts was approved by the Ethical Committee in the Hiroshima City Asa General Hospital in 1987. The retrospective review of the records was approved by the institutional review board in 2009.
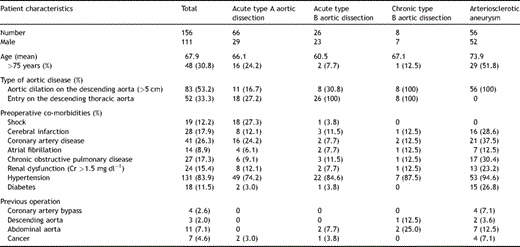
Informed consent was required in each case. Between September 1997 and September 2008, 156 patients (mean age 67.9 years) with different pathologies from the aortic arch to the extended descending aorta in 100 dissections (acute A/acute B/chronic B = 66/26/8) and 56 thoracic arteriosclerotic aneurysms (TAAs) had the frozen elephant technique performed upon them. All operations were done by two surgeons (Ishihara and Uchida). The patient cohort consisted of 111 men and 45 women aged from 32 to 84 years, with a mean age of 67.9 years and 48 (30.8%) patients were older than 75 years. Preoperative patient profiles with four pathologies have been summarised in Table 1 . The proximal descending aorta was dilated more than 5.0 cm in 83 patients (acute A/acute B/chronic B/TAA = 16.7%/30.8%/100%/100%) and the primary entry was located on the proximal descending aorta in 52 (33.3%) patients (acute A/acute B/chronic B = 27.2%/100%/100%).
1.2 Operative indications
1.2.1 Acute type A aortic dissection
We principally undertook resection of the intimal tear and open distal anastomosis as an operative procedure for acute type A aortic dissection. Ascending aortic or hemiarch replacement was the procedure performed in the tear-oriented surgery. However, a FET procedure was added for secure anastomosis and early thrombosed closure of the false channel in the descending aorta. The operating surgeon made the final decision as to which of the two procedures to use in consideration of preoperative patient characteristics (e.g., physical conditions, age, collapsed false lumen and location of primary entry). The primary tear was distal to the left subclavian artery (DeBakey subtype III-D) in 18 (27.2%) patients, and the primary tears were covered by the stent graft.
1.2.2 Acute type B aortic dissection
The indications for acute type B aortic dissection were complicated diseases with occlusion or near occlusion of the true lumen on the descending thoracic aorta with the patient developing abdominal pain or lower limb pain. Furthermore, uncomplicated type B aortic dissection was indicated for the purpose of preventing dissection-related events in the future and compression of the true lumen by the enlarged patent false lumen. The criteria for surgery were: (1) age ≪70 years, (2) primary entry site within 5 cm of the left subclavian artery origin, (3) descending thoracic aorta diameter >5 cm and (4) visceral arteries receiving blood supply from the true lumen.
1.2.3 Chronic type B aortic dissection and TAA
The distal arch aneurysms could not be treated with trans-catheter endovascular stent grafting via peripheral artery (TEVAR) involving the left subclavian artery and performing distal anastomosis in the median approach extending to the upper middle descending aorta was deemed difficult. The diameter of the distal normal edge was less than 35 mm on preoperative computed tomography (CT).
1.3 Stent graft
A self-expanding Z-shaped stent with the tip 5 cm on the distal side was attached to a polyester fabric prosthesis (UBE graft, Japan) with a diameter between 24 and 37.5 mm using 5/0 monofilament sutures. These stent grafts were prepared preoperatively for use.
1.3.1 Aortic dissection (types A and B)
The size and length of the stent graft were determined by means of intra-operative measurement with a ball-shaped sizer inserted into the true lumen of the descending aorta through a transverse incision under trans-oesophageal echocardiographic guidance. The distal edge of the stent graft was usually positioned at the level of the main pulmonary trunk.
1.3.2 TAA
The stent graft was set at 110% of the diameter of the distal descending aorta at 5 cm longer than the length from the distal normal edge on preoperative three-dimensional CT scans. This was verified by intra-operative trans-oesophageal echocardiography.
1.4 Operative procedures
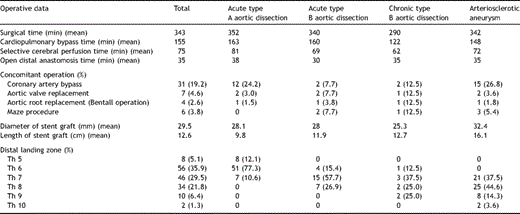
The FET technique has been described in previous reports [5,8]. When it was confirmed that the rectal temperature had decreased to 26–28 °C, using a median sternotomy and an arterial perfusion from the right axillary artery, the aortic arch was incised longitudinally until immediately before the origin of the left subclavian artery under lower circulatory arrest with selective cerebral perfusion. A ball-shaped valve sizer had been inserted into the true lumen of the descending aorta from the transverse incision under trans-oesophageal ultrasound guidance to verify the correct size (both diameter and length). The stent graft was placed through a 30F introducer, which was inserted into the descending aorta. The stent-graft delivery was technically successful in all 156 patients. As for TAA, in the first 22 patients up to July 2002, left subclavian bypass from the ascending bypass was achieved except for cases with dilatation of the ascending aorta. In the latter 34 patients from August 2002, onwards ascending and total arch replacement was routine performed. After January 2002, cerebrospinal fluid drainage (CSFD) was performed the day before the operation in elective cases with a history of aortic repair in the thoraco-abdominal or abdominal aorta or when the pathologic condition required stent-graft delivery lower than the ninth thoracic vertebra.
Concomitant procedures included 31 coronary artery bypass grafts, seven aortic valve replacements, four aortic root replacement and six Maze procedures for chronic atrial fibrillation. The average operative, cardiopulmonary bypass, selective cerebral perfusion and circulatory arrest times of the lower body were 343, 155, 75 and 35 min. The stent graft was delivered to the fifth thoracic vertebra level (Th5) in eight, Th6 in 56, Th7 in 46, Th8 in 34, Th9 in 10 and Th10 in two patients (Table 2 ).
1.5 Patient follow-up
The follow-up with CT imaging was obtained at 1 month and 6 months after the operation and yearly thereafter. A follow-up CT image was available for 96.0% (143/149) of patients who survived longer than 12 months. Follow-up clinical status was obtained by medical records from an outpatient clinic. Follow-up was 100% complete, averaging 63.3 ± 39.2 months (maximal follow-up 144 months, 78 patients remaining at risk at 5 years). The postoperative change of the aneurysm was classified as showing increase, no change, shrinkage or obliteration. When the size of false lumen or aneurysm decreased more than 3 mm, this was defined as shrinkage. When the thrombus out of false lumen or stent graft became less than 3-mm thick, this was defined as obliteration.
1.6 Statistical analysis
All data were reviewed retrospectively. Continuous data were expressed as mean ± standard deviations. Categorical data were expressed as counts and proportions. Survival, freedom from overall aortic event, including an endoleak, and freedom from events on the site of stent graft were estimated by the Kaplan–Meier method.
2 Results
2.1 Early results
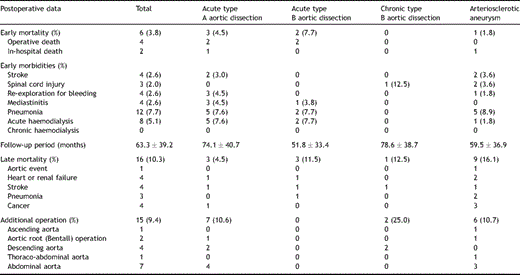
Operative mortality within 30 days was 4 of 156 (3.8%); cause of death was deep infection in two and multiple organ failure in two. In addition, there were two in-hospital deaths. One patient, who had stenting to the left main bronchial lesion due to atelectasis resulting from compression by an enormous aneurysm 3 months after the operation, died suddenly owing to aorto-oesophageal fistula 7 months after FET. The other died from pneumonia 2 months after FET. Perioperative morbidity included four (2.6%) cases of strokes, three (2.0%) of spinal cord injury, 12 (7.7%) of pneumonia, four (2.6%) of re-exploration for bleeding and eight of acute haemodialysis (Table 3 ). The first patient out of the three cases of spinal cord injury, who was also the fifth recipient of the FET for TAA in 1998, had complete paraplegia because the stent graft was distally positioned on Th9 level without the perfusion through left subclavian artery. On the basis of this experience, we routinely used selective cerebral perfusion through three neck vessels including the left subclavian artery from the sixth case onwards. The second patient, who was 26th case of FET for chronic type B dissection in 2000, had paraplegia caused by intra-operative hypotension because of bleeding with distal positioning on Th9 level of the stent graft in spite of the perfusion through left subclavian artery. The third patient, who was the 61st case of the FET technique for TAA in 2002, had paraparesis because he had had a previous operation for abdominal aortic aneurysm. After this experience cerebrospinal fluid drainage (CSFD) was performed the day before the operation in elective cases with a history of aortic repair in the thoraco-abdominal or abdominal aorta or when the pathologic condition required stent-graft delivery lower than the ninth thoracic vertebra from January 2002. CSFD was administered preoperatively in 12 cases.
2.2 Late results
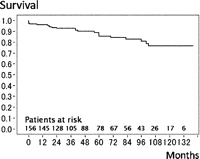
During the mean follow-up period of 63.3 ± 39.2 months, 16 late deaths (10.3%) caused by acute type A dissection (n = 1, that was in-hospital death 6 months after dissection operation) in TAA and non-aortic events (n = 15) such as heart failure (n = 4), stroke (n = 4), pneumonia (n = 3) and cancer (n = 4). The survival rate was 80.7% at 8 years (Fig. 1 ). The patient with acute type A dissection in whom reconstruction from the ascending aorta to left subclavian artery without ascending and total arch replacement in primary FET procedure had been achieved, experienced a new intimal tear at the ascending aorta 64 months after FET that was not associated with previous anastomosis. Fifteen patients (9.4%) required an additional aortic operation: ascending aortic replacement for acute type A dissection (n = 1), Bentall operation for root dilatation (n = 2), descending aortic replacement (n = 4; two replacement and two TEVARs), thoraco-abdominal replacement (n = 1) and abdominal aortic replacement (n = 7) (Table 3). The freedom from overall aortic event, including an endoleak, was 79.5% at 8 years (Fig. 2 ). Three patients (2.1%) had stent-graft-related events: two of endoleaks (Type I endoleak) after 30 months in chronic type B dissection and after 42 months in TAA, one of aortic rupture after 18 months in chronic type B dissection. All three patients were rescued by additional replacement or TEVAR. Freedom from events including on the site of stent graft was 93.8% at 8 years (Fig. 3 ).
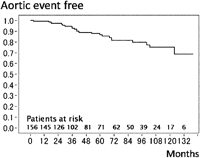
Overall aortic event. The overall 8-year event free rate was 79.5%.
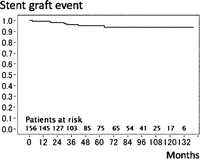
Aortic event on the site of stent graft. The 8-year event free rate on the site of stent graft was 93.8%.
A follow-up CT image was available for 96.0% (143/149) of patients who survived longer than 12 months. The size of aneurysm increased in four patients (2.8%), was unchanged in 20 patients (14.0%), shrank in 66 (46.2%) and was completely obliterated in 53 (37.1%) (Table 4 ).

3 Discussion
Since initial publication by Borst and co-workers [1] in 1983, the elephant trunk operation has been employed in two conditions: aneurysms and dissection of the aortic arch and the descending thoracic aorta involving the respective downstream portions of the vessel. In the conventional elephant trunk operation, the perigraft space around the elephant trunk remains perfused thereby promoting further dilatation of the aortic segment. Etz and co-workers [9] in the Mount Sinai School reported staged repair using long elephant trunk technique; 65% required two-staged complete repair, and the low mortality after stage one justifies liberal use of the elephant trunk technique to facilitate future open or endovascular repair of the distal aorta. However, it would be preferable if one-step repair were possible. To avoid these potential problems of a long elephant trunk graft, Kato and co-workers inserted a graft, the distal part of which was sustained by an intraluminal stent into the descending aorta [2]. The FET obtains distal portion by substituting the expansile force of the stented graft. Therefore, the FET can close primary entry via the aortic arch avoiding left thoracotomy and, in addition, kinking and flapping action of the graft can be prevented [10]. The perigraft space around the FET graft can be completely obliterated. Karck and Kamiya [11] reviewed the FET in 215 patients from eight reports and the mortality rate of this procedure, including acute aortic dissection, was 15/215 (7.0%) and concluded that the FET technique might become the next standard treatment for extended thoracic aorta instead of its conventional variant. This study reported the long-term results of FET for four different pathologies: acute type A aortic dissection, acute type B aortic dissection, chronic type B aortic dissection and arteriosclerotic aneurysms.
3.1 Acute type A aortic dissection
In recent years, the results of surgery for acute type A dissection have improved. Considering the long-term prognosis, total arch replacement is often performed for long thoraco-abdominal aortic dissection in younger patients, and good results have been reported [12,13], even if the primary intimal tear is located in the ascending aorta. However, an anastomotic leakage or a small tear in the proximal descending thoracic aorta can exist after replacement of aortic arch; the false lumen is susceptible to dilation due to shear stress acting on the proximal descending aorta. The stent graft has been introduced for the purpose of obliterating the false lumen in the distal part of the descending thoracic aorta. There are two reasons for this outcome. The first is that it is possible that stent compression prevented anastomotic leakage more effectively. The second reason is that a stent graft inserted into the true lumen on the pulmonary artery level can completely close second tears because bronchial or intercostal arteries were dissected; this can be confirmed by intra-operative trans-oesophageal echogram. We usually perform the FET for acute type A dissection in patients under 70 years considering the surgical risks and the long-term natural prognosis.
3.2 Type B aortic dissection
Medical treatment is usually performed as the initial approach for acute type B dissections. Patients with life-threatening complications of acute type B aortic dissection require emergency treatment using open surgical aortic graft replacement, thoracic aortic stent grafting, interventional or surgical abdominal fenestration, or catheter reperfusion or extra-anatomic surgical bypass, or both [14]. Recently, excellent results of entry closure by endovascular stent-graft repair and direct non-covered stent treatment to dissected visceral arteries were reported that excellent interventional technology supported. We recommended in a previous paper that a central aortic operation for the purpose of entry closure should be performed to collapse true lumen and also that organ reperfusion treatment with extra-anatomical bypass or non-covered stent directly to symptomatic branch vessel obstruction extending into visceral arteries should be performed [15]. TEVAR as a central aortic operation has anatomical limitations, such as a tortuous and narrow iliac artery or aorta, and there is difficulty managing the arch vessels using special materials such as fenestrated grafts or branch grafts. In addition, the proximal diameter differs from the distal diameter of a stent graft inserted into the aortic dissection with a collapsed true lumen. The FET, which is achieved by direct suturing to the aortic arch and allows easy anchoring across the arch vessels, is effective for type I endoleaks. The FET with sternotomy allows concomitant heart surgery; total arch replacement, coronary artery bypass grafting and Bentall operation. The technical learning curve and material improvement in TEVAR has led to the same results as those of the FET. We consider that FET is one option for endovascular stent-graft repair of acute type B dissection.
Uncomplicated type B aortic dissection was indicated for the purpose of preventing dissection-related events in the future and compression of the true lumen by the enlarged patent false lumen. Akutsu and co-workers [16] have demonstrated that patency of the false lumen is a strong independent prognostic factor of dissection-related death and dissection-related events and, in addition, the location of the most dilated aortic segment at the distal arch is a significant risk factor in the patients with a patent false lumen. We had an excellent outcome of the FET for acute type A dissection. There is the risk of a new tear caused by the stent graft affecting the fragile intima, but a 1-year upper limit from the time of dissection is considered to be the maximum window of opportunity with respect to aortic plasticity because the intima may become too rigid late after chronic dissection to allow for safe stent-induced aortic remodelling. These considerations support the FET as soon as possible or at least within 3 months of dissection. In patients with type B ‘chronic’ dissection, the false lumen showed early thrombosis after the FET; however, the thrombosed false lumen with chronic aneurysmal dilation persisted without absorption because of retrograde flow from re-entry in the abdominal aorta. In addition, the true lumen was dilated at the central portion of the graft, which might increase turbulent flow by interaction with the stent. Such flow may damage the intima of the true lumen and lead to ulceration in the mid-term postoperative period. We recommend that FET should not be performed for chronic type B dissection.
3.3 New intimal tear
Use of a stent graft for acute dissection has problems such as the risk of intimal damage or selection of size and is controversial [17,18], but we want to emphasise that direct sizing through an incision in the aortic arch is very important. We insert a ball-shaped valve sizer into the true lumen of the descending aorta from the transverse incision under trans-oesophageal ultrasound guidance and choose the correct size. Therefore, we have stent grafts of every size always ready. We minimise the danger of intimal damage by inserting a stent graft of the exact size under circulatory arrest and have not experienced any new intimal tears after stent grafting so far during long-term follow-up. We have experience of the FET for Marfan’s syndrome in only one case of acute complicated type B dissection and the long-term prognosis is unknown. Therefore, it seems that use of a stent graft for Marfan’s syndrome patients should be carefully considered.
3.4 Thoracic arteriosclerotic aneurysms
On the contrary, the FET had several disadvantages such as risk of distal endoleak and spinal cord injury compared with the conventional replacement. We experienced one case of late endoleak 42 months after operation. This case involved the migration towards aortic arch of a fusiform aneurysm with a diameter of 65 mm. We should caution that the FET for the fusiform aneurysm with a large diameter holds the risk of distal endoleak for the period of long-term follow-up. Stent graft for the FET should be improved such as stent-graft design avoiding migration until long-term follow-up.
3.5 Spinal cord ischaemia
Spinal cord ischaemia is known to be a devastating complication of the FET [4,5,11,19]. The incidence of paraplegia after implantation of FET is 3.3% according to a review report by Karck and Kamiya [11] Spinal cord injury after the FET was associated with multiple factors, such as the thoracic vertebral level, where the distal end of the FET was deployed, the history of downstream aortic operation, intra-operative hypotension and each patient’s individual pathology (aneurysm or acute dissection) [20]. Our series showed three (2.0%) cases of spinal cord injury because of the deep positioning of stent graft, the simple clamp left the subclavian artery without perfusion, the hypotension after stent grafting and the previous operation of abdominal aortic aneurysm. The cerebrospinal fluid drainage (CSFD) was performed before the operation in elective cases with a history of aortic repair in the thoraco-abdominal or abdominal aorta or when the pathologic condition required stent-graft delivery lower than the ninth thoracic vertebra from January 2002. No spinal cord ischaemia has been observed since then.
3.6 Limitations of this study
This is a retrospective cohort study. A prospective study would be required for a full analysis of the precise advantages of the FET technique.
4 Conclusions
The FET could be an attractive treatment for extended aortic arch disease to the extended descending aorta for ‘acute’ aortic dissection, except ‘chronic’ dissection, as well as TAA.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
Appendix A
Conference discussion
Dr C. Hagl (Hannover, Germany): We are all aware that treatment of such complex arch pathologies can be challenging, especially if the disease extends into the descending aorta. Furthermore, there is an ongoing controversial discussion, and Dr Bachet was mentioning it before, concerning the question how to treat patients with acute type A aortic dissection. Should we always replace the aortic arch or even approach the proximal descending aorta? The major question, ‘Is perfect an enemy of good?’ has not been answered yet.
In their study, the group from Hiroshima analysed 156 patients over an 11-year period with different pathologies of the aortic arch. Sixty-six were treated for acute aortic dissection, 26 for acute type B aortic dissection, and 56 had atherosclerotic aneurysms. The mortality rate of 3.8% in the whole group, 4.5% in acute type A's, and 7.7% in acute type B's is remarkable. This is also true for a very low incidence of neurological complications.
On reading the manuscript, which I appreciate having received in advance, I was wondering about the very small number of aortic root or aortic valve replacements in the acute type A aortic dissections. There were only 3 patients out of 66.
Furthermore, I missed the information concerning the real patient selection. What was the proportion of acute type A's and B's who were treated with the frozen elephant trunk compared to those who were treated with a more limited approach? How do you decide on the individual surgical approach?
Taking your excellent data into account, we could speculate that every patient presenting with acute type A aortic dissection should be approached by using a hybrid prosthesis. This may be an elegant approach, but we know from the Essen group that this approach is not free from possible complications despite the fact that they have quite an expertise in this field. I would appreciate if you can comment on that.
The second question is on your technique of cerebral and spinal cord protection, since your circulatory arrest times are frequently longer than 60 min in all groups. What is the temperature you use, what type of selective perfusion, and what is your opinion on CSF drainage?
Dr Uchida: Concerning the first two questions about patient selection for acute type A dissection, we select according to patient age lower than 70 years old and tear located downstream of the arch and diameter of thoracic descending aorta over 40 mm and, in addition, narrow true lumen. We select operative indication regarding these four factors.
And Marfan syndrome, we have only one case for acute type B dissection using frozen elephant trunk. Using elephant trunk technique for Marfan syndrome should be carefully considered.
And the second question, spinal cord injury, is very complicated. I believe hypotension into the operation is major cause of spinal cord injury. In addition, for patients of deep replacement lower than the 9th level, and history of abdominal aortic operation, we usually use CSF drainage and subclavian artery perfusion.
Dr Hagl: Thank you. Can I come back to the first question: How many type A aortic dissections were treated in your institution in this 11-year period? How many, overall, type A aortic dissections were treated?
Dr Uchida: 50% for acute type A dissection, we select the frozen elephant trunk. Other 50%, for example, 83 years old, and early thrombosed dissection, we use hemiarch replacement only.
Dr Hagl: But the percentage of patients with a dilated descending aorta is relatively low, right, in acute dissections?
Dr Uchida: No. All cases diameter not over 40 mm. But if the patient is young or narrow true lumen, we select frozen elephant trunk technique
Dr Hagl: I think the most important thing is to distinguish between the groups. You have 3 different groups and 3 different pathologies, and I think that makes a big difference how you treat these patients.
Dr R. Bonser (Birmingham, United Kingdom): The study lacks a comparator group of patients undergoing what we might call a conventional repair. In the previous reports, the conventional repair is being reserved for the patients who are actually at high risk, those with tamponade, those with preoperative malperfusion phenomena, and the older patients, and so therefore, it's essential to see if this treatment is going to be generalisable and become a standard for Debakey type I dissection, that we also declare what is happening with the patients having a standard repair and that we match the patients to make sure that a sicker patient having a standard repair is not compared against a younger, fitter patient having this more extended repair. Would you like to comment upon that, please.
Dr Uchida: The study has a limitation that it is not prospective study comparing standard repair and frozen elephant trunk. Concerning acute type A dissection, surgical strategy is selected according to preoperative condition by primary surgeon. However we believe frozen elephant trunk technique is very useful for acute type A dissection concerning both early and late results. It is very easy to have hemostasis and to prevent malperfusion in the early phase and the prognosis of long-term result is very good. Therefore we prefer frozen elephant trunk technique if the patient has catastrophic state for example severe brain damage and intestinal necrosis.
References
- aortic arch
- dissection of aorta
- stents
- echocardiography
- computed tomography
- hypothermia, natural
- descending aorta
- distal aortic dissection
- cerebrovascular accident
- aneurysm
- ascending aorta
- abdominal aorta
- tissue dissection
- elephants
- follow-up
- paraparesis
- paraplegia
- safety
- spinal injuries
- survival rate
- tissue transplants
- morbidity
- endoluminal grafts
- aortoplasty
- supraaortic valve area
- cerebral perfusion




