-
PDF
- Split View
-
Views
-
Cite
Cite
Ines Florath, Alexander Albert, Andreas Boening, Ina Carolin Ennker, Juergen Ennker, Aortic valve replacement in octogenarians: identification of high-risk patients, European Journal of Cardio-Thoracic Surgery, Volume 37, Issue 6, June 2010, Pages 1304–1310, https://doi.org/10.1016/j.ejcts.2009.12.025
Close - Share Icon Share
Abstract
Objective: This study identifies high-risk octogenarians for surgical aortic valve replacement (AVR) because with the current advances in transcatheter valve therapy, a definition of patient selection criteria is essential. Methods: Between 1996 and 2006, 493 consecutive octogenarians with symptomatic aortic stenosis underwent AVR with and without (51%) concomitant coronary artery bypass grafting (CABG). To identify high-risk patient groups, risk factors of 6-month mortality were determined using multivariable logistic regression. Results: The 30-day mortality rate was 8.4% and it increased up to 15.2% until 6 months after AVR. Independent risk factors of 6-month mortality were patients older than 84 years (odds ratio (OR): 2.2 (1.29–3.61)), left ventricular ejection fraction ≪60% (OR: 2.5 (1.35–4.61)), body mass index (BMI) ≪24 (OR: 2.0 (1.22–3.36)), creatinine (OR: 1.6 (1.04–2.53)) and blood glucose (OR: 1.01 (1.001–1.009)). High-risk groups were patients older than 84 years with an ejection fraction ≪60% (6-month mortality 28%) and patients younger than 84 years with an ejection fraction ≪60% and a BMI ≪24 (6-month mortality 23.2%). These high-risk groups comprised 37% of the patient population. After isolated AVR, the 30-day mortality and survival at 1 and 5 years was 11.6%, 69% and 35% in this high-risk group, respectively. In octogenarians with an STS score >10 and an EuroScore >20, the 30-day mortality and survival at 1 year was 10.5% and 80%, 11.6% and 77%, respectively. Conclusions: In most octogenarians, AVR is a safe and beneficial procedure. In high-risk octogenarians, identified by STS score >10, EuroScore >20 and by simple three risk factors (age >84 years, ejection fraction ≪60% and BMI ≪24), the mortality after surgical AVR was no different from the currently reported outcome after transcatheter AVI.
1 Introduction
The European Heart Survey on valvular heart disease has shown that 33% of patients with severe symptomatic aortic stenosis did not undergo surgical intervention because of an expected excessive operative risk due to advanced age or presence of significant co-morbidities [1]. The development of transcatheter aortic valve implantation (TAVI) offers the option of treatment of severe symptomatic aortic stenosis in these patients. Most of the patients undergoing TAVI were older than 80 years [2–4]. In recent studies, patients were selected for TAVI by age (over 80 or 75 years) and by current scoring systems such as the European system for cardiac operative risk evaluation (EuroScore) or the Society of Thoracic Surgeons (STS) Score [2–4].
The European Association of Cardio-thoracic Surgery (EACTS) and the European Society of Cardiology (ESC) formulated a position statement recommending four steps of patient selection: confirmation of severity of symptoms of aortic stenosis; evaluation of symptoms; assessment of the feasibility and exclusion of contraindications for TAVI; and the establishment of a high surgical risk by clinical judgement in association with a more quantitative assessment based on scoring systems [5]. The usefulness of scoring systems for TAVI feasibility studies depends on the accuracy of these algorithms to identify high-risk patients. Recently, it has been demonstrated that the EuroScore overestimates 30-day mortality after surgical aortic valve replacement (AVR) and especially inaccurately predicts mortality in high-risk patient [6,7]. The STS score was found to underestimate but predict the observed mortality most accurately [8].
Our intention was to identify high-risk groups for surgical AVR in octogenarians and to estimate their operative risk. Because it has been shown that hospital and 30-day mortality rates underestimate the operative risk in cardiac surgery and a longer time period (e.g., 180 days) was proposed [9], we estimated predictors of 6-month mortality.
2 Patients and methods
2.1 Patient population
Between 1996 and 2006, 493 consecutive octogenarians with aortic stenosis underwent AVR with and without (51%) concomitant coronary artery bypass grafting (CABG). The study population included patients receiving mechanical (10%, N = 52) and biological stented (46%, N = 239) and stentless valves (44%, N = 224), but excluded patients with valve repair, multivalve replacement and replacement of the ascending aorta. Preoperative and operative characteristics are shown in Table 1 . Preoperative and intraoperative data were collected on all patients as a part of a national quality assessment trial of cardiothoracic surgery (Quadra: Quality Assurance Data Review Analysis) using standardised protocols of the German Society of Thoracic and Cardiovascular Surgery.

Patient characteristics and univariate association between risk factors and 6-month mortality.
The patients were followed up till September 2008. The follow-up information was obtained by mailed questionnaires and completed by telephone interviews and was 99% complete. The retrospective study was approved by the Ethics Committee of the General Medical Council of the State of Baden-Württemberg (Germany). The approval includes written informed consent regarding participation in the study.
2.2 Statistical analysis
Data were statistically analysed using the software package SPSS (SPSS Inc., Chicago, IL, USA). All continuous data were expressed as mean values (±one standard deviation) and, in general, compared by the Mann–Whitney test. Body mass index was also compared by Student’s unpaired t-test. Dichotomous variables were evaluated by the univariable chi-square test and Fisher’s exact test.
Predictors of 6-month mortality were identified by multivariate logistic regression analysis. For variable selection minimisation of the Akaike information criterion, the formula (AIC = deviance of the model + 2 × number of included parameters) was used. AIC was calculated for variables showing a difference for the outcome variable with a p-value smaller or equal to 0.25 (Table 1). Variables with the minimal AIC were included in multiple regression models in a stepwise manner. The AIC was calculated each time a variable was included. The final model was reached when no more reduction in AIC was observed.
To verify the linearity assumption of the logistic regression model for continuous variables, the logit of probability of death within 6 months after AVR was computed specifically for each continuous variable using rectangular kernel functions to smooth the curves (Fig. 1a–e) [10]. If a non-linear relationship was observed, the continuous variable was transformed (e.g., into a dichotomous variable). A possible cut-off to define a dichotomous variable was determine by maximisation of the Youden index (=sensitivity + specificity − 1) and presented as vertical lines in Fig. 1. For the different transformations of the continuous variable, the AIC was determined (Table 2 ). If the AIC of the model containing the transformed variable was lower than for the model containing the continuous variable linearly, the transformed variable was included in the final model.

Relationship between logit of the probability of death within 6 months after AVR (p) and several continuous variables (a) age, (b) body mass index, (c) ejection fraction, (d) blood glucose, (e) creatinine. The vertical lines represent possible cut-offs used to transform the continuous variable into a dichotomous one.
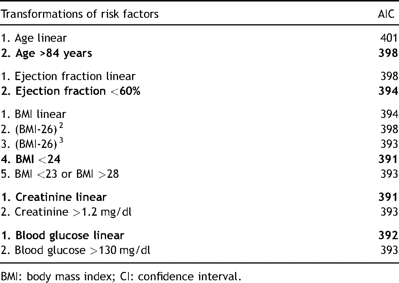
Akaike information criterion for several transformations of risk factors. Transformation with the lowest AIC and included into the final model was highlighted in bold letters.
The discriminative power of the final models was estimated by the c-index, the area under the receiver operating characteristics (ROC) curve.
EuroScore was calculated according to the rules described at the web site: http://www.euroscore.org. The mortality predicted by the model of the Society of Thoracic Surgeons from 2009, the STS score, was calculated using the regression coefficients for the isolated aortic valve – and the aortic valve plus CABG – model provided in the most recent publications [12,13].
3 Results
The 30-day and 6-month mortality in octogenarians was 7.6% and 14.3% after isolated aortic valve replacement and 9.5% and 16.6% after AVR with concomitant CABG, respectively. Risk factors of 6-month mortality were age over 84 years (odds ratio (OR): 3.4 (2.23–5.21)), body mass index (BMI) less than 24 kg/m2 (OR: 2.1 (1.56–2.73)), left ventricular ejection fraction less than 60% (OR: 2.1 (1.54–2.79)), creatinine (OR: 1.4 (1.24–1.55)) and blood glucose concentration (OR: 1.005 (1.003–1.007)). The model had a good predictive ability with a c-index of 0.71.
For several combinations of identified risk factors, the observed 6-month mortality was almost or greater than 25% (Fig. 2 ). We assigned patients younger than 84 years with an ejection fraction less than 60% and a BMI less than 24, and patients older than 84 years with an ejection fraction less than 60% to a high-risk group. Although the 30-day mortality in this high-risk group was acceptable with 13%, 26.5% of these patients died within 6 months after aortic valve surgery, whereas the 6-month mortality in the low-risk group was only 8.8% and 30-day mortality 5.9%.

Observed 6-month mortality for several combinations of identified risk factors.
The impact of the continuous variables blood glucose and creatinine on 6-month mortality is shown in the risk profiles (Fig. 3a and b ). Whereas the mortality increases with increasing glucose concentration in both risk groups (Fig. 3a), the 6-month mortality increases with increasing creatinine concentration only in the high-risk group (Fig. 3b).

Risk profiles for continuous risk factors blood glucose (a) and creatinine (b).
In Table 3 , we compared the observed 30-day mortality with the mortality predicted by our own regression model and by the STS and logistic EuroScore model. For the presented study population of octogenarians, the EuroScore model overestimates the 30-day mortality, whereas the STS did not discriminate well between high- and low-risk patients identified by our analysis.

Observed and predicted 30-day mortality by the presented risk model and by STS and EuroScore for high and low risk patients. .
In octogenarians with an STS score higher than 10 and an EuroScore higher than 20, the observed mortality was 10.5% and 26.3%, 11.6% and 23.3%, respectively, 30 days and 6 months after isolated AVR. Survival at 1 and 5 years after isolated AVR was 80.2 ± 2.9% and 41.5 ± 4.1%, 77.4 ± 2.0% and 44.9 ± 2.9%, respectively, for octogenarians with an STS score higher than 10 and with an EuroScore higher than 20.
The overall survival at 1 and 5 years was 83.9 ± 2.3%, 58.8 ± 3.7% and 79.2 ± 2.6%, 45.7 ± 3.8, respectively, for isolated AVR and AVR with concomitant CABG. The survival rates after isolated AVR at 1 and 5 years were 69.0 ± 3.4%, 34.7 ± 4.3% and 89.2 ± 1.8%, 62.6 ± 2.9, respectively, for the high- and the low-risk groups. The survival curve (Fig. 4 ) shows that the main decrease was observed within the first 6 months. Thereafter, the course of the survival curves is nearly parallel. The median survival time of the 6-month survivors after isolated AVR was 4.9 years (4.2–5.5) and 7.0 years (5.9–8.0), respectively, for the high- (mean age: 84 years) and low-risk group (mean age: 82 years).

4 Discussion
The overall 30-day mortality was 8.4% and slightly lower than the recently published rates, which varied between 9.2% and 12% in populations larger than 200 patients [13–16]. A comparison of mortality and survival rates after AVR in octogenarians is provided in Table 4 . The survival rate observed in the presented patient population was quite similar to that reported by Melby et al. [14] and Leontyev et al. [16], whereas it was lower than that reported by Kolh et al. [15] and Asimakopulos et al. [13]. The survival rate largely depends on the incidence of risk factors in the studied population. For example, the incidence of two important risk factors such as diabetes (12%) and renal failure (4%) was much lower in the study by Kolh et al. [15] than in the present study population.
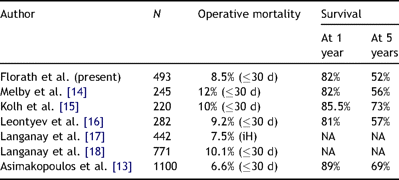
Comparison of observed mortality and survival by several authors.
Whereas hospital mortality reflects institutional habits concerning postoperative hospital stay, 30-day mortality allows inter-institutional comparison because a follow-up procedure is required [9]. Nevertheless, both parameters underestimate the operative risk and a longer time period (e.g., 180 days) is recommended [9]. This is supported by the survival curve presented in Fig. 4. There is a steeper decrease within the first 6 months than later.
Therefore, we identified the risk factors of 6-month mortality, which were age >84 years, left ventricular ejection fraction ≪60%, BMI ≪24 kg/m2, blood glucose and creatinine concentration. Age, ejection fraction, creatinine and blood glucose or diabetes are well-known risk factors presented in the recent risk models predicting operative mortality [11,12,19,20]. An adverse impact of small BMI on operative outcome after AVR in large study populations has also been reported previously [20,21].
For validity reasons of logistic regression models, the number of events (e.g., death) per risk factor is recommended to be greater or even 10 [22]. That means, considering a mortality of 10%, the study population must contain more than 400 patients (death = 40) for valid estimation of p-values and confidence intervals of four risk factors. There are only a few studies on AVR in octogenarians with study populations larger than 400 patients.
In a study population of 771 patients, the following multivariate predictive factors of 30-day mortality were found: renal insufficiency, left-heart failure and NYHA class IV, whereas age and ejection fraction were not identified [18]. These discrepancies to the presented results may be explained by the different endpoints (death after 30 days or 6 months), because the continuously increasing number of high-risk patients due to the progress in surgical and anaesthesiological management causes the prolongation of early risk after cardiac surgery [7].
Our intention was to identify octogenarians with a high operative risk after AVR, who may benefit from transcatheter aortic valve implantation. In one-third of the octogenarians undergoing surgical AVR at our institute, a high 6-month mortality of 26% was observed. The 30-day mortality in this high-risk group was 11.6% and the 1-year survival was 69% after isolated AVR. In high-risk patients assessed by STS score higher than 10 and EuroScore higher than 20, the 30-day mortality was 10.5% and 11.6% and the 1-year survival 80% and 77% in octogenarians after isolated AVR, respectively. The mean age of patients currently undergoing TAVI is over 80 years and the observed 30-day mortality varies between 8% and 12.4% [2–4,23], whereas the observed early mortality was 2–3 times higher for the transapical than for the transfemoral approach [4,24]. The 1-year survival rate after TAVI was similar (74–78%) to that observed in high-risk octogenarians in the present study assessed by STS score, EuroScore and the proposed risk factors [4,25]. At the current stage, the mortality after TAVI and surgical AVR was not different.
Nevertheless, in the near future, high-risk patients may benefit from TAVI if the outcome results could be further improved, as recently reported: the 30-day mortality decreased from 12.3% to 3.6% and from 25% to 11.1% comparing the first and the second half of the treated patients, respectively, for the transarterial and the transapical approaches [4] and the 1-year survival rate increased from 60% in the first 25 patients versus 93% in the last 50 patients [25]. Meanwhile, a high surgical risk should be established by quantitative assessment based on scoring systems to make outcome after surgical AVR and TAVI comparable.
For the current study population of octogenarians, EuroScore overestimates the operative risk (Table 3), previously demonstrated in several studies [6,7], also in octogenarians [16]. The STS score overestimated the risk in the low-risk group and underestimated it in the high-risk group, identified by the presented risk factors (Table 3). Further attempts for risk assessment in high-risk patients undergoing AVR are necessary. In our large population of octogenarians, we found two simple risk factors, age over 84 years and ejection fraction below 60%, which when simultaneously present in patients increase the 6-month mortality to 28% from 12% when none of these factors was present, whereas the 30-day mortality was acceptable and within the range previously reported (10.5%, Table 4). This clearly shows that the 30-day mortality underestimates the operative risk in high-risk patients and that a longer time period of evaluation is required to assess the operative risk and to compare the outcome after surgical AVR and transcatheter AVI.
Presented at the 23rd Annual Meeting of the European Association for Cardio-thoracic Surgery, Vienna, Austria, October 18–21, 2009.
Appendix A
Conference discussion
Dr P. Kolh (Liege, Belgium): I have, first, questions and comments concerning your model. Indeed, the validity of your model is an important issue because you are attempting to identify risk factors. As a multivariable model, in this case using Akaike criterion, is developed, I would like to question the robustness of your model. When you want to validate a multivariable model, you should use, for example, samples of patients randomly chosen within the group, for example, one hundred times take different samples of patients and see whether the model holds or not. This is important, because you report results that we need to question, first regarding the body mass index. I think it is very interesting, but a normal body mass index is somewhere between 19 and 24. Patients considered to be really underweight would be with a BMI less than 19. Also, I am puzzled by the fact that you did not find high body mass coming out as a significant parameter. And similarly, the association of AVR and CABG is not significant in your model. I think, if I understand correctly your manuscript, 51% of the patients had isolated AVR and 49% had associated AVR and CABG. This variable has been shown significant in numerous other reports. So if you would like to answer these comments regarding your model, I would appreciate it.
Dr Ennker: Thank you very much, Dr Kolh. Of course, I read your own paper and read all the defined statistics. I am the senior surgeon, but I can quote also the statistical analysis of my co-worker, Dr Florath. And I can tell you that the predictors of 6-month mortality and survival times were identified by multivariate logistic regression analysis and Cox regression, respectively. For variable selection, minimization of the Akaike criterion was used. The AIC was calculated for variables showing a difference with a p-value smaller or equal to 0.25. Variables with minimal AIC were included in mitral regression models in a stepwise manner. The AIC was calculated each time a variable was included. The final model was reached when no more reduction in the Akaike information criterion was observed. The discriminative power of the final models was estimated by the c-index, the area under the receiver operating characteristics (ROC) curve.
‘To verify the linearity assumption of the logistic regression model for continuous variables, Kernel functions were calculated. If a non-linear relationship was observed, the continuous variable was transformed. If the AIC of the model contained in the transformed variable was lower than for the model containing the continuous variable linearly, the transformed variable was included in the final model. All continuous data were expressed as a mean value and in general compared by the Mann–Whitney test. Body mass index was also compared by Student's t-test. Dichotomous variables were evaluated by the univariate -
Dr Kolh: May I interrupt you? I’m sorry. I know what is written in the manuscript, and we can have a discussion later on with your statistician, if you want, but what about BMI specifically and maybe BMI over 30, would you not expect that to be a significant risk factor?
Dr Ennker: The myocardial infarction or what?
Dr Kolh: No, body mass index, higher body mass index.
Dr Ennker: It is well written in the literature that even patients with increased body mass index have a better outcome in at least coronary artery surgery. So far, no studies in aortic valve replacement are known to me, but I presume it is the same. If a patient is a little or more overweight, he is in a better position. If the cut-off line is a body mass index of 38 or even higher, then they are in a poorer condition, and patients who are underweight have a poorer outcome than normal or overweight patients.
Dr Kolh: Second and final question. The follow-up was well done, 98%, but only through mailing and questionnaires, and I did not find anywhere the causes of death. So we may very well end up with a six-month mortality including a lot of non-cardiac causes. Obviously in this case what we want to identify in these elderly patients is cardiac causes of death. Do you have that information?
Dr Ennker: Well, in the high-risk group we had an early dip in survival, as I showed on one of the slides. In the high-risk groups the age was over 84, mean age was 86, they die also of other causes, but we have to still look out for this group.
Dr R. Przybylski (Zabrze, Poland): I would like to ask one question. You have shown us 30-day mortality, which is, as everybody knows, a biased assessment of treatment results for octogenarians. Could you tell us how many patients in each group were discharged home?
Dr Ennker: We don’t discharge back home because they have a high risk and then they have to go to a special rehab, especially in these octogenarians. We keep them longer in intensive care and we also keep them longer in our hospital, because we know that a lot of patients are lost due to early dismissal to inadequate surroundings. So especially in this age group, we have to take care.
References
- aortic valve stenosis
- left ventricular ejection fraction
- coronary artery bypass surgery
- body mass index procedure
- aortic valve replacement
- creatinine
- surgical procedures, operative
- blood glucose
- mortality
- ejection fraction
- octogenarians
- european system for cardiac operative risk evaluation
- society of thoracic surgeons risk calculator




