-
PDF
- Split View
-
Views
-
Cite
Cite
Nabil Saouti, Wim J. Morshuis, Robin H. Heijmen, Repke J. Snijder, Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension: a single institution experience, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 6, June 2009, Pages 947–952, https://doi.org/10.1016/j.ejcts.2009.01.023
Close - Share Icon Share
Abstract
Objective: Pulmonary endarterectomy (PEA) for chronic thromboembolic pulmonary hypertension (CTEPH) is the first treatment of choice with good short-term results. Only limited data are available concerning the long-term outcome after PEA. The purpose of this study is to evaluate the long-term survival and functional outcome after PEA with nearly 10 years experience. Method: In the period of December 1998 and December 2007 120 patients with CTEPH were referred to the St Antonius Hospital (Nieuwegein, The Netherlands) of whom 72 underwent PEA. The clinical data are collected retrospectively. Results: In-hospital mortality was (5/72) 6.9%. Since 2004 one patient died in the hospital (1/38, 2.9%). Two patients died during long-term follow-up with a median observation of 3 years. The overall 1-, 3- and 5-year survival rates were 93.1%, 91.2% and 88.7% respectively. Prior to surgery patients were in New York Heart Association functional class III (58) and IV (14) with a mean pulmonary vascular resistance of 572 ± 313 dynes s cm−5. The following data were compared before and after operation: mean pulmonary artery pressure (mPAP) decreased from 42 ± 11 to 22 ± 7 mmHg (p = 0.0001), NT-pro BNP improved from 1527 ± 1652 to 160 ± 3 pg/ml (p = 0.0001), 6 min walk distance (6MWD) from 359 ± 124 to 518 ± 11 m (p = 0.0001), and almost all patients returned to functional class I or II (p = 0.0001). Conclusion: Pulmonary endarterectomy for patients with CTEPH has shown a dramatic improvement of clinical status with excellent long-term survival.
1 Introduction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a rare, although increasingly recognized, disease caused by obstruction of the pulmonary arteries after single or recurrent episodes of pulmonary embolism without complete resolution. These vascular obstructions lead to an increased pulmonary vascular resistance (PVR), eventually resulting in right ventricular failure [1]. Left untreated this disease has a poor prognosis. Only a 5-year survival of 14% was reported in patients with a mean pulmonary artery pressure (mPAP) exceeding 50 mmHg [2]. The preferred treatment for this disease is the surgical removal of the organized thromboembolic material from the major pulmonary arteries by pulmonary endarterectomy (PEA). This surgical procedure is pioneered and developed by the team in San Diego [3–5] and now performed worldwide. It is potentially curative because it restores the pulmonary hemodynamics to normal or near normal.
The direct postoperative mortality rates of the PEA procedure reported since 1996 range from 4.4% to 24%, with the lowest mortality rate by the San Diego team with their extensive experience [6–14]. Recent published data about the long-term outcome after PEA have shown satisfying results in terms of mortality and morbidity [15–17].
Our PEA program was initiated in 1998. The purpose of this study was to analyze the results of a consecutive series of PEA and to identify factors that influence mortality.
2 Materials and methods
We studied the charts from all consecutive patients between 1998 and 2007 who were referred to our institution (St Antonius Hospital, Nieuwegein, The Netherlands) from across the country for consideration of PEA. All patients were analyzed for PEA according to our protocol. They all underwent preoperative right and left heart catheterization, conventional pulmonary angiography, ventilation/perfusion scintigraphy and CT pulmonary angiography. Furthermore, in the clinical diagnostic work-up patients underwent laboratory investigations for thrombophilia and N-terminal pro brain natriuretic peptide (NT-pro BNP) levels, they were classified according to the New York Heart Association (NYHA) functional class and had to perform a 6-min walk test (6MWD). All data of the patients were reviewed by a team consisting of a cardiothoracic surgeon, pulmonologist, cardiologist and radiologist. Patients were considered suitable for surgery when they were symptomatic, had an elevated PVR (>250 dynes), segmental or more proximal lesions and no severe comorbidity. Severity of hemodynamic disease was not a reason to decline a patient for surgery. At the beginning of our PEA program severely compromised hemodynamic patients were preoperatively treated with prostacyclin. Since 2003 high-risk patients with PVR >1000 dynes s cm−5 were treated with oral disease modifying therapy consisting of phosphodieesterase-5 inhibitors or/and endothelin receptor antagonist (ERA).
Since our clinical work-up was not standardized until 2001 we could not obtain 6MWD in 10 (14%) patients. NT-pro BNP was obtained in 42 (58%) patients, since NT-pro BNP measurement was not available until the end of 2003.
All patients were consistently operated by two surgeons (MWJ and HRH) using standardized technique [3,4]. The surgical procedure involves a median sternotomy with extracorporeal circulation and intermittent circulatory arrest (CA) in deep hypothermia (DHCA). Before cardiopulmonary bypass is initiated the head is wrapped around with ice to cool the head up to 4 °C enabling cerebral protection. Transcranial cerebral oximetry (TCO) is used to monitor cerebral saturation oxygenation. After the patient is cooled to 20 °C with cardiopulmonary bypass the surgeon starts with the endarterectomy in the right pulmonary artery. If bronchial back bleeding obscures optimal vascular visibility circulatory arrest is initiated up to 20 min max. If necessary this period of CA is prolonged, after an intermittent period of 10 min of cold reperfusion, to assure complete endarterectomy. The same procedure is repeated for the left pulmonary artery. Mean duration of DHCA was 55 ± 2.7 min. The operation was performed bilaterally unless the patient had unilateral disease on the basis of angiography and objectived intraoperatively (n = 10). During the rewarming period additional cardiac procedures were performed in 22 (31%) patients; closure of persistent foramen ovale (n= 16), closure of atrial septal defect type 2 (n= 1), coronary artery bypass (n= 3), mitral valve reconstruction (n= 1), tricuspid valve reconstruction (n= 1).
After the operation the patients PAP was monitored by a ‘simple’ pressure wire for the purpose of postoperative pressure measurement that was inserted intraoperatively by the surgeon and usually removed the second or third day. The postoperative care consisted of mechanical ventilation with pressure control and positive end expiratory pressure. Mean systemic arterial pressure was maintained between 60 and 80 mmHg with inotropics. If the systemic pressure dropped below this level norepinephrine was added. As soon as hemodynamics stabilized patients were aggressively treated with diuretics to achieve a cumulative fluid balance equally to preoperative level within 24 h. After the fluid balance of zero the patient was weaned from ventilator support and extubated. Anticoagulation was started with heparin immediately after the operation. Six hours postoperatively oral coumadin was restarted with a target INR of 2.5–3.5.
Follow-up of the patients was performed 8 weeks after discharge from the hospital followed by visits to the outpatient clinic after 3 months and every half-year thereafter. Investigations consisted of clinical examination by means of assessing the NYHA functional class, transthoracal echocardiography, ventilation/perfusion scanning, 6MWD and assessing the NT-pro BNP value. If persistent pulmonary hypertension was likely right-heart catheterization (RHC) was performed (n = 7).
3 Statistics
Baseline parameters between survivors and non-survivors were compared using independent samples t-test for continuous variables, χ2-test for categorical variables and test for one proportion for the NYHA classification. NT-pro BNP was skewed to the right and was normally distributed after log transformation. The NT-pro BNP values reported were transformed back to original values after the tests. For the comparison of pre- and postoperative data samples t-test (paired) were used for continuous variables and test for one proportion for the improvement of NYHA classification. Univariate analysis based on the proportional hazard model was used to examine the relations between survival and selected demographic, clinical measures and hemodynamics measured at baseline. Optimal cut-off values assessed with receiver operating curve (ROC) analysis were used to separate the patients on both sides into two groups. Survival curves were derived by Kaplan–Meier method. Groups were compared by the log-rank test.
Survival was estimated from the date of operation until December 31, 2007 or the date of death. A p value <0.05 was considered statistically significant.
4 Results
Of the 120 patients 72 (60%) underwent surgery. Of the 48 patients who did not proceed for surgery 31 (65%) had too distal, surgically inaccessible disease, 8 (17%) refused surgery, 8 (17%) were declined due to severe comorbidity (e.g. cancer and severe chronic obstructive pulmonary disease) and 1 (1%) was considered too old (81 years).
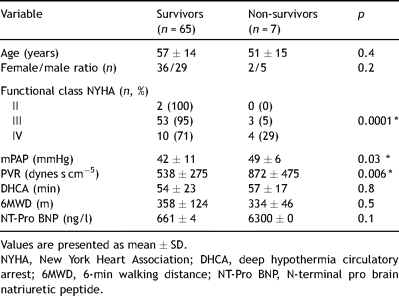
The baseline patient clinical characteristics, pulmonary function and hemodynamics are summarized in Table 1 . The age ranged from 23 to 74 with a mean age of 56 ± 14 and the ratio of male/female was almost equal. The majority of the patients were in NYHA functional class III (78%). Almost all patients had a history of pulmonary embolism (94%). The mean duration between the onset of symptoms and diagnosis of CTEPH was 59 months. Coagulation disorder was present in 33% of the patients. The preoperative hemodynamic parameters showed that patients had pulmonary hypertension with a PVR of 572 ± 313 dynes s cm−5.

Baseline clinical characteristics, pulmonary function and hemodynamics (n = 72).
Table 2 shows the difference in baseline parameters between survivors and non-survivors. The hemodynamic status at baseline was worse compared to survivors (significantly higher mPAP and PVR). Ninety-five percent of the patients in NYHA functional class III survived and from the patients in NYHA class IV 71% survived (p = 0.03). There was no difference between survivors and non-survivors in age, female/male distribution, time of DHCA and 6MWD. Although NT-pro BNP was much higher in non-survivors reflecting the wall stress of the RV, it did not reach statistical significance probably due to the wide spread.
4.1 Postoperative outcome
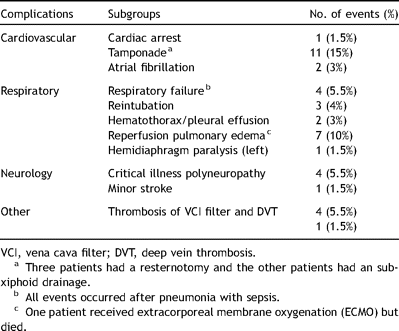
The postoperative mPAP decreased from 42 ± 11 to 22 ± 7 (p = 0.0001). Sixty-two percent were extubated within 24 h. Mean duration on the intensive care unit (ICU) stay was 7 ± 8 days with a range from 1 to 40 days. The complications in the postoperative period are summarized in Table 3 .
Of the clinical measures assessed three months after surgery the exercise tolerance (6MWD) improved significantly and the NT-pro BNP levels returned to normal (p = 0.0001). Most patients improved 2 NYHA functional classes (58%), 12% changed 3 classes and 28% changed 1 class. Of the survivors one patient remained in the same functional class III postoperatively after 5 years (Table 4 ).
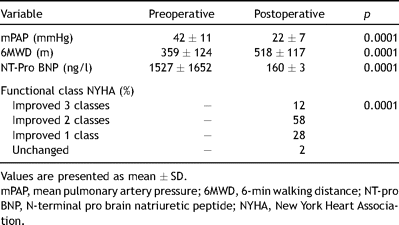
Comparing pre- and postoperative hemodynamic and clinical measures (n = 72).
4.2 Survival
In the overall period five (7%) patients died in the hospital, no patient died intraoperatively. In the period 2004–2007 only one of the 38 operated patients (2.9%) died in the hospital. Three patients died as a consequence of reperfusion pulmonary edema of which one was treated with ECMO. The fourth patient died as a consequence of respiratory failure after septic pneumonia. The fifth patient died after multi organ failure. Two patients died during long-term follow-up after 2 and 4 years with a median observation of 3 years. One patient was found dead 48 h after delivery of a healthy infant. Before the pregnancy recurrent pulmonary hypertension was excluded by right-heart catheterization in rest and during exercise. The autopsy could not identify the cause of death. The second patient who died had persistent PH treated with an ERA and died from cerebral bleeding. No patient was lost to follow-up. The overall 1-, 3- and 5-year survival rates for the total cohort including late deaths was 93.1%, 91.2% and 88.7% respectively (Fig. 1 ).
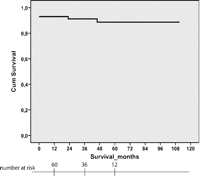
Kaplan–Meier overall survival curve of patients who underwent pulmonary endarterectomy.
4.3 Risk analysis and predictors of postoperative hospital death
To distinguish survivors from non-survivors according to baseline parameters, groups were created based on the optimal cut-off values determined by ROC analysis and Kaplan–Meier curves were compared for the different groups. In the group with a preoperative mPAP exceeding 46 mmHg six patients died versus one patient in the group with an mPAP les than 46 mmHg (log rank test, p = 0.03; Fig. 2a ); and in the group with a PVR exceeding 645 dynes s cm−5 six patients died versus one patient with a PVR less than 645 dynes s cm−5 (p = 0.006; Fig. 2b).

Kaplan–Meier curves according to the optimal cut-off value derived by ROC analysis for pulmonary vascular resistance (PVR; panel a), mean pulmonary artery pressure (mPAP; panel b).
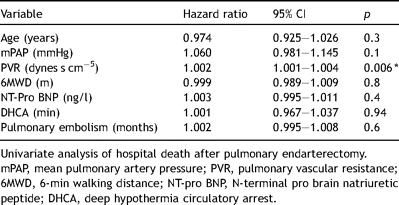
Univariate analysis revealed that PVR is the only factor associated with an increased risk of death (Table 5 ). The hazard ratio for a 50 dynes s cm−5 difference in PVR 1.11 (95% CI: 1.00–1.22). Mortality was not associated with age, mPAP, 6MWD, NT-pro BNP and time of DHCA.
5 Discussion
The present study demonstrates that the short- and long-term outcome and survival after PEA is very satisfying, with a very good long-term survival. Persistent pulmonary hypertension is not seen very frequently. Furthermore, we showed that preoperative cut-off values of PVR and mPAP are very good predictors of survival.
Increasing number of centers in the world are performing this technically demanding surgery with good to excellent results in terms of clinical outcome and survival. The study from Japan by Matsuda et al. in 102 patients showed a hospital mortality rate of 7.8% and 3- and 5-year survival rates of 90.9% and 84% respectively [18]. The recent study from a national referral centre in the UK showed an in-hospital death rate of 21% in 291 patients with 3- and 5-year survival rates of 74.6% and 72.7% respectively [17]. Corsico and colleagues showed in a very recent study in 157 patients a hospital mortality of 11.5% and a cumulative survival of 84% at 5 years [16]. Our results, with an in-hospital death rate of 6.9% and an actuarial 5-year survival rate of 93.1%, are similar with these recent published studies. As in other studies, we observed a learning curve [9,16]. The study by Jamieson and colleagues showed a decline in mortality from 8.8% (between 1994 and 1998, n = 500) to 4.4% (1998–2002, n = 500). In our study, all hospital deaths occurred in the early years. Since 2004 only one patient of the 38 operated died (2.6%). In our opinion several factors have contributed to this good survival. As patient selection remained grossly unchanged, pretreatment of high risk patients, improved surgical experience in an operation consistently performed by the same two surgeons, and improved postoperative care contributed to our almost zero mortality over the recent years.
Previous studies have suggested that the treatment of high-risk patients to reduce PVR as a bridge to surgery may have a positive influence on survival [19,20]. This study, however, was not randomized and therefore it is not possible to relate successful surgery to the use of preoperative IV prostacyclin. It can be speculated whether preoperative treatment with vasodilating agents is useful while the obstructing material is still in place. In our institution, patients are pretreated with a PDE-5 inhibitor or endothelin-receptor antagonist while they are on the waiting list for surgery. The rationale for this is that these oral disease modifying drugs have a potential beneficial effect on PVR. It is speculated that, although the exact mechanism is unclear, it reduces the vascular remodeling in the distal pulmonary vasculature.
Nonetheless, in the end the key to success remains the removal of all obstructing material.
Furthermore, patients with CTEPH and splenectomy, chronic intravenous catheters or chronic inflammatory disease were considered not suitable for operation because of reported bad results in the literature during the last years [15]. The death of the first three patients was related to reperfusion pulmonary edema. The frequency of reperfusion pulmonary edema showed a progressive decline due to aggressive maintenance of a negative fluid balance and early extubation.
We have tried to identify predictors of postoperative hospital mortality retrospectively. The univariate analysis showed that mPAP and PVR are good predictors of hospital mortality. Until now a few studies have studied the risk factors for perioperative mortality and found NYHA functional class, age older than 70 years, degree of raised RA pressure and duration of PH to be associated with increased risk of postoperative mortality [8,9,14,21,22]. The study by Ogino et al. showed that an age more than 60 years to be significantly associated with hospital mortality [23]. Beside these factors, other studies showed that preoperative PVR is an important predictor of hospital mortality. These studies have shown that a preoperative PVR exceeding 1000 till ∼1150 dynes s cm−5 have a adverse influence on hospital survival [8,9,14,24]. The present study found a preoperative cut-off value of 645 dynes s cm−5 to be discriminative between high and low risk mortality. This can be explained by the lower average PVR in our study compared to previous studies. Although patients with a high preoperative PVR do worse, mortality is strongly related to the postoperative PVR. These patients with high residual PVR have most probably inoperable secondary vasculopathy [9]. Additionally, we found that a mPAP of more than 46 mmHg was associated with a high probability of hospital mortality. The cut-off value for mPAP of 46 mmHg in our study is similar to the cut-off value of 50 mmHg in the study by Hartz et al. [8].
A limitation of this study is its retrospectively observational design. The small number of events made it impossible to identify predictive factors with multivariate analysis. The result of NT-pro BNP and 6MWD should be interpreted cautiously as these data were not complete in all patients.
In conclusion, PEA can be performed safely with very satisfying long-term outcome and survival adding more evidence to the current literature about the beneficial outcome after PEA. Furthermore, PVR and mPAP can be indeed used as preoperative risk stratification factors for surgery.
Presented at the 22nd Annual Meeting of the European Association for Cardio-thoracic Surgery, Lisbon, Portugal, September 14–17, 2008.
N. Saouti: Financially supported by the Netherlands Organisation for Scientific Research, Mozaiek grant, project number 017.003.019.
References
- thromboembolic pulmonary hypertension
- brain natriuretic peptide
- follow-up
- hospital mortality
- netherlands
- surgical procedures, operative
- survival rate
- heart
- surgery specialty
- pulmonary vascular resistance
- walking distance
- pulmonary artery endarterectomy
- 6-minute walk test
- pulmonary artery mean pressure




