-
PDF
- Split View
-
Views
-
Cite
Cite
Mehmet Kayrak, Osman Sonmez, Mehmet A. Vatankulu, Mehmet S. Ulgen, Completely asymptomatic proximal aortic dissection and massive bullous lung disease: coincidence or is there any etiologic link?, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 6, June 2009, Pages 1102–1104, https://doi.org/10.1016/j.ejcts.2009.02.022
Close - Share Icon Share
Abstract
This case report focuses on a completely asymptomatic proximal aortic dissection in a middle-aged male smoker with bullous lung disease. The possibility of a relationship between A1-antitrypsin (A1AT) deficiency and aortic dissection is discussed in light of the recent data.
1 Introduction
Aortic dissection (AD) is the most common disease of the aorta and requires urgent diagnosis and treatment. Although it is a rare condition, a misdiagnosis or patient-related diagnostic delay can lead to devastating results. It is known that the mortality rate for AD increases 1–3%/h, reaching 70% after 48 h [1]. Bullous lung disease (BLD) is an uncommon cause of respiratory distress and includes emphysematous and nonemphysematous morphology [2]. The authors were presented with a case of an asymptomatic patient with a giant right apical bulla occupying nearly one-third of the right hemithorax, along with acute proximal aortic dissection
2 Case report
A 42-year-old asymptomatic male consulted the family physician on ways to quit smoking. The physician examined routine laboratory tests, including a lipid panel, hemogram, and PA chest radiogram. The chest radiogram revealed the absence of apical bronchoalveolus on the right side. The patient was referred to the chest surgery clinic for advanced diagnostic assessment. A thorax computed tomography (CT) was planned for the first step. The thorax CT revealed a giant apical bulla occupying nearly one-third of the right hemithorax, with normal parenchyma and ascending aorta evaluated as normal (Fig. 1 ). The patient was assessed by a cardiologist before noncardiac surgery. He was asymptomatic and had no risk factors for coronary artery disease, AD, or BLD except for smoking and gender. There was no history of intermarriage or Marfan syndrome (MS) or AD among his close relatives. Physical examination found right and left arm blood pressure to be 110/70 mmHg and 115/70 mmHg, respectively. A severe holodiastolic murmur could be heard in the mesocardiac area. His height of 173 cm, body weight of 75 kg, and arm span of 176 cm fell within normal percentiles. He had no arachnodactyly or ectopia or subluxation of lentis. ECG was normal.
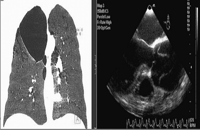
Frontal thorax CT view of lung and TEE image show dilated aortic root and dissection.
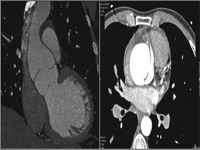
Because aortic regurgitation was suspected, transthoracic echocardiography was performed and revealed severe aortic regurgitation, aortic root dilatation, and a dissection flap in the ascending aorta. Left ventricular systolic function and end systolic and diastolic dimensions were normal. Ejection fraction was calculated at 65% by modified Simpsons’ method. Transesophageal echocardiography (TEE) performed to confirm the diagnosis of AD revealed a type A dissection flap beginning at right sinus valsalva level and terminating at the upper region of the ascending aorta (Fig. 1). Multislice CT was performed to verify the borders of the dissection (Fig. 2 ). The results of multislice CT were consistent with TEE. Patient’s laboratory test results were normal, including A1AT levels: 120 pg/ml (60–200 pg/ml). PiMM homozygous genotype was detected for A1AT by PCR method.
The patient was referred to surgery. A stapling resection for bulla and Bental procedure for AD were successfully performed in one session. The patient was discharged five days later. Smoking cessation and pulmonary rehabilitation were recommended to the patient.
3 Discussion
Most patients with AD suffer from severe chest pain. Among young patients under the age of 40, hypertension and atherosclerosis are rarely seen as risk factors; however, MS, a bicuspid aortic valve, and prior aortic surgery are commonly seen [3]. The prevalence of silent aortic dissection is unknown. Original asymptomatic cases are very rare. Silent (asymptomatic and uncomplicated) aortic dissection was first discovered by Khan in a patient with MS [4]; however, the present patient had no Marfanoid habitus or other characteristics such as archnodactyly and ectopia or subluxation of lentis. Although MS is an autosomal dominant disorder of connective tissue, the patient had no history of MS among close relatives
Bullous lung disease is an uncommon cause of respiratory distress. In patients with severe emphysema, discrete emphysematous bulla have been shown to functionally impair pulmonary mechanics and result in diminished exercise capacity and even acute respiratory distress. BLD is characterized by the formation of blebs, bullae, and emphysema. The term bulla is used to describe an air-filled space within the lung parenchyma resulting from deterioration of the alveolar tissue [2]. Most patients with bulla have a significant history of cigarette smoking, although cocaine smoking, pulmonary sarcoidosis, A1AT deficiency, A1 antichymotrypsin deficiency, Marfan syndrome, Ehlers-Danlos syndrome, and inhaled fiberglass exposure has been shown to be associated with emphysematous lung bullae [2]. Smoking causes the development of bullous emphysematous or nonemphysematous pulmonary parenchymal pathologies even with no respiratory complaint. The patient had no Marfanoid habitus; thus we emphasized cigarettes and A1AT deficiency, which are etiologic risk factors. Cigarettes and A1AT deficiency have been recognized as causes of bullous lung disease in numerous studies [5].
The A1AT protein is encoded by the protease inhibitor (PI) locus located on chromosome 14q32.1. Normal levels of A1AT protein protect the tissues against enzymatic degradation by neutrophil elastase, and normal serum levels of A1AT are associated with the M allele, with reduced levels commonly associated with the S and Z alleles. Z mutation leads to misfolding of the protein, with accumulation in liver cells and severely reduced plasma A1AT levels [6]. A1AT is an important serum protein that limits the activity of a number of proteinases in tissues, and its deficiency is well known to promote connective tissue degeneration, most prominently in the lung. A1AT deficiency is a genetic and systemic disorder characterized not only by lung and liver disease but also by vascular manifestations, including spontaneous cervical artery dissection, fibromuscular dysplasia, and aneurysms [7]. In AD, however, cigarettes and A1AT deficiency have not been clearly defined in current publications, but few data have demonstrated a relationship between A1AT and endothelial cell apoptosis [8]. Blair et al. described an acute thoracic aortic dissection occurring 18 months following lung transplantation for α1-antitrypsin deficiency emphysema [9]. Ogushi et al. pointed out the importance of A1AT deficiency in the time-dependent kinetics of smokers’ lung by comparing the risk of developing emphysema among cigarette smokers with normal A1AT levels to the emphysema risk among individuals with the A1AT phenotype MZ [10]. MZ heterozygotes who do not smoke are not at greater risk of emphysema than nonsmoking MM homozygotes, despite their markedly lower levels of A1AT. In obvious contrast are MM individuals who smoke, in whom the risk of emphysema is significantly increased. This observation highlights the importance of diminished function of A1AT in MM phenotypic patients who smoke, as in our case [10]. Interestingly, it has been shown that circulating levels of A1AT range from 10% of normal in homozygous (A1AT phenotype PiZZ) to 60–70% of normal in heterozygous (A1AT phenotype PiMZ) individuals [7]. Cigarette smoking decreases the efficacy of A1AT, resulting in a situation similar to genetically determined A1AT deficiency. We speculate that A1AT deficiency plays a role in the etiopathology of both diseases.
A1AT may represent a novel etiopathological mechanism relevant to disease processes characterized by excessive structural endothelial cell apoptosis and damage, oxidative stress, and inflammation by activating proteolytic enzymes such as bullous lung disease and aortic dissection.
4 Conclusion
No common original cause to explain both diseases was discovered; however, considering the recent data, we conclude that cigarette smoking may be the cause of both diseases, resulting in A1AT deficiency in the time-dependent kinetics of smokers’ lung, inhibiting other proteolytic enzymes, and thus causing endothelial apoptosis and damage. In our opinion, both asymptomatic giant bullous lung and proximal AD can contribute to our knowledge of the morphology and clinical and etiopathologic spectra of both diseases.




