-
PDF
- Split View
-
Views
-
Cite
Cite
Elisa Meacci, Alfredo Cesario, Stefano Margaritora, Venanzio Porziella, Adele Tessitore, Giacomo Cusumano, Amelia Evoli, Pierluigi Granone, Thymectomy in myasthenia gravis via original video-assisted infra-mammary cosmetic incision and median sternotomy: long-term results in 180 patients, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 6, June 2009, Pages 1063–1069, https://doi.org/10.1016/j.ejcts.2009.01.045
Close - Share Icon Share
Abstract
Objective: The clinical outcome of 180 non-thymomatous myasthenia gravis (MG) consecutive cases surgically treated is reported herein. The original surgical access, consisting of a video-assisted infra-mammary cosmetic incision and median sternotomy, has originally been designed and described by our group. Methods: The in-hospital patients’ charts and the outpatients’ clinic follow-up information of the 180 cases have been extensively reviewed. In addition to the strictly surgical benchmark referral, data on the rate of cure of the MG (complete stable remission – CSR; pharmacological remission – PR) as indicated by the Myasthenia Gravis Foundation of America (MGFA) have been analysed as recorded at the 12 months after surgery checkpoint. Cosmetic outcome was evaluated as well. Results: Female to male ratio was 156 (86.7%):24 (13.3%). Mean age: 29.1 ± 10.9 years. Preoperative MGFA score: stage I: 4 patients (2.2%); IIa: 57 (31.7%); IIb: 32 (17.8%); IIIa: 41 (23.3%); IIIb: 42 (23.3%); IVa: 2 (1.1%); V: 2 (1.1%). Median operative time was 110 min (70–130 min) and median postoperative hospital stay was 4 days (3–10 days). Postoperative mortality was nil and morbidity occurred in seven patients (3.8%). Final pathology was consistent with: 146 hyperplastic thymus (81.1%); 28 involuted thymus (15.6%) and 6 normal thymus (3.3%). Ectopic thymic tissue was found in 68% of the patients. Mean follow-up was 62.9 ± 34.6 months. A CSR was obtained in 55%; PR in 18.3%; improvement in 39.9%, unchanged in 3.5%, worse in 1.1% and died in 0.5%. Kaplan–Meier estimates of CSR were 34.1% and 75.8% at 5 and 10 years, respectively. The preoperative therapy was the only parameter significantly associated with Kaplan–Meier CSR rates (univariate analysis – p < 0.001). Remarkably, 171 (95%) patients judged their cosmetic results to be excellent or very good. Conclusions: Thymectomy in MG patients via video-assisted infra-mammary cosmetic incision and median sternotomy has shown to be a useful surgical approach as demonstrated by the good functional and very good aesthetic results, associated with a very low morbidity and no mortality. Patients with preoperative mono-therapy have higher CSR rates. CSRs are durable, as the CSR rate improves with extended follow-up.
1 Introduction
Myasthenia gravis (MG) is a rare autoimmune disorder occurring predominantly in young women. The thymus gland plays a central role in the pathogenesis of the MG and thymectomy is recognised as an effective surgical therapy in MG, complementing medical therapy. It is well known that MG is frequently associated with thymic abnormalities such as thymoma in 10–15% of patients and follicular hyperplasia in 60–65%. The hyperplastic thymus is considered to be the site of immunisation against acetylcholine receptors (AChRs) and a source of anti-AChRs antibodies, while the pathogenic role of thymoma remains less clear [1]. Thymectomy is considered a mainstay therapy for MG (commonly accepted but, so far, with no clear evidence based proof of concept), leading to clinical improvement in 70–80% of cases and to clinical remission in 33–38% of cases [2]. The first report of positive clinical improvement after thymectomy in MG patients was by Blalock et al. in 1939 [3]. With time, thymectomy gained widespread acceptance in the treatment of MG in association with medical treatment (immunosuppression/plasmapheresis). Several surgical accesses have been adopted during the past 80 years to carry out a thymectomy, these being divided into two major categories: trans-sternal or trans-cervical, or a combination of both. In recent years some video-assisted minimally invasive thoracoscopic approaches have been reported. Independently of the surgical approach, an extra-capsular radical resection of the thymus is ideally performed; this includes the mediastinal fatty tissue, with sharp dissection from the pericardium. This is supported by the fact that (a) thymic tissue left after an incomplete thymectomy often leads to persistence or aggravation of the disease and (b) gross and microscopic foci of thymic tissue are widely distributed in cervical and mediastinal fat outside the thymic gland and can be found in 39.5–98.0% of patients undergoing thymectomy. These and other important thymectomy-related issues have been extensively described by Jaretzky and Wolff [4]. Aside from the completeness of data supporting the surgical indication and the level of completeness of the thymectomy, a substantial lack of clear evidence of the advantages of one approach with respect to the others (no properly controlled trials available) leaves the discussion forum on this topic alive and kicking; in fact a certain degree of optimal to suboptimal value has been demonstrated by the vast majority of the proposed surgical techniques. The functional and cosmetic impact (drawback) of the total sternotomy is a clear issue when thymectomy is planned via an open sternotomic approach. By the way the total sternotomy is clearly the type of approach that gives the best access to those mediastinal regions otherwise surgically reachable with great difficulty. In 1993, with the double aim of achieving the best and optimal mediastinal exposure via an open sternotomy and to obtain a more than acceptable cosmetic outcome we have designed an original technique (published in 1999 [5]) we refer to as ‘video-assisted infra-mammary cosmetic sternotomic thymectomy’. This was inspired by the work of Orringer as described in [6]. Herein we report our 13-year experience with this approach in 180 consecutive non-thymomatous MG patients. Long-term data after thymectomy are scarce so this long-term study was designed to:
validate our technique,
to identify the criteria which stratify patients into the various degrees of clinical response to thymectomy and
to detect those prognostic factors potentially useful for the prediction of survival and postoperative outcome; of course, in this setting, special attention has been put to MG-related symptoms.
2 Materials and methods
This is a retrospective evaluation of data stemming from normal routine clinical behaviour. No additional ethical approval was sought for at the institutional review board. At the moment of surgery all patients have given their informed consent for, among the other procedures, the use of data for research purposes. In the period from January 1993 to December 2005, 195 patients with non-thymomatous MG (NTMG) underwent thymectomy via video-assisted infra-mammary cosmetic sternotomic thymectomy in our division. For the homogeneity of the cohort only data coming from the charts of patients with definitive pathology histologically confirmed NTMG were included in this study. Preoperative clinical staging was assigned according to the guidelines of the MGFA clinical classification system [7]. Criteria for the assessment of the postoperative status during the follow-up have been referred to the definitions given in the MGFA clinical research standards (http://www.myasthenia.org/docs/ClinicalResearchStandards.pdf), and, as well, reported previously [7] (Table 1 ). These were assessed at the end of a minimal period of 12-month follow-up. No criteria for patients’ exclusion were met except in 15 cases: in 9 preoperative data were incomplete and 6 patients did not show up at the follow-up scheduled outpatient visits because they referred themselves elsewhere. Finally, the clinical outcomes of 180 cases have been considered for the purpose of this analysis. The follow-up, including a cosmetic evaluation, has been obtained through outpatient clinic visits and phone conversations. The mentioned MGFA criteria have been extensively adopted for the assessment of any of the significant clinical outcomes evaluated (treatment and surgery related morbidity, mortality and, as already stated, post-interventional status at follow-up).
2.1 Surgical technique and procedure [5]
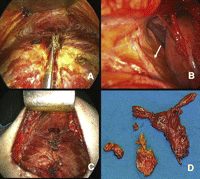
The patient’s position is illustrated in Fig. 1 . A wide cutaneous-subcutaneous flap is created superiorly to the notch, inferiorly to the linea alba and laterally to the hemiclavear lines. The periosteum of the sternum is cut with the electrocautery along the middle line and the posterior face of the bone is prepared by means of digital manoeuvre. A total median sternotomy is accomplished, the sternum is cut from the xyphoid upward using an orthopaedic chisel, a small retractor is placed and the two halves are separated (Fig. 2A ). The incision of the thyroid’s muscle sternal insertion and the cervical fascia completes the exposure of the anterior mediastinum. Once the pericardial fat has been dissected, the area of the left pleuro-pericardial angle is explored and all the fatty tissue, to the exposition of the left phrenic nerve (arrow), is dissected (Fig. 2B). The thymus is then separated from the left brachio-cephalic vein, with the surrounding fatty tissue; the left upper pole of the thymus is set free by traction of serially applied clamps and gauze-pledge dissection, after the section of the Keynes’ veins. The same procedure is performed on the right side. The thymus is removed en bloc with all the mediastinal fatty tissue (Fig. 2D). Pleural membranes ideally remain un-open. The cutaneous-subcutaneous flap, which interferes with direct vision, affects the preparation of the phrenic nerves, the thymic veins and the gland’s upper poles. Then, for these steps and for the fatty tissue radical dissection, the use of the thoracoscope proves to be really helpful. In fact, through the use of the thoracoscope the anatomic structures are enhanced and dissection manoeuvres can easily be performed with the usual surgical instruments while looking at the monitor. Pleural drainage is not used unless the pleurae are inadvertently entered; single mediastinal drainage is employed. The sternum is routinely closed using re-absorbable sutures (Fig. 2C). The subcutaneous flap is obliterated and a tight dressing is put after the suture of the cutaneous incision to prevent the formation of seromas. A movie of the operation can be viewed at www.rm.unicatt.it/timectomia.
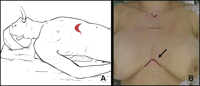
(A and B) Patient’s position. (A) The patient is supine with the arms resting along the body and the head hyperextended. (B) The cutaneous incision is horizontal, with a length of 5–6 cm, lightly upward curvilinear, 4–5 cm above the xyphoid (arrow).
The ad hoc policy of postoperative care for MG patients in our institution is to monitor them during a 24 h intensive care unit (ICU) recovery. Stabilised patients are thereafter transferred to normal care ward and discharged from the hospital 1 day after the removal of the mediastinal drainage and radiological control (chest X-rays). The assessment of the therapeutic response to surgery has been determined by comparing the preoperative clinical status with the status recorded at the first follow-up check visit and classified again according to the MGFA post-intervention status [7]. Mean follow-up was 62.9 ± 34.6 months. Taking into account the duration of the follow-up, the CSR rate was calculated using the Kaplan–Meier method. The univariate analysis of variables potentially affecting CRS including:
age at operation (<40 years/>41 years)
gender
preoperative disease duration (<12 months/>13 months)
preoperative therapy and
histology
Histology was calculated using the Kaplan–Meier method and the survival curves were compared using the log-rank test [SPSS (version 13, SPSS Inc., Chicago, IL)]; a p value <0.05 was considered as statistically significant. The cosmetic outcome was assessed administrating the patients a single five items questionnaire:
excellent;
very good;
good;
poor;
very poor.
3 Results
Female to male ratio was 156 (86.7%):24 (13.3%). Mean age: 29.1 ± 10.9 years. Preoperative MGFA score: stage I: 4 patients (2.2%); IIa: 57 (31.7%); IIb: 32 (17.7%); IIIa: 41 (22.8%); IIIb: 42 (23.3%); IVa: 2 (1.1%); V: 2 (1.1%). Mean preoperative duration of symptoms was 22.9 ± 24.7 months. The preoperative medical treatment regimen consisted of single drug therapy in 87 patients (74 pyridostigmine [PYR] and 13 steroids [ST]). Two-drug or two-drug multi-strategy based regimens: 63 patients (PYR/ST or PYR/plasmapheresis or PYR/azathioprine [AZ]). Three-drug or three-drug multistrategy regimens: 20 patients (PYR/ST/plasmapheresis or PYR/ST/AZ). Four-drug or four-drug multi-strategy regimens: eight patients. Two patients underwent surgery without any previous medical treatment.
3.1 Surgical findings
Median operative time was 110 min (range 70–130 min). Median postoperative hospital stay was 4 days (range: 3–10). Mediastinal drainage was left in site 3 days (range: 2–5). Intra- and postoperative 30-day mortality was nil. No procedure was converted (skin incision enlarged). Tracheostomy was never necessary. Postoperative morbidity occurred in seven patients (3.8%) and consisted of: one haemothorax treated by chest drainage placement, two cases of pneumothorax after mediastinal drainage removal, two wound suppuration and two subcutaneous haematomas. All complications were treated conservatively (did not require surgical re-intervention).
3.2 Pathology
The median weight of the specimen was 73 g (range 25–185 g). Histologic diagnosis was: hyperplastic thymus in 146 patients (81.1%); involuted thymus in 28 (15.6%) and normal thymus in 6 (3.3%). Foci of ectopic thymic tissue (confirmed as containing the Hassal’s corpuscles) were present in 68% of specimens, location being prevalent in the perithymic fat (45%).
3.3 Clinical outcome
The overall CSR rate was 55.0% (99 patients); 66 patients (36.6%) resulted asymptomatic off/medication and 33 (18.3%) experienced a pharmacological remission (PR). Seventy-two patients (39.9%) improved and six (3.5%) remained no change, two (1.1%) got worse and one (0.5%) died of MG. The CSR rate calculated using the Kaplan–Meier method taking into account the duration of the follow-up is shown in Fig. 3 . The Kaplan–Meier analysis showed CSR rates of 34.1% (C.I.: 26.5–41.7) and 75.8% (C.I.: 66.6–85), at 5 and 10 years, respectively. No patient suffered relapse after an initial CSR. Survival curves were compared using the log-rank test according to the variables gender (p = 0.378), duration of disease <12 months/>13 months (p = 0.343), age at surgery <40/>41 (p = 0.314), histology (p = 0.607) and preoperative MGFA class (p = 0.586) (Fig. 4 ). The only factor associated with a statistically significant probability of CSR was the preoperative therapy (p < 0.001). No differences in inducing postoperative CSR have been detected when the match was set for preoperative use of steroids or pyridostigmine alone (Fig. 5 ). Regarding patient’s cosmetic satisfaction, 171 (95%) judged their cosmetic results to be excellent or very good; 6 (3.4%) good, 2 (1.1%) poor and 1 (0.5 %) very poor (Fig. 6 ).
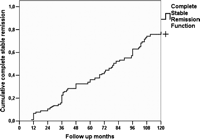
Kaplan–Meier curve for time to complete remission (CSR). The CSR rate in our study group was only 7% at the end of the first year after surgery but increased to 34% at the end of the fifth year. A further increase of the CSR rate to 76% by the end of the tenth year was seen.
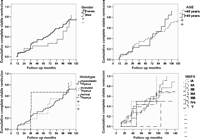
Univariate analysis results for influence of various factors on 5-year and 10-year Kaplan–Meier (KM) complete stable remission (CSR).
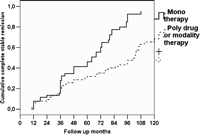
CSR according to preoperative therapy. Single drug preoperative therapy with pyridostigmine or steroids showed a better chances of CSR than multi-drug therapy (p < 0.001).

4 Discussion
Since the first report by Blalock et al. [3], thymectomy has been widely accepted for the treatment of patients with MG. Still, no clear indication of which patient should undergo surgical treatment exists, along with a clear criterion to judge the best surgical approach. Many surgeons advocate minimal procedures to reduce morbidity and others are in favour of a more radical approach that provides a better mediastinal exposure, and, as such, allows the complete removal of the gland and all the mediastinal fatty tissue at the price of a greater morbidity (and mortality). Although a radical ‘maximal’ thymectomy has been advocated by investigators such as Masaoka et al. [8], this approach remains unpopular among surgeons routinely performing thymectomy, as clearly stated by Jaretzki and Wolff [4] in 1988.
According to the prevalent indications in the English literature, thymectomy may be effectively carried out within the setting of the therapeutic approach to MG, by (a) median sternotomy [9] (with or without a transversal cervical extension), (b) partial sternotomy [10], (c) trans-cervical incision [11], (d) trans-cervical incision combined to partial sternotomy [12,13], and more recently, (e) video-assisted thoracoscopy [14,15]. A comparison of the data reported in the literature clearly shows that the maximal thymectomy is the most radical and effective approach for thymectomy in MG, but an extended thymectomy through a sternotomic approach without cervicotomy allows similar results to be obtained. Controversies are raised, however, regarding the extensiveness and completeness (defined as the removal of the whole thymus gland and mediastinal fatty tissue) when less invasive techniques are adopted. On the basis of the reported evidences we have considered a median sternotomy as the approach to be preferred in NTMG patients. In 1992, Orringer [6] described an access to the anterior superior mediastinum performed through a 5 ± 1 cm curvilinear smile incision just below the sterno-manubrial junction and a partial upper sternal split. From Orringer’s report we derived the idea of moving few centimetres down the line of incision and to create a subcutaneous flap to perform a complete median sternotomy and obtain a valuable cosmetic result. The aid of video-thoracoscope in the dissection of the upper region of the anterior mediastinum is added because the flap affects the direct vision of the area. The upper mediastinum and the lower neck regions can be well examined because of high resolution and magnification of the current image processing video systems. The brachiocefalic vein can be easily identified in the upper mediastinum and traced back to its conjunction with the superior vena cava by meticulous dissection of the thymus; the intervening thymic veins can be easily divided between endoclips. With this procedure we have a clear vision of the entire thymus and the pericardial adipose tissue along both phrenic nerves; dissection can be performed safely. In addition, the thoracoscopic aid avoids potential pitfalls associated with trans-cervical thymectomy such as the crowding of instruments into a narrow access incision and restricted viewing of the operative field. Total sternotomy allows an excellent exposure and diminishes the likelihood of re-exploration due to persistent refractory symptoms.
The kind of view that is in fact given by total sternotomy is the best available for adequate bilateral mediastinal fatty tissue dissection; this is the truest due to the fact that a correct surgical plan implies the removal of all the bilateral pericardial fat tissue lying among the phrenic nerves that can be easily removed under direct and toracoscopic view. As far as the adequacy (adequate extent) of thymectomy is concerned, we believe this can be best reflected by the weight of the resected specimen. Using the thoracoscopic aided approach, we had an average specimen weight of 73 g (range: 25–185 g). We advocate that the removal be deemed unsatisfactory whenever the thymus gland is torn by surgical traction and when the gland is withdrawn in pieces. In fact at present, persistence of symptoms in patients with MG who have undergone previous thymectomy has been directly and clearly attributed to thymus remnants. This is a long discussed issue: in 1983, Rosenberg and colleagues [16] reported that 85% of the thymic tissue was found in patients who underwent re-operation by trans-sternal approach and 67% of them improved clinically after the second procedure. Scott and Detterbeck [17] reported good results (remission or improvement of symptoms in 78% of cases) with no mortality on the total median sternotomy approach, with the opening of both pleurae in 100 consecutive patients. The advantage of the bilateral opening of the pleurae is commonly believed to lie in the fact that it provides extremely good visualisation of the phrenic nerves and surrounding region; this therefore decreases the probability of neural damage and allows for an extensive (complete) resection of the fat adjacent to the thymus. Conversely we believe that systematic opening of the pleura is not required, our belief being corroborated by long-term validated results (a comparison of our results with those reported by other authors using more invasive techniques shows that the remission rate in our series is consistent with the referral estimates reported in literature [Table 2 ]). On the other hand, the systematic opening of the pleurae may increase the incidence of postoperative pleural complications [10] and certainly increases the pain resulting from the drainage of the pleural spaces. It is clear that an approach without pleural opening allows continuous bilateral ventilation. This is true, as well, when considering the advantages of the approach we propose matched against the unilateral thoracoscopic ones where single lung ventilation (sometimes for long periods) is required. Our technique is characterised by a very low morbidity (3.8%) and no mortality; postoperative course can be further optimised by the adoption of innovative rehabilitation techniques currently being investigated; furthermore, the cosmetic outcome we obtained was clearly valued by patients when directly questioned about their perception. With regard to factors influencing the outcome of the patients undergoing thymectomy, the question regarding which is the most significant remains, despite a comprehensive analysis of data stemming out from our series with no clear answers (as in Nieto’s one [9]). In fact, no gender-related differences in achieving CSR existed (p = 0.31) and patients with an earlier onset age (<40/>41) (p = 0.32) and preoperative duration of symptoms (<12 months/>13 months) (p = 0.60) had the same chances of achieving remission after surgery than the others. By contrast, Masaoka et al. [8] demonstrated that young age (<35 years) and preoperative symptom duration (<24 months) were favourable prognostic factors (along with absence of thymoma). On this line the report from Huang et al. [21] showed that the younger group (35 years or less) had a significantly better CSR.
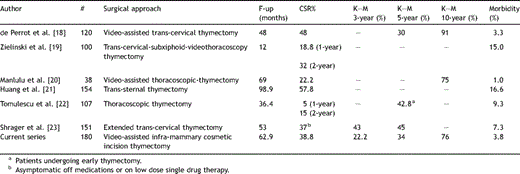
Although some high rates of remission have been reported in patients affected by hyperplasic thymus [24,25], our results did not point to any advantage for this histologic finding: this is in line with similar results as reported by Jaretzki and Wolff [4] and Tomulescu et al. [22]. Undoubtedly the heterogeneity used by the different authors and groups in the classification (clinical and, to a significant extent, histological) introduces a bias in the interpretation of the results rendering a final precise assessment rather difficult. In fact, the clear effect (bias) introduced by the use of non-standard classification systems becomes readily apparent in (a) clearly establishing coherent classes of initial stage of disease at the moment of surgery and (b) assessing a coherent definition of specific outcomes as in the case of CSR which is, instead, a criterion detailed and precise in its characteristics. This bias being defined, however, it is possible to identify in the preoperative medical treatment a common positive (and significant) indicator of CSR. The patients included in the analysis described in this report underwent a preoperative medical treatment regimen consisting of diverse regimens (see Section 2 for details). Those undergoing single drug therapy (corticosteroids or pyridostigmine alone) showed a better outcome (p < 0.001) when compared with multidrug therapy; however, the preoperative MGFA class was not significantly correlated with the clinical outcome (p = 0.18). These findings are not in line with the evidence reported by Huang et al. [21], where patients without steroid treatment before operation had a better complete remission rate, though it was not significant in multivariate analysis elaborated by the author [21]. Finally we underline the optimal cosmetic outcome obtained in our series, which adds up to a completely uneventful postoperative course with regards to the wound healing (including sternum closure) in our series. Of course this was particularly appreciated by young female patients.
5 Conclusions
Thymectomy in MG patients performed with our original video-assisted infra-mammary cosmetic incision and median sternotomy has shown to have overall functional results (including morbi-mortality) in line with those referred as the standard in the prevalent literature. Patients with preoperative mono-therapy have higher CSR rates. CSR are durable, as the CSR rates improves with extended follow-up. The cosmetic outcome was excellent.
Presented at the 16th European Conference on General Thoracic Surgery, Bologna, Italy, June 8–11, 2008.
References
- patient referral
- myasthenia gravis
- benchmarking
- esthetics
- follow-up
- hyperplasia
- infectious mononucleosis
- outpatients
- preoperative care
- surgical procedures, operative
- thymectomy
- morbidity
- mortality
- pathology
- pharmacology
- thymus gland
- treatment outcome
- sternotomy, median
- cell cycle checkpoint
- univariate analysis
- disease remission




