-
PDF
- Split View
-
Views
-
Cite
Cite
Jeffrey H. Shuhaiber, Sieuw Yen Ho, Michael Rigby, Babulal Sethia, Current options and outcomes for the management of atrioventricular septal defect, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 5, May 2009, Pages 891–900, https://doi.org/10.1016/j.ejcts.2009.01.009
Close - Share Icon Share
Summary
A wealth of experience has been gained in the management of atrioventricular septal defect (AVSD) since the first complete correction of this malformation in 1955. The success of surgical therapy followed an enhanced understanding of morphology and physiology as well as major improvements in imaging of this congenital heart defect. Therapeutic success in the management of patients with AVSD has been extended to include those with associated lesions such as tetralogy of Fallot, double outlet right ventricle and relative degrees of ventricular hypoplasia. Although operative mortality is low and long-term survival is relatively good, important detrimental residual or AVSD-related complications such as left atrioventricular valve regurgitation, left ventricular outflow tract obstruction still carry significant late morbidity in a proportion of patients. This article reviews our current understanding of the morphology of this defect, aspects of diagnosis and surgical treatment options.
1 Introduction
When Becker and Anderson published their editorial concerning atrioventricular septal defects ‘What’s in a name?’ they chose to dismiss the terms endocardial cushion defect, atrioventricular defect and atrioventricular canal defect on the basis that they were morphologically unsound and inaccurate [1]. Although it was their intention to provide a precise and accurate way of describing hearts with an atrioventricular septal defect (AVSD) the terms continued to be used imprecisely by some.
2 Morphological aspects
2.1 Basic features
The basic morphological features that are present in all hearts are deficiency of the atrioventricular septum and a common atrioventricular junction guarded by a common atrioventricular valve in the setting of biventricular atrioventricular connections. In most cases the valve guarding the common atrioventricular junction consists of five leaflets: superior (or anterior) and inferior (or posterior) bridging leaflets, each of which overrides the interventricular septum and has chordal attachments to both ventricles, a left mural leaflet, a right mural and a right antero-lateral leaflet. There are two common variations: those with a common valvar orifice (so-called complete form) and those with separate right and left valve orifices (so-called partial or incomplete form, or ostium primum ASD). In a partial AVSD a tongue of tissue joins the otherwise free distal margins of the superior and inferior bridging leaflets dividing the common valve into two valves, although retaining continuity between the left and right sides of the bridging leaflets. The trileaflet valve, left atrioventricular valve bears no resemblance to the mitral valve. The so-called cleft in the mitral valve is merely the line of apposition between the superior and inferior bridging leaflets at their septal attachment. This may or may not be the site of left atrioventricular valve regurgitation. Frequently, the line of apposition is at an acute angle rather than perpendicular to the septum. The papillary muscles are in antero-superior and postero-inferior relationships instead of the diagonal relationship of the antero-lateral and postero-medial locations in the normal heart.
Different to the normal conotruncal morphology, the aortic valve in an AVSD, is located more anteriorly in an un-wedged position. Measurements made in heart specimens have shown disproportion between the inlet and outlet length in the left ventricle. The inlet length is approximately 0.7 less than the outlet length [2].
2.2 Variations in valvar orifice and leaflet attachments
While there are two major categories in the arrangement of the common atrioventricular valve (common orifice and separate orifices), hearts with AVSD have further variations in attachments of the valvar leaflets. The latter will determine the levels of shunting through the AVSD.
In so-called complete form of AVSD there is usually an inter-atrial communication (primum component) between the inferior margin of the true atrial septum and the atrial surface of the common atrioventricular valve, and a large inter-ventricular communication (ventricular component) between the crest of the inter-ventricular septum and the ventricular surface of the common valve. The inferior bridging leaflet may or may not have direct attachments to the septal crest. Thus, there is usually, a septal deficiency beneath the inferior bridging leaflet and inter-ventricular shunting is possible through tight inter-chordal spaces [3]. The defect beneath the attachments of superior bridging leaflet is usually larger. The right ventricular attachments of this leaflet form the basis of the Rastelli classification in complete AVSD [4].
Another variant of AVSD is for the superior and inferior bridging leaflets to be fused to the free margin of the atrial septum, leaving a persistent interventricular communication only. Thus an AVSD can masquerade as a perimembranous inlet VSD. An even rarer form of AVSD is that without interatrial or interventricular communications. The atrial septum and the crest of the ventricular septum sandwich the leaflets of the atrioventricular valve [5]. The septal tissues to either side of the valve tend to be thin, suggesting late spontaneous closure of previous communications [6].
2.3 Associated malformations
Other morphological features can also profoundly influence clinical manifestations, outcome and treatment. Dysplastic atrioventricular valve leaflets and deficient leaflet are encountered frequently. Variants of a partial AVSD may co-exist with stenosis of the left sided orifice.
Hearts with isomeric atrial arrangement are frequently associated with AVSD; there are then anomalies of systemic or pulmonary venous connection. Abnormalities of concomitant ventricular arterial connection include double outlet right ventricle and infundibular hypoplasia with or without pulmonary atresia. Common arterial trunk, transposition of the great arteries, Ebstein malformation are exceedingly uncommon anomalies.
2.3.1 Variations on valvar morphology
There may be additional orifices in the right or left valve in addition to the common and separate orifice AVSD. More commonly the left valve has one or more accessory orifices. Dual orifices may result from accessory leaflet tissue joining together two leaflets. Small accessory orifices are usually due to fenestrations in leaflets.
As discussed above, one of the hallmarks of AVSD is a trileaflet arrangement of the left atrioventricular valve. The annular insertion of the mural leaflet occupies approximately one third of the left component of the common atrioventricular junction, or one fifth of the left valvar orifice if the septal junction is included [2].
When this arrangement is distorted it becomes a major surgical significance. In some cases the mural leaflet is diminished or lacking, the two groups of papillary muscles are indistinguishable, and the chords from the bridging leaflets attached mainly to one papillary muscle group resembling insertions of parachute strings. Rarely may all three leaflets be fused together to form an imperforate membrane.
2.3.2 The left ventricular outflow tract
The left ventricular outflow tract in an AVSD is intrinsically narrow. Hearts with separate atrioventricular orifices and hearts with a Rastelli Type A tend to have direct adhesions of the superior bridging leaflet to the septal crest thus creating a funnel shape to the outlet [8]. Even cases of chordal attachments to the septal crest tend to have leaflets that are tightly bound down. By contrast, hearts with free-floating superior bridging leaflet are less likely to have ventricular outflow obstruction. Measurements on heart specimens have shown that the hearts with separate orifices have smaller outflow tracts than hearts with common orifices [9]. Any additional structures, for example, accessory muscle bundles or strands of fibrous tissue, in the area beneath the outlet valve will result in outflow obstruction [10]. Hearts with subvalvar obstructions often have obstructive lesions and are more likely to be associated with coarctation of the aorta.
2.3.3 Tetralogy of Fallot
In this combination, the ventricular arterial connection can be concordant or double outlet right ventricle depending on the degree of override of the aortic valve [11]. The outlet ventricular septal defect of the Fallot malformation is confluent with the ventricular component of the AVSD. Usually there is a common valve orifice and Rastelli type C attachment of the superior bridging leaflet. As with regular tetralogy of Fallot outflow tract the surgeon needs to be cognisant of any anomalous courses of a left coronary artery arising from the right aortic sinus.
2.3.4 Ventricular dominance
In most cases, the common atrioventricular valve in AVSD is more or less equally shared between the morphological right and left ventricles, i.e. balanced [12]. When the common atrioventricular valve junction is dominantly committed to one or other ventricle resulting in marked ventricular disproportion, the smaller ventricle will not support the respective circulation and biventricular repair is not possible. A total cavopulmonary connection or other variant of the Fontan procedure will be the only alternative surgical option. When more than 35% of the common atrioventricular valve is committed to one ventricle, the atrioventricular connection becomes double inlet ventricle.
When the valvar orifice and atrioventricular junction are unequally shared and less than 75% to one ventricle, there remains biventricular atrioventricular connections. The inequality may favour either the right ventricle (right dominance) or the left ventricle (left dominance). When most of the common junction is committed to the right ventricle, the left ventricle and the left component of the atrioventricular are hypoplastic. The left ventricle may be so small as to form part of the spectrum of hypoplastic left heart syndrome. This is almost always associated with aortic outflow obstruction and coarctation/tubular hypoplasia. When the junction is predominantly connected to the left ventricle it is the right ventricle that is hypoplastic. This combination is usually seen in either hypoplasia or atresia of the main pulmonary artery.
Irrespective of dominance, the ventricular septum always extends to the cardiac crux and the atrioventricular conducting system follows the general path of AVSD (see below).
2.3.5 Atrioventricular conduction tissues
The atrioventricular node is displaced posteriorly and inferiorly away from the regular triangle of Koch. The proximity of the coronary sinus to the atrioventricular junction determines whether or not there is enough room to place the interarterial patch and leave the coronary sinus draining to the right atrium without damaging the atrioventricular node [13–15]. Owing to this arrangement, together with the un-wedged location of the left ventricular outflow tract, heart block is an unlikely complication should there be the need to relieve future sub-aortic obstruction via the left ventricular outflow tract. In some hearts both an anomalous antero-lateral node and a ‘regular’ postero-inferior node give rise to penetrating bundles and atrioventricular bundles to form a sling of conduction tissues [16]. When AVSD is associated with atrial isomerism dual atrioventricular nodes with conduction tissue sling are also found in those with right-hand ventricular topology [17].
3 Surgical management
Thorough echocardiography is mandatory to establish the diagnosis of AVSD and any other associated anomalies. It is our practice to perform cardiac catheterisation, only when there is a clinical concern about the possibility of a significantly elevated pulmonary vascular resistance, usually in patients with a late presentation who are greater than six to eight months old .The average age at which definitive repair of complete AVSD is normally performed has decreased from late childhood to around three to four months of age [18–20]. However, duration of ventilation and length of intensive care unit stay increases with decreasing infant weight [21] as well as residual defects in infants below 5 kg [22]. Observational studies have confirmed that late intervention in AVSD results in worsening valve function as well as a propensity for the development of pulmonary hypertension [23,24].
Similarly, in the presence of associated lesions such as tetralogy of Fallot or in children with complex AVSD (unbalanced ventricle or double outlet right ventricle) surgery may be more easily carried out at a lower risk in the older infant or young child [25]. Nonetheless, rare combinations of AVSD and TAPVD, unroofed coronary sinus and multiple VSDs can carry a relatively higher hospital mortality although positive contemporary outcome of lone neonatal AVSD repair with only occasional case reports may suggest that tissue fragility, presence of dysplasia or combination thereof favour medical therapy until an appropriate body weight is achieved [26].
3.1 Surgical technique of AVSD repair
Palliation for AVSD with pulmonary artery banding remains an important option particularly in children with low birth rate or major extra cardiac morbidity [27]. Palliative strategy may also be appropriate when a definitive repair may require a valved conduit; particularly in the presence of severe right ventricular outflow tract obstruction/hypoplasia and hypoplastic pulmonary arteries. In patients with AVSD and severe hypoplasia of one ventricle a strategy leading to a functional single ventricle may be the next best option [28,29]. The precise timing and nature of staged procedures will inevitably be determined by both anatomical and physiological considerations as well as the experience of the congenital heart disease unit treating the child.
The classical repairs of complete AVSD have utilised either single patch (Fig. 1 ) to close both the atrial and ventricular components [30–32] of the defect or a two-patch technique (Fig. 2 ) [33] in which the ventricular component is closed separately from the atrial component thus avoiding division of the bridging leaflets. More recently, the modified one-patch technique described by Dr Wilcox in the early 1990s avoids the need for a ventricular septal patch in most patients [34]. Equivalent results to classical series have been demonstrated in larger series [35] (Fig. 3 ). Repair of complete AVSD without any patch material has been successfully reported in three cases (two small and one large VSD component) [36]. The authors claim that patchless repair lowers the level of the left AV valve implantation at the crest of the septum, increasing the coaptation height and reducing both ischaemic and total pump times. Prêtre et al. [37] states that without the patch there is reduced atrial volume that may help in preventing the occurrence of postoperative arrhythmias. However, with the exception of small residual septal defects we remain concerned about applying tension on the valve tissues with possible increased risk of valvular disruption. Although it seems that it is possible to directly close atrial defects, long-term outcome and reconfirmation by other surgical units is awaited before patchless repair becomes more widely adopted.
![Figure illustrates principal aspect of single patch repair by surgical division of the superior bridging leaflet. This allows improved exposure of the ventricular crest. In some cases both superior and inferior bridging leaflets are incised (reproduced from Ref. [32] with permission from Elsevier Inc.).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/35/5/10.1016_j.ejcts.2009.01.009/1/m_891.S1010794009000268.gr1.gif?Expires=1747853871&Signature=3Kw4x4xgpknrn366NF0pDqsZHJKttnl7tUeWa8Ad~DoVrjnscLAHEwANBZgJdfwaM8SWyRLaviJIOi4mhlRFrcLhjiEglEdyRplBBfj08gVqlWtxjTg3HwGLvGPlEA6Q9LqNfGzQ-9P~bSUyEUZGhRpd6VadkXOWvhMo5PJEtXxtW6CO5Bg3VJMVlT2N-D9DroLqUu7NamcPz0tYWChFzg0iEjRg6VWGjEhzmdbVq68tHfQsLwEOvZ1PffowO9DQRoeonKUE~sg8lgAuDzAIN0UnRDZeorGgOP2xRJZMrGp816NOzWLsS3oZsl1xXY7qwdsV3gAtR0BRtJxsofPK1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure illustrates principal aspect of single patch repair by surgical division of the superior bridging leaflet. This allows improved exposure of the ventricular crest. In some cases both superior and inferior bridging leaflets are incised (reproduced from Ref. [32] with permission from Elsevier Inc.).
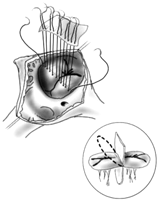
Figure illustrates double patch repair of AVSD. Notice the sutures extending through the bridging leaflets from within the ventricular patch (encircled inset). These sutures will then be passed through the pericardial patch to close the atrial septal defect (reproduced with the permission of CTSNet, Inc.).
![Figure illustrates the modified single patch. Note the sutures secure the atrioventricular leaflets to the septal crest directly with out a patch (lower inset). (reproduced from Ref. [35] with permission from Elsevier Inc.).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/35/5/10.1016_j.ejcts.2009.01.009/1/m_891.S1010794009000268.gr3.gif?Expires=1747853871&Signature=HI1hZX83oTjyOFyyjXxwEajaXhjVb5dNMBm0C1QPpkzZsAVwWlUX6DeW9SsotI8JF5LY-5a7~zlh-r7ECSjuul1-09c1PHspkwknpQ13c6uOWYtrpLDIEjTlsV~uSXuwCU9JilqzyUp1OYgKpYd1vtZgb0HmtAvItxmxB9~DLFW5X-CCAQDU9Yr4-mvmW3wUqDx2TdAbSdJXl8yWI3UGn6c3JiCyA6gBWygoVpBAkO3Kc~0VeA0sDuMAqBdC3JmsW9b1b6FCSkHEjaGLEFP-VEh-0Y6zt7jpuDsaYuWs8YSYRfWsQeC9~Px45oyzjIeBpZof0W9-kTi4DMpWXv5ZVQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure illustrates the modified single patch. Note the sutures secure the atrioventricular leaflets to the septal crest directly with out a patch (lower inset). (reproduced from Ref. [35] with permission from Elsevier Inc.).
Important surgical principles during attachment of the ventricular and/or atrial septal patch are to maximise the competence of both AV valves, avoid ventricular inflow obstruction and to minimise residual septal communications. Whichever technique is used it is important to avoid damage to the penetrating AV bundle which, as described above, is displaced near the coronary sinus in AV septal defects. This can be achieved by first identifying the conduction territory followed by careful placement of stitches either medial (usually at the based of the left AV valve mural/inferior leaflet) or lateral to the coronary sinus. The latter will direct coronary sinus venous drainage to the left atrium. When the aortic valve is difficult to visualise, the superior bridging leaflet can be detached for careful placement of VSD stitches, confirming absence of injury to aortic cusps by lack of cardioplegia effluent through the aortic root. When the aorta arises from the right ventricle it may be necessary to modify the single-patch technique utilising a small patch to close the superior component of the ventricular septal defect while directly closing the inferior defect thus avoiding undue tension following repair as well as minimising the potential for left ventricular tract obstruction [38,39]. When the two-patch technique is performed in the context of right ventricular narrowing, a comma-shaped Dacron patch is inserted with the widest dimension fashioned towards the left ventricular outflow tract to minimise the risk of ventricular outflow tract narrowing [40].
Critical appraisal of comparative techniques despite the limitations of era, surgeon and institutional experience has demonstrated that the three techniques are all very feasible to common atrioventricular junction morphologies. The single, double and modified patch have demonstrated equivalent efficacy in short- and intermediate-term outcomes with respect to left AV valve regurgitation and left ventricular outflow tract and residual septal defects [38–40]. While infants with Downs have less operative re-intervention than non-Downs, no single technique is proven to be superior to another [39].
From a practical standpoint, the double patch as opposed to the single patch repair allows more selective adjustment of the height of the VSD, ventricular outflow tract and angle of the AV leaflet to the VSD patch as well without the need to divide the bridging leaflet(s). Given these advantages, the double patch technique has been the most widely published repair modality for both simple and complex AVSD associated with DORV, TOF and relative ventricular hypoplasia. The main papers reporting single patch repair as well as modified patch techniques focused on simple balanced AVSD and excluded complex AVSD from their cohort of patients analysed.
The modified single-patch outcome is comparable with the two-patch technique in younger patients with similarly sized ventricular septal defects [39], however there remains some theoretical concerns with this approach and long-term results from many centres are awaited. In our practice, we think the stresses and strains placed on the lower zone of approximation of the inferior and superior bridging leaflet is at two levels; leaflet to leaflet and leaflet to ventricular crest. This dual strain effect can increase substantially when the VSD component is large and available leaflet tissue is limited somewhat. This will result in injury to the valve leaflets with propensity for worsening AV valve regurgitation. Moreover, plastering the leaflets to the septal crest increases the outlet to inlet axial length ratio creating a potential substrate for sub-aortic stenosis especially when the leaflets already have attachments to the septal crest. Again, though these are theoretical concerns, objective multicentre data is awaited.
A variety of manoeuvres are available to minimise the risk of postoperative left AV valve regurgitation. These include suturing of the so-called cleft of the left AV valve, plication of the left AV valve annulus, chordal replacement with Gortex suture and augmentation of the valve leaflets where there is deficiency of leaflet tissue [41]. Some features of the left atrioventricular valve (large mural leaflet, dystrophic tissue) represent a challenge for repair of atrioventricular septal defects without postoperative regurgitation. Creating a double-orifice left atrioventricular valve in such circumstances has been promising in small series [41] (Fig. 4).
Importantly left AV valve competence has also been accomplished by maximising coaptation height of the leaflets. One technique has been by incising both anterior and posterior bridging leaflets and joining the free edge of the ventricular patch at a right angle to the base of the left AV valve [42]. In newborns and young infants additional valvar support has been achieved using a small Gortex tube parallel to the VSD placed across the bridging valve leaflets [43]. Our preference remains to avoid left AV valve replacement using prosthetic valves in young children wherever possible, even if this entails multiple operations over a period of years to achieve satisfactory left AV valve performance.
3.2 Re-operation for left AV valve regurgitation
Left AV valve regurgitation following AVSD repair occurs at a rate of 3–18% for partial and 6–14% for complete forms over 5–40 years [44–46]. Chronic left AV valve insufficiency worsens with time and results in considerable morbidity, more often noted in cases with partial AVSD than those with complete AVSD. Re-operation in this group is more likely when the cleft was not repaired at the original operation [47]. Contemporary clinical practice usually recommends surgical closure over a zone of apposition between the superior and inferior bridging leaflets in most cases [40]. In a study of 54 patients undergoing re-operation for left AV valve regurgitation following AVSD repair, determinants of successful conventional repair were being young, having mild to moderate leaflet dysplasia and presence of cleft incompetence [48]. Before dwelling on another re-repair, the surgeon should study the echocardiogram carefully and revisit any prior operative notes describing the conduct of primary repair. A myriad of re-repair techniques should be considered according to the underlying cause of post AVSD regurgitation along the spectrum of annuloplasty, chordal replacement to the more recently advocated leaflet edge-to-edge technique (Fig. 4 ) [41,49]. In recent years the impact of patch augmentation of the left AV valve on valve dynamics has been studied. When regurgitation is associated with insufficient valve leaflet tissue, anterior leaflet pericardial patch extension repair including the zone of apposition has been recommended with good results (Fig. 5 ) [48]. Roman et al. compared annular areas of eccentricity annulus stiffness and compliance between a patch of left AV valve versus conventional and normal annuli [50]. Transoesophageal and three dimensional echocardiography was acquired preoperatively and postoperatively with a main follow-up of 27 months for the patch and 12 months for those of a conventional repair; patch repair showed no evidence of shrinkage or expansion [50]. Interestingly, neither repair technique established normal annular eccentricity. In a further retrospective study Moran et al. found no predictor for replacement in 46 patients with severe left AV valve regurgitation [51]. Freedom from re-operation at 9 years for valvuloplasty in replacement groups were 78.5% and 85.7% respectively. Predictors for reoperation within the valvuloplasty group included the presence of moderate or worsening regurgitation in the early postoperative period. Down’s syndrome has not been found to be a predictor of outcome following primary AVSD repair. The valve tissue in Down’s is usually more versatile, abundant and robust. Given the inherent risk for developing pulmonary hypertension among Down’s patients, reported experience with biventricular repair remains superior to single ventricle physiology [52,53]. Despite limited reported experience of left AV valve regurgitation following AVSD repair, reintervention appears to be later in life for Down’s than non-Down’s [54,55].
![Figure illustrates double orifice repair of the left AV valve. The hatched areas are the papillary muscles. Approximation of the zone of apposition of the bridging leaflets and bilateral commissure annuloplasties were performed (reproduced from Ref. [41] with permission from Elsevier Inc.).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/35/5/10.1016_j.ejcts.2009.01.009/1/m_891.S1010794009000268.gr4.gif?Expires=1747853871&Signature=fvAosjBSCzDtDFpS6PNQEgAru~J6bHPmvD7gv69s5uCTbkOpz1TNnyT9NF2JsAcC97cJnwqrc~KFJgXbi83nqT3OM4IeJYiQG1NNpWlS458Y2r6ffNxV7zPOoCdozcu6ouxoauJenHSB88PgofOYgOpDkg5D8j5~-Q68uOWR3YZlLRoexqKwyDRHiRefjtEfM4t82rfEVSf0LHNGFabSbcgXiDmtKQPdKJgd7etNQir99v7z5bxaWbVdmwfO3NP-Amie2GKc4xb0fohwiKzIlMIFYLRwtUUAdrpYHj-D4LJvNAGSM1ya~o62MEQrjIdPkGx8UU8JMjpl4awRQJgi0A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure illustrates double orifice repair of the left AV valve. The hatched areas are the papillary muscles. Approximation of the zone of apposition of the bridging leaflets and bilateral commissure annuloplasties were performed (reproduced from Ref. [41] with permission from Elsevier Inc.).
![Figure illustrates the insertion of an autologous pericardial patch in the anterior leaflet of left AV valve and completion closure of the zone of the apposition between the bridging leaflets. These two manoeuvres reduce the tension and increase the coaptation height minimising the regurgitation jet (reproduced from Ref. [48] with permission from Elsevier Inc.).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/35/5/10.1016_j.ejcts.2009.01.009/1/m_891.S1010794009000268.gr5.gif?Expires=1747853871&Signature=dZY3oiqcPDLC3I5DJG8Qx1rq6C6TRrDVfJ52mzBTbjEfmAZlm7YMBI8awfFPVaTmrxLiJ-zFOHOkZCak~HsVngrIliw7kIoqNYqtx8gL6vbH4OwURuvxMc8vASmd95tpw9BcvyRCbTwBFoAWcGqOqXxhWI9naWYtEJiRr24Ac1zviKXhiK5R9f1kuSTgtwdJLQ8xkS0TqokVm-th6cuYL0Ilecof1zuD61OXW9Z1fGUZw2u2mvug~QxLeCREM13VWrjrI~RnlLW8IAm8YlUJdz0VL~4zWN7rzbSQOy~mpIdXp42PNjfDX1qWWoVfG-Yo-ldQ5i9S7zYRBII3cKx1wg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure illustrates the insertion of an autologous pericardial patch in the anterior leaflet of left AV valve and completion closure of the zone of the apposition between the bridging leaflets. These two manoeuvres reduce the tension and increase the coaptation height minimising the regurgitation jet (reproduced from Ref. [48] with permission from Elsevier Inc.).
Should left AV valve replacement be required a number of techniques may be utilised in a process of replacing the left AV valve. John Kirklin detailed a technique by elevating the postero-superior annulus with a Dacron patch cut out from a conduit along the plane of the prosthetic valve away from the left ventricular outflow tract [56]. Other techniques for valve replacement in this situation include supra-valvar insertion [40].
The mortality following left AV valve replacement has decreased from 30% prior to 1990 to less than 4% in recent series [57,58]. The major surgical concerns following replacement include injury to the conducting system with associated complete heart block and the development of left ventricular outflow tract obstruction. Long-term survival following mitral valve replacements AVSD is reported between 50% and 65% at 10 years [58]. Half of the survivors require subsequent replacement within 10 years with low mortality rate.
3.3 Complete AVSD and tetralogy of Fallot
The incidence of tetralogy of Fallot with complete AVSD lies around 6–16% [59,60]. It is particularly important in this group of patients to achieve satisfactory exposure of the ventricular septal defect and to ensure that the VSD patch is appropriately contoured thus avoiding residual RV outflow tract obstruction. In this group of patients the presence of both right and left AV valve regurgitation coupled with pulmonary regurgitation postoperatively, can lead to poor outcome in terms of risk of death and postoperative morbidity [61,62]. Contemporary awareness of these perioperative aspects has reduced the mortality rate of this rare combination of defects from 29–40% to 0–11% [63–65].
3.4 Complete AVSD and double outlet right ventricle
Complete AV septal defect with double outlet right ventricle (DORV) occurs in less than 5% of patients [66]. Usually the AVSD is a Rastelli type C with a large sub-aortic VSD; the left ventricle can be tunnelled to the aorta as long as there are sufficient ventricular infundibular fold dimensions to minimise RVOT narrowing. Biventricular repair (tunnelling of the VSD to the aorta and RV to the PA via a conduit) is feasible in the absence of contraindications such as straddling atrioventricular valves or severe heterotaxy with anomalous pulmonary venous drainage [67,68]. When AVSD occurs with a sub-pulmonary VSD an arterial switch procedure with tunnelling of the left ventricle to the aorta is the procedure of choice [69]. In cases with a non-committed VSD component it may be possible to modify the intraventricular pathway from the LV to the aorta by surgically shaving a generous portion of the interventricular and outlet septum [70–72]. Should this challenge be inappropriate however, the option of a single ventricle type of repair may be considered [73].
3.5 Left ventricular outflow tract obstruction
As has been described above the AVSD provides a substrate for LV outflow tract obstruction on purely anatomical grounds [7,8]. This substrate may be aggravated in causing LVOTO by accessory chordal attachments, development of fibro-muscular ridges in the outflow tract and accessory valve tissue in this area.
Partial AVSD is associated with LVOTO more often than in complete AVSD [7]. This is due to narrowing of the LVOT as well as a superior bridging leaflet of the left AV valve, which forms the larger portion of the LVOT. There are various surgical options available to manage this difficult lesion in the context of patients with AVSD [74]. Each of these have to be tailored to the individual patient but may well include the use of a modified Konno procedure (with preservation of the aortic valve) [75,76]. In a series from Toronto, 19 children underwent surgery for AV septal defect and associated sub-aortic stenosis. In those patients attempts were made at fibrous resection of the sub-aortic obstruction with myomectomy. There was a 38% incidence for further re-operation for previously treated sub-aortic stenosis at a mean interval of 5 years [77]. One important group relates to the presence of fixed sub-aortic stenosis in the context of unbalanced AVSD which has been considered a risk factor for surgery especially when the criteria for biventricular repair is borderline. In a series of 69 consecutive patients [78] right ventricular dominance was found in 29% with a borderline of left ventricle in 13% and truly hypoplastic left ventricle in 7 patients. Biventricular repair was always favoured in a case of borderline left ventricle and precluded when LV/RV ratio was less than 0.33. At 10 years actual survival of 51% in a group with complete AVSD was achieved but there was a 38% re-operation rate in this group. The authors concluded that biventricular repair should be avoided in patients presenting with sub-aortic stenosis especially when there are borderline LV dimensions.
3.6 Unbalanced AVSD
The decision to proceed with biventricular repair is based on presence of several unvalidated morphometric parameters. To allow a left ventricle to fully function as the systemic pump, an indexed mitral valve area >4.75 cm2/m2, left ventricular inflow dimension >25 mm, ratio between the apex-to-base left ventricular dimension and right ventricular dimension >0.8, and aortic annulus >6 mm, has been put forward [79]. Morphologically we should also address the presence or absence of insufficient valve tissue, ventricular non-compaction and presence of sufficient cavity (an indexed left ventricular end diastolic volume >20 ml/m2 of body surface area) [80] before recruiting the left ventricle.
Nonetheless, the use of echocardiography including three-dimensional (3D) imaging, Cine magnetic resonance technology and angiography will help in providing more accurate estimation of the structure, function and dimensions of the systemic and pulmonary ventricle. One important echocardiographic approach is using the potential left ventricular volume based upon the interventricular septal position. In a study using this technique, bi-ventricular repair was accomplished with preoperative indexed left ventricular end diastolic volume <15 ml/m2 of body surface area and non apex-forming left ventricle. The authors concluded that apparent underfilling of the left ventricle does not necessarily confirm true ventricular hypoplasia; the ventricle may simply be ‘squashed’ [81,82].
When LV hypoplasia is deemed severe a palliative strategy should be incorporated with the addition of a bidirectional cavopulmonary shunt. This is because conventional repair increased probability of hospital death and with more than 60% mortality in children with complete atrioventricular septal defect for a right ventricular dominance with right/left ventricular volume = 2.0, and more than 80% mortality for a right/left ventricular volume = 2.5 [57]. Although this data is historical, it remains a cautious reminder of risks involved in repairing an unbalanced AVSD. In more recent series using echocardiography, left ventricular dominance is usually defined when the right AV valve area to left AV valve ratio is less than 0.5 resulting in RV to LV length ratio of less than 81% [83]. In a study of 38 children from Toronto [83] with left ventricular dominance 32 patients underwent biventricular repair and 6 proceeded to single ventricle palliation. The restricted atrial fenestration was created in 11 patients. There were 4 operative deaths (three in the small RV-biventricular repair group with an AVV high less than 0.43, and one death in single ventricle palliation group). However over a 10-year follow-up only three of the six patients committed to a single ventricle circulation had achieved full palliation largely on account of the presence of severe pulmonary hypertension in three of the cases.
More difficult is estimating the size of the left ventricle in AVSD.
In the presence of right ventricular dominance (LAVV − RAVV area = less than 0.67) 11/26 underwent a Norwood type of procedure and 15 underwent a biventricular repair in a report from Philadelphia [29]. Risk factors for mortality in this biventricular repair group included the presence of a large VSD, transverse arch hypoplasia with ductal-dependence circulation and narrowing of the left AV valve. The authors recommended that single ventricle palliation is more appropriate than biventricular repair in the presence of either significant left AV valve stenosis or insufficiency [29].
Attempts at right ventricular cavity recruitment has been achieved adopting some of the key surgical principles of the ventricular ‘overhaul’ approach for intact ventricular septum and pulmonary atresia [84] by releasing the septal papillary muscle to free up the right AV valve subvalvar apparatus. Although such techniques may be important when biventricular repair has better implications than single ventricle physiology (e.g. Down’s syndrome), long-term outcome of these techniques have yet to be reported systematically. Also atrial fenestration should be utilised when attempting complete repair of AVSD in the context of patients with RV hypoplasia.
Although there has been a growing move towards achieving biventricular repair, it is important to appreciate that some biventricular hearts, following repair of AVSD, fail balanced ventricular metrics due to dominance of aberrant morphology and residual defects [85].
3.7 Double orifice left AV valve
The additional orifice is usually located in the mural leaflet but can occur elsewhere in the bridging leaflets. Such an orifice can be closed uneventfully if the primary valve orifice is of adequate size. Some surgical attempts at closure have been associated with higher than expected postoperative mortality [86]. Al-Hay et al. had found that 11 patients out of 147 patients with AV septal defect presented with double orifice left AV valve and that this finding was robust enough to predict an increase in the 30-day mortality [87]. Long-term follow-up of double orifice AV valve has been associated with higher rate for left AV valve re-intervention in partial rather than the complete AVSD subtype [88] This atypical malformation of the AVSD has an association with single papillary muscle which may require fenestration though conservative management is recommended [89].
3.8 Absent posterior (mural) leaflet
This is a rare and seldom reported anomaly. Repair should err on the side of committing small valve tissue to the left with the aim of achieving biventricular left AV valve without closure of the zone of apposition [90].
3.9 Repair in univentricular atrioventricular connections
The combination of AVSD and heterotaxy is not uncommon and in the absence of biventricular repair they are at risk of regurgitation. This can occur early in infancy following a systemic to pulmonary shunt leading to volume overload or late when palliated with cavopulmonary circulation. Repair options include pericardial patch augmentation of a common atrioventricular or direct leaflet approximation. The lateral free edges of the bridging leaflets are sutured together and the patch is sutured to the free edges of the superior and inferior leaflets [91]. Occasionally when leaflets are less mobile, secondary or primary chords have been to detached and the freed edges of the leaflets sutured together [92]. Others have described bridging annuloplasty (Fig. 6 ) [93]. While some authors advocate repair of AVSD regurgitation during single ventricle palliation [94,95], the degree of regurgitation can improve alone with extracardiac rather than lateral tunnel connection [96]. Long-term results have shown that repairing the regurgitation can prevent the development of supraventricular arrhythmias [97,98] in most but not all published series [96].
![Figure illustrates insertion of annuloplasty rings well as prosthetic material bridge across the bridging leaflets at the zone of coaptation during single ventricle palliation (reproduced from Ref. [93] with permission from Elsevier Inc.).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/35/5/10.1016_j.ejcts.2009.01.009/1/m_891.S1010794009000268.gr6.gif?Expires=1747853871&Signature=htj-8dxOPVtp1LMy9PSzoQpo2Tshv-7RyG9SDwQhBI21NAVQg2KiT9ToQIlJbuWRUY7tqAbRThj0w2nqy-uDtoCUY54bBOG0trkq1VI5d53WtPXYK97fNJAJ~0fJKTh359KNn3pX50Ldr-8exM35xJi9WRs4-UDams9uKAi8fW3El~cw-mj9hgutYAB1hBt6is4nigVtUmMTHkaYC0gz1nZ5GP8Q~WvZLMCcOUzsrU2uohlxwJ~PDlVb26zGybZoRv4FrhZRzcuR4dacIWth-Fdklvrj~IW255FEijL7en3c2kjHZxA8fOdlWt9SH24XIRp4X9lxv6A7JfdTrdFZqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Figure illustrates insertion of annuloplasty rings well as prosthetic material bridge across the bridging leaflets at the zone of coaptation during single ventricle palliation (reproduced from Ref. [93] with permission from Elsevier Inc.).
4 Future prospects
Recent advances in imaging utilising real time higher definition imaging as in 3D echocardiography may provide necessary resolution of AVSD defect in the context of direct valve motion. A recent advance in the field of computer simulation is augmented reality innovations. This modality combines 3D computer-generated objects and text superimposed onto real images and video, all in real time. Augmented reality so far promises us additional information that cannot be detected by the five senses of a human being. This technology has the ability to provide experienced surgeons the benefit from such systems by extending the limit of a safe area to allow for more complete and radical operative therapy, while providing orientation and key surgical landmarks to trainees [99]. However, despite early successes in VSD patch with robotic arms [100], further research is needed to be able to utilise this technology for patients with AVSD.
5 Conclusion
There is now a significant body of evidence to support the concept that anatomical early repair of uncomplicated AVSD should be undertaken where feasible. In the presence of associated lesions the complexity and risks of surgery are increased but the early outcomes remain good in the majority of patients. Surgical strategies must take account of individual surgical experience and are predicated on the availability of detailed comprehensive clinical assessment and perioperative echocardiographic imaging. Sub-standard results at the conclusion of a primary procedure for repair of AVSD are unacceptable, as such patients will inevitably require early re-operative intervention.




