-
PDF
- Split View
-
Views
-
Cite
Cite
Ignacio Garutti, Federico González-Aragoneses, Maria Teresa Biencinto, Emma Novoa, Carlos Simón, Nicolás Moreno, Patricia Cruz, Carmen Benito, Thoracic paravertebral block after thoracotomy: comparison of three different approaches, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 5, May 2009, Pages 829–832, https://doi.org/10.1016/j.ejcts.2009.01.025
Close - Share Icon Share
Abstract
Background: Thoracic paravertebral block (TPVB) is a regional block technique increasingly used for the early management of post-thoracotomy pain. We compare three different postoperative analgesic approaches based on TPVB: anesthetist, anesthetist plus surgeon, and surgeon. Materials and methods: We randomized 54 patients undergoing elective thoracotomy to three different postoperative analgesia groups: paravertebral percutaneous catheter (PVA group), paravertebral percutaneous catheter plus incisional (subcutaneous) catheter (PVA + Inc), and paravertebral catheter under direct vision (PVS group). During early postoperative 48 h, we measured pain intensity, intravenous morphine afforded by the patient-controlled analgesia pump, and the spirometric test. Results: There were no statistically significant differences among the collected preoperative data. No significant differences were observed on postoperative spirometric values. Analgesic quality was better in PVA + Inc group at 12 and 24 postoperative hours. In this group, intravenous morphine use to improve analgesia was significantly lower from 8 h until 48 h postoperative. Conclusions: Association of thoracic paravertebral block to continuous infusion of a local anesthetic in the surgical incision area affords a better pain relief than paravertebral block alone (introduced by the surgeon or the anesthetist).
1 Introduction
Thoracic paravertebral block (TPVB) is a regional block technique increasingly used for the early management of post-thoracotomy pain (PTP). Its efficacy compared with thoracic epidural analgesia (TEA), regarded as the gold standard in chest surgery, is similar in terms of the quality of the analgesia afforded. Compared with TEA, however, TPVB can reduce the postoperative pulmonary complications and the complications associated with the analgesic technique (hypotension, urinary retention) [1]. Another characteristic differentiating TPVB with respect to TEA is the fact that the former can be performed intraoperatively by the surgeon under direct vision [2,3]. To our knowledge, no studies to date have compared the effectiveness of TPVB (under direct vision or otherwise).
Continuous infusion elastomeric pumps have recently been introduced as another analgesic option in different forms of surgery [4–6]. These pumps allow the administration of low volumes of local anesthetics (LA). Local anesthetics are delivered by multiorificed catheters inserted around, near or at the surgical incision site.
Anti-inflammatory properties of LAs have been clearly demonstrated in vitro as well as in vivo [7,8]. Decrease of local inflammation on injured chest wall can improve analgesia. We postulate that LA delivered in the surgical zone of the chest wall associated to paravertebral analgesia could improve the quality of analgesia and facilitate improved postoperative respiratory physiotherapy. The present study compares three different postoperative analgesic approaches based on TPVB: anesthetist, anesthetist plus surgeon, and surgeon.
2 Materials and methods
After obtaining research ethics committee approval and informed written consent, we randomized 54 patients undergoing elective thoracotomy to three different postoperative analgesia groups: paravertebral percutaneous catheter, paravertebral percutaneous catheter plus subcutaneous catheter, and paravertebral catheter under direct vision. Patients were recruited during the period between 2006 and 2008. Exclusion criteria were ASA physical status above III, a patient age of under 18 years, acute cardiopulmonary disease (NYHA stage III/IV), allergy to local anesthetics or opioids, abnormal coagulation test results, or the inability to comprehend or perform verbal and physical instructions.
On the day before surgery, patients received instructions on how to use a patient-controlled analgesia (PCA) device, perform simple spirometry with a portable monitoring device through a mouthpiece, and measure pain on a visual analog scale (VAS) – with 0 representing no pain and 10 representing the worst pain imaginable. Baseline spirometry, including forced vital capacity (FVC), and forced expiratory volume in 1 s (FEV1) were recorded. The best score of three successive trials was recorded for each hand. The same test instrument was used for pre- and post-testing. Patients were premedicated the night before surgery with lorazepam 1 mg p.o., and 1 h before surgery with midazolam 7.5 mg p.o. Patients with chronic obstructive pulmonary disease (COPD) were premedicated with inhalatory salbutamol and ipratropium bromide 30 min before surgery.
In all patients a percutaneous paravertebral catheter was placed in the paravertebral T5-T6 space ipsilateral to the thoracotomy. A test dose of 3 ml of 2% lidocaine with 1:200,000 epinephrine was injected through the catheter. After a negative response to the test dose was confirmed, general anesthesia was induced with propofol 2 mg/kg, fentanyl 3 μg/kg and rocuronium 0.8 mg/kg, and maintained with a minimum 1–1.5% sevoflurane alveolar concentration (MAC). Intraoperative analgesia was provided with a continuous perfusion of 0.5% bupivacaine (0.15 ml/kg/h).
Patients were randomized to three groups. (1) Paravertebral approach by the anesthesiologist (PVA group). In this group TPVB was performed preoperatively by the anesthetist. Postoperative analgesia was provided with a continuous paravertebral infusion of 0.25% bupivacaine (0.15 ml/kg/h). (2) TPVB plus incisional analgesia (PVA + Inc). The approach in this case was the same as in the first group, with the particularity that at the end of the operation, the surgeon inserted two multiple orifice catheters measuring 12.5 cm in length in the surgical area. The catheters were placed above and below the anterior extremity of the thoracotomy, and were guided backwards. The first catheter was positioned along the rib as close as possible to the intercostal nerve, until reaching the costotransverse junction. The second catheter was placed in the subcutaneous tissue plane, above the latissimus dorsi muscle, over its entire length. Postoperative analgesia in this group was provided with 0.25% bupivacaine (0.15 ml/kg/h) through a percutaneous paravertebral catheter. An elastomeric pump connected to the subcutaneous catheter was used for continuous perfusion of 0.25% bupivacaine (4 ml/h). (3) Paravertebral approach by the surgeon (PVS group). In this group, the percutaneous paravertebral catheter inserted by the anesthetist was removed at the end of surgery. Subsequently, a paravertebral catheter was placed by the surgeon under direct vision into the paravertebral T5-T6 space ipsilateral to the thoracotomy. Two catheters measuring 6.5 cm in length were placed through an introducer sheath. The catheters were positioned behind and below the posterior extremity of the thoracotomy incision. Blunt extrapleural dissection was carried out to create two tunnels reaching the head of the ribs, anterior to the transverse process, in the paravertebral space, at the height of the thoracotomy. The tunnels were directed up and downwards so that the extremity of one catheter reached at least two to three intercostal spaces above, and the other two to three spaces below. Postoperative analgesia was provided only by the continuous infusion of 0.25% bupivacaine with an elastomeric pump (4 ml/h).
All patients received dexketoprophen 50 mg i.v. every 8 h and ondansetron 4 mg i.v. every 4 h in the case of nausea or vomiting. Intravenous PCA was added with a device programmed to deliver morphine in bolus doses of 1 mg, with a lockout duration of 10 min and a restricted total dose of 4 mg/h.
The patients remained in the postoperative care unit for the first 24 h. Discharge was decided based on the criteria usually employed by the physicians in the unit, following inspection of the patient, arterial blood gas testing and a chest X-ray study.
After 48 h the administration of bupivacaine via any route was suspended, and nonsteroidal anti-inflammatory drugs (NSAIDs) were continued via the oral or intravenous route, depending on patient oral tolerance at the time.
Data were collected 4, 8, 12, 24, 48, and 72 h after surgery. The recorded parameters included the total amount of morphine/kg (PCA consumption), VAS scores for pain at rest, movement and cough, FVC and FEV1. Side effects were also recorded, such as postoperative nausea and vomiting (PONV), hypotension and sedation.
The presence of unilateral sensory loss to cold (ice) was confirmed in all patients in the early postoperative period, although no formal assessment of this was made.
All continuous data were presented as the mean and standard deviation. A sample size of 17 patients per group would allow us to detect a difference ≥0.15 mg/kg in morphine consumption at the end of the study. We assume a standard deviation of 0.15 and α = 0.05 and β = 0.2. The assumption of normality was checked using the Kolmogorov–Smirnov test before applying repeated measures analysis of variance (ANOVA) to assess differences between groups in terms of morphine consumption, VAS scores for pain and spirometric variables. The post-hoc Bonferroni test was used for multiple comparisons. Categorical data were examined by Fisher’s exact test. We considered it significant if p < 0.05. The SPSS version 13.0 statistical package for MS Windows (SPSS Inc., Chicago, IL, USA) was used throughout.
3 Results
Eighteen patients were included in each analgesia group. One patient in the PVA group was excluded from the study due to an insufficient number of blocked dermatomes. There were no statistically significant differences among the collected preoperative data (Table 1 ).
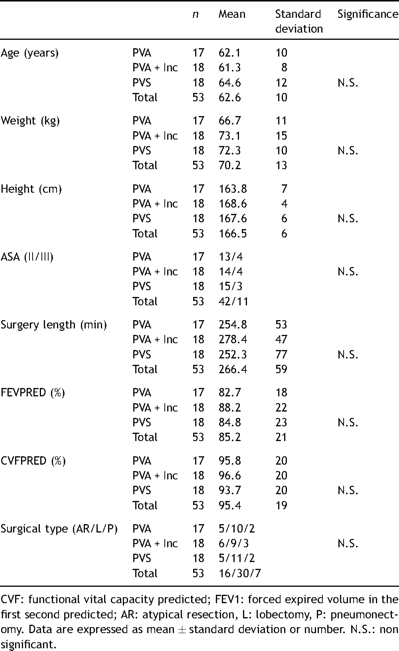
Spirometry before surgery and on the three days following surgery showed a decrease in FEV1 and FVC of about 50% in all three groups of patients. No significant differences were observed between groups on comparing these parameters among the different analgesic techniques analyzed (Table 2 ).
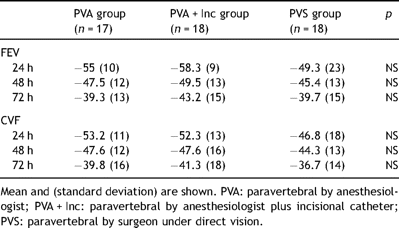
Postoperative porcentual changes (CVF and FEV1 predicted) during first three postoperative days.
Analgesic quality as reported by the patient was generally greater in the group in which a subcutaneous catheter was used to supplement the paravertebral analgesia. The differences proved significant 12 and 24 h after surgery, as regards resting pain, pain with movement or pain on coughing (Table 3 ).
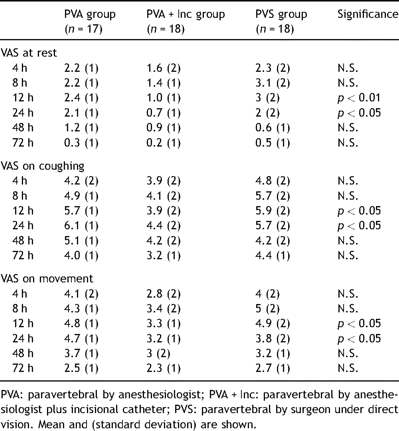
Visual analog scores (VAS) for pain at rest, on movement and coughing. VAS on movement.
Daily morphine use to improve analgesia was significantly lower in the patients who had received only incisional analgesia during all study except in the first 4 h (Table 4 ).
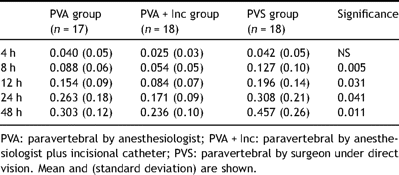
Postoperative cumulative intravenous morphine (mg/kg) administered.
4 Discussion
This randomized and controlled study was designed to compare three regimens of thoracic paravertebral analgesia for post-thoracotomy pain. The administration of local anesthetics in the area of the surgical incision associated to TPVB proved useful for lessening both the intensity of pain in the first 12 postoperative hours and cumulative consumption morphine. In addition, the analgesia obtained through the placement by the surgeon of two paravertebral catheters was similar to that afforded by catheterization of the paravertebral space by the anesthetist, but involving almost half the local anesthetic dose.
This analgesic improvement afforded by the addition of continuous incisional analgesia to TPVB could come at the risk of inducing higher plasma anesthetic concentrations. Local anesthetic absorption towards the bloodstream is high with both types of analgesia. We have not measured bupivacaine plasmatic concentrations in our study. This combined analgesic technique could be lower than the safety profile, although we did not have any evident problem of local anesthetic toxicity.
Incisional analgesia has been shown to be useful in a recent meta-analysis, and its use is presently growing with the introduction of different multiple orifice catheters connected to elastomeric pumps. This technique presents a good safety profile, and has also been used in chest surgery (thoracotomy or videothoracoscopy), with satisfactory results [9,10].
Liu et al., in a recent meta-analysis of over 40 randomized and controlled studies using incisional analgesia, reported a reduction of resting pain or pain associated to movement, a lesser need for postoperative opioids, and a shortening of hospital stay [11].
The administration of local anesthesia in an area already blocked by TPVB could seem redundant. Some authors had previously reported the beneficial effect in terms of post-thoracotomy analgesia of adding intercostal block to thoracic epidural block at the time of closing the chest wall [12].
The direct application of local anesthetics in the surgical region may offer analgesia via different mechanisms. On one hand, the sodium channel block suppresses the neuronal signals, while on the other hand local anesthetics are known to have properties that are not regarded as directly analgesic [13]. In effect, some experimental studies have shown that local anesthetics reduce inflammatory mediator release by the neutrophils, reducing adhesion of the latter to the endothelium, and reducing the formation of free oxygen radicals [14–17]. We consider the cause for the analgesic improvement obtained with the administration of local anesthetic in the surgical incision zone to be related to its anti-inflammatory effects, which may sensitize the nociceptive receptors and contribute to pain and hyperalgesia after the operation.
We have observed the important postoperative impairment in the respiratory function produced by posterolateral thoracotomy. Forty-eight hours after the intervention, the mean values in all three groups of patients were similar but below 75% of the respective preoperative values, in coincidence with the observations of Richardson et al. in a meta-analysis [18].
Lesser intravenous opioid consumption was observed among the patients in whom incisional analgesia was added to paravertebral analgesia. At the end of the study these patients consumed half of the morphine than surgeon’s paravertebral group and less than 20% with anesthesiologist’s paravertebral group.
To our knowledge, this is the first study to compare the analgesic qualities of two different approaches to the thoracic paravertebral space. We found no differences in intravenous opioid use between them. However, the bupivacaine doses used were approximately two to three times less in the PVS group. The success rate was 100% in the patients of this group.
One of the limitations of our study is that it was not blind. The patients in the group subjected to incisional analgesia plus paravertebral block required three pumps (an elastomeric pump for the surgical incision, another pump for the paravertebral catheter, and a third for the intravenous administration of morphine), compared with only two pumps in the other two groups.
In conclusion, the association of thoracic paravertebral block to continuous infusion of a local anesthetic in the surgical incision area affords a better pain relief than paravertebral block alone (introduced by the surgeon or the anesthetist).
Sources of funding: DISMED company (representing to ON-Q PainBuster in Spain) has collaborated to develop this study.
References
- morphine
- analgesics
- patient-controlled analgesia
- anesthesia, conduction
- anesthesia, local
- anesthetics, local
- pain
- preoperative care
- surgical procedures, operative
- thoracotomy
- vision
- pain management
- catheters
- percutaneous catheters
- intravenous infusion, continuous
- injection of anesthetic agent around paravertebral thoracic nerve
- paravertebral block
- postoperative analgesia




