-
PDF
- Split View
-
Views
-
Cite
Cite
Pierre-Emmanuel Falcoz, Jalal Assouad, Françoise Le Pimpec-Barthes, Marc Riquet, Lobectomy for metachronous lung cancer after pneumonectomy, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 2, February 2009, Pages 373–374, https://doi.org/10.1016/j.ejcts.2008.11.012
Close - Share Icon Share
Abstract
Second primary non-small cell lung cancer (NSCLC) is a well-known disease in patients having undergone successful NSCLC resection. Surgery for patients with cancer in the residual lung after pneumonectomy should not be excluded automatically. However, surgery on a single residual lung is usually done by wedge resection or segmentectomy, whereas lobectomy remains somewhat exceptional. We report the cases of two right upper lobectomy patients, alive and doing well at 5- and 6-year follow-up, with a FEV1 equal to 36% and 35% of predicted value, respectively.
1 Introduction
Second primary non-small cell lung cancer (NSCLC) is a well-known entity in patients having undergone successful NSCLC resection. Surgery on a single residual lung is usually done by wedge resection or segmentectomy. Lobectomy remains controversial [1] and, as shown by the lack of published literature on this topic, somewhat exceptional. From the 14 single residual lung surgery patients in our department, we present the cases of two lobectomy patients alive and well at 5-year follow-up. To the best of our knowledge, long-term survival with only the right middle and lower lobes has not yet been published.
2 Case report
2.1 Patient 1
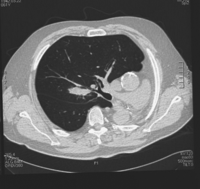
A 52-year-old man underwent a left pneumonectomy in 1996 for well-differentiated squamous cell carcinoma, pT2N2. He was given a postoperative radiotherapy with 56 Gy. He presented a right upper nodule discovered during postoperative follow-up surveillance in 2002. A CT chest scan confirmed a suspect single pulmonary mass, 4 cm in diameter, located deep in the parenchyma, with no other anomalies, so lobectomy appeared feasible (Fig. 1 ). Bronchoscopy and bronchial biopsies were normal. Full-body PET-CT showed an intense uptake of the right pulmonary mass with no other anomalies. The patient was asymptomatic, active and deemed to be in good physical condition. He had smoked 20 cigarettes a day for 30 years, until 1996. Spirometry showed decreases in forced expiratory volume in 1 s (FEV1) to 1.53 l (44% of predicted) and a diffusing capacity of 49%. Arterial blood gas analysis on room air was normal. Pulmonary scintigraphy perfusion was homogenous. At thoracotomy (standard posterolateral), frozen extemporaneous examination revealed a large cell carcinoma. Therefore, a right upper lobectomy with radical systematic lymphadenectomy was performed, with no complications. Pathologic examination revealed a pT2N0M0 well-differentiated squamous cell carcinoma. The patient was discharged on postoperative day 9 without supplemental oxygen, and recovered progressively. At 6-year follow-up he is doing well with a FEV1 equal to 35% of predicted value. Fig. 2 shows chest radiography at 6-year follow-up.
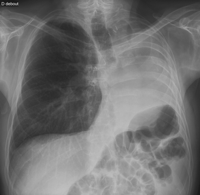
Chest radiography at 5-year follow-up. See the left-sided loss of volume with shift of the mediastinum and chest wall (ribs) and left hemidiaphragm towards the ‘empty’ left hemithorax.
2.2 Patient 2
A 61-year-old man underwent a left pneumonectomy after two regimens of induction chemotherapy in 1996 for a pT2N1M0 squamous cell carcinoma. In 2003, under surveillance, he was found to have a right hilar opacity diagnosed by chest radiography. He was clinically asymptomatic. A CT scan of the chest confirmed a suspect mass 3 cm in diameter located at the back of the right upper bronchus near the vascular structures. Bronchoscopic visualization was carried out and the bronchus appeared macroscopically normal, as did histological studies of bronchial biopsies. Full-body PET-CT showed an intense uptake of the right upper pulmonary mass with no other anomalies. He was judged to be in fair physical condition. After having smoked 20 cigarettes a day for 40 years, he stopped in 1996. The findings from preoperative spirometry showed a decrease in FEV1 to 2.02 l (61% of predicted). Arterial blood gas analysis on room air was normal. Pulmonary scintigraphy of ventilation-perfusion showed a defect in the right upper lobe at the level of the mass. At thoracotomy (standard posterolateral), a wedge resection was deemed impossible, so a right upper lobectomy associated with radical systematic lymphadenectomy was performed. The postoperative course was uneventful and the patient was discharged on postoperative day 10. Pathologic examination revealed a pT1N0M0 squamous cell carcinoma. He regained a normal physical and functional status without supplemental oxygen. At 5-year follow-up he is doing well with a FEV1 equal to 36% of predicted value.
3 Discussion
Surgery for patients with cancer in the residual lung after pneumonectomy should not be excluded automatically even when a lobectomy seems indicated. With this in mind, two particular points deserve mention.
First, it is the surgeon’s responsibility to determine whether the proposed resection (lobectomy) is likely to enhance survival with a good quality of life. Strict clinical evaluation of the candidates is therefore mandatory, as are full-body PET evaluation and routine pulmonary function testing (spirometry, diffusing capacity, arterial blood gas). The criteria for adequate pulmonary reserve for additional resection remain to be defined. In our department, the policy is to consider surgery only if the patient’s predicted postoperative FEV1 is at least 33% of the predicted value, that is within the range of values recommended in the literature [2].
Another point is that during the lobectomy, the operated lung was ventilated via a single-lumen endotracheal tube with minimal tidal volume, and gentle hand ventilation or intermittent periods of apnea. In our experience, specialized ventilation (temporary ventilation of the lobe not undergoing resection [3] or high frequency jet) and extracorporeal membrane oxygenation [4] are not necessary.
To conclude, we have shown that lobectomy on a single residual lung is not only technically feasible, with excellent immediate postoperative results [5] and, for the first time in the literature, that it may provide long-term survival in highly selected patients. We therefore recommend not contraindicating this type of surgery when preoperative evaluation authorizes it.




