-
PDF
- Split View
-
Views
-
Cite
Cite
Masahiko Higashiyama, Kazuyuki Oda, Jiro Okami, Jun Maeda, Ken Kodama, Akemi Takenaka, Tomio Nakayama, Gen-ichiro Yoneda, Prognostic value of intraoperative pleural lavage cytology for lung cancer without carcinomatous pleuritis: importance in patients with early stage disease during long-term follow-up, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 2, February 2009, Pages 337–342, https://doi.org/10.1016/j.ejcts.2008.10.013
Close - Share Icon Share
Abstract
Purpose: The clinical significance of intraoperative pleural lavage cytology (PLC) for lung cancer has been insufficiently elucidated. We therefore reviewed the surgical results of lung cancer patients without carcinomatous pleuritis followed up over the long term to elucidate PLC implications. Patients and methods: PLC was performed immediately after thoracotomy in consecutive lung cancer patients without carcinomatous pleuritis undergoing tumor resection between 1988 and 1997. Postoperative follow-up was generally performed for at least 5 years while checking tumor recurrence and survival. Results: Eighty-nine (13.1%) of 679 patients had positive PLC findings, which were observed more frequently in patients with advanced stage, larger tumor size, higher involvement of the pleura, lymph node, lymphatics and vessels. The overall 5- and 10-year survival rates in PLC-positive patients were 43% and 25%, respectively, while those in PLC-negative patients were 66% and 58%, respectively (p ≪ 0.0001). Among 395 patients with stage I disease, 35 (8.9%) showed PLC-positive findings, and their overall survival rate was significantly poor compared with those with PLC-negative findings (p ≪ 0.0001). In contrast, such differences were not observed among patients with more advanced stage diseases. In regard to histological type, a difference in the postoperative survival rate according to PLC status was statistically found in adenocarcinoma type (p ≪ 0.0001), but not in squamous cell carcinoma type (p = 0.24). According to multivariate analysis, PLC was an independent prognostic factor for all tested patients (p = 0.007, hazard ratio = 0.60) as well as for those with stage I disease (p = 0.0135, hazard ratio = 0.51). When examining postoperative pleural recurrence, the rate for PLC-positive patients was statistically higher than that for PLC-negative patients (p ≪ 0.0001, hazard ratio = 0.08). Interestingly, late pleural recurrence more than 5 years occurred in five (5.6%) of PLC-positive patients, all of whom were included in stage I. Conclusions: Based on the present analysis of long-term follow-up after operation, PLC may also be an independent prognostic factor. In particular, the PLC status of patients with stage I disease or adenocarcinoma type has an important impact on survival. PLC-positive findings may be a high risk for postoperative pleural recurrence. For PLC-positive patients with stage I disease, careful serial follow-up for more than 5 years is required while paying attention to late pleural recurrence.
1 Introduction
Despite many recent reports regarding intraoperative pleural lavage cytology (PLC) for lung cancer [1–8], the clinical significance has been undetermined in several points. First, it is considered that PLC may be a prognostic factor, but does this apply to all surgical cases of all stage diseases? [1,6] Second, PLC-positive findings may be a potential risk factor for recurrence [2,6], but the recurrence pattern has not been analyzed based on sufficiently long follow-up. Third, several investigators have shown the significance of PLC in lung adenocarcinoma [4,6,7], but how does this relate to squamous cell carcinoma of the lung? Thus, many controversial questions remain to be answered.
In our preliminary study [9], we reported the clinical importance of PLC immediately after thoracotomy and before closure of the pleural cavity for lung cancer patients without carcinomatous pleuritis. Next, we analyzed the postoperative recurrence pattern of PLC-positive patients but in these studies the follow-up periods after operation, as well as the number of patients tested, were insufficient to reach conclusions about the PLC significance [10]. Therefore, we reviewed the surgical results of lung cancer patients without carcinomatous pleuritis followed up for a long period, and re-analyzed the PLC implications, especially for prognosis and recurrence patterns.
2 Patients and methods
From December 1987 to December 1998, PLC was performed immediately after thoracotomy in 679 consecutive patients undergoing pulmonary resection for lung cancer without apparent findings of carcinomatous pleuritis. Patients in whom pleural dissemination or malignant effusion was intraoperatively found were excluded from the present analysis. Some of the present tested patients were included in the previously reported studies [9,10].
The method of PLC immediately after thoracotomy was previously described [9,10]. Briefly, the pleural cavity was washed with about 200 ml of physiological saline solution, most of which was then collected into a glass bottle with a mixture of heparin. After centrifugation, sediment was stained using the Papanicolaou, Giemsa and Alcian blue methods on several glass slides. The cytological results were generally classified into two categories: PLC-negative or -positive. Patients with borderline positive results, in whom a few cells with highly severe atypia or possible tumor cells (usually one or two cells) were detected in the specimen, were classified as PLC-positive.
The pathological staging was determined according to the international staging system [11], and other clinicopathological factors were quoted according to the general guidelines of the Japan Lung Cancer Society [12]. Lymphatic and vascular invasions of tumor cells were determined histopathologically, and were classified as positive and negative.
Postoperative follow-up was generally performed as follows: within 3 years after operation, systemic and local screening examinations using blood tests, chest computed tomography (CT), abdominal ultrasound echogram and bone scintigram were performed every 6 months. Brain CT or magnetic resonance imaging (MRI) was performed as needed. Between 3 and 5 years after operation, such intensive examinations were performed every year, and 5 years postoperatively; chest X-ray and blood tests were performed every year. Intensive examinations, including chest CT and abdominal ultrasound echogram, were performed when necessary. Follow-up was continued over a long period or at least more than 5 years after operation to check for tumor recurrence and survival. Survival was calculated using the Kaplan–Meier method, and differences in survival were determined by the log-rank test. Multivariate analysis of several prognostic factors was carried out using the Cox proportional hazards regression model. Zero time was the date of pulmonary resection, and the terminal event was death due to cancer or non-cancerous causes. The chi-square test was used to examine the associations between PLC results and clinicopathological factors.
3 Results
Patient clinicopathological characteristics are shown in Table 1 . There were 476 men and 203 women whose ages ranged from 21 to 87, with a median of 64 years. The resected tumors ranged from 5 to 140 mm, with a median of 31 mm.

Eighty-nine (13.1%) of 679 patients had positive PLC findings. Table 2 shows an association between PLC-positive findings and clinicopathological factors. Positive PLC findings were observed more frequently in patients with more advanced T factor (T1,2 vs T3,4, p ≪ 0.001), N factor (N0 vs N1–3, p = 0.024) and stage (I vs II–IV, p ≪ 0.001), larger tumor size (smaller than 31 mm vs larger than 30 mm, p = 0.034), greater involvement of the pleura (P0,1 vs P2,3, p ≪ 0.001), and more aggressive lymphatic (negative vs positive, p = 0.031) and vascular (negative vs positive, p ≪ 0.001) invasion. As for histological type, positive PLC findings were observed marginally more frequently in patients with adenocarcinoma type, in comparison with squamous cell carcinoma type (p = 0.082).
Because of intraoperative PLC-positive findings, postoperative intrathoracic chemothermotherapy (PICT) was selectively performed for seven patients within the period. This therapeutic modality was previously described in detail by Kodama et al. [13,14].
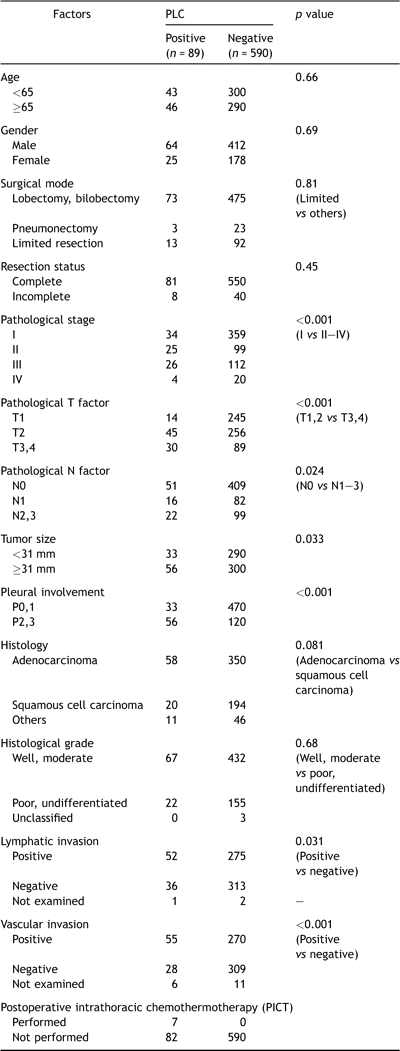
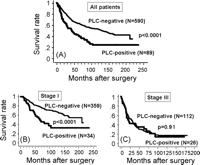
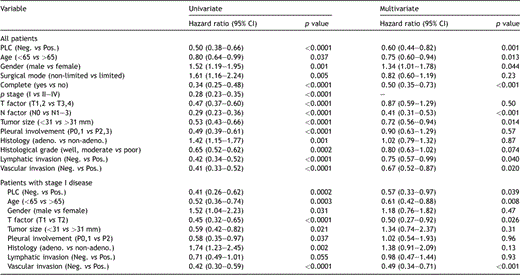
Among the 342 surviving patients in the database top, 58 (17.0%) were lost to follow-up during the initial 5-year postoperative period, but the rest (83.0%) could be followed up for more than 5 years. The median follow-up period for all patients was 6.7 years, ranging from 0.5 month to 19.6 years. The overall 5- and 10-year survival rates in all patients in the present series were 62% and 51%, respectively. The overall 5- and 10-year survival rates in PLC-positive patients were 43% and 25%, respectively, while those in PLC-negative patients were 66% and 58%, respectively (p ≪ 0.0001) (Fig. 1A ). Among 393 patients with stage I disease, 34 (8.7%) showed PLC-positive findings, and their 5- and 10-year overall survival rates were 57% and 33%, respectively, which was significantly poor compared with those with PLC-negative findings (80% and 68%, p ≪ 0.0001) (Fig. 1B). However, these clear differences disappeared as the disease stage progressed; the difference in the overall survival rate was p = 0.10 among patients with stage II disease, p = 0.91 with stage III disease (Fig. 1C), and p = 0.40 with stage IV disease, respectively. According to univariate analyses, all the listed 14 clinicopathological factors, age, gender, surgical mode, resection status, T factor, N factor, stage, tumor size, pleural involvement, histology, histological grade, lymphatic and vascular invasions and PLC findings had a statistical influence on the postoperative prognosis (Table 3 ). According to multivariate analyses using 13 prognosis-associated factors, except for stage (Table 3), age, gender, resection completeness, N factor, tumor size, lymphatic or vascular invasions, or PLC finding were independent prognostic indicators in all the tested patients. As for patients with stage I disease, 9 factors, age, gender, T factor, tumor size, pleural involvement, histology, lymphatic and vascular invasions, and PLC findings were potential prognosis-associated factors according to univariate analyses (Table 3). Multivariate analyses showed that age, T factor, vascular invasion and PLC findings were independent prognostic indicators in this population. According to the prognostic analysis of PLC based on histological type, while PLC findings for patients with adenocarcinoma have a strong impact on survival, they are lacking in prognostic value for squamous cell carcinoma. While 5- and 10-year overall survival rates of PLC-positive patients with adenocarcinoma were 42% and 22%, respectively, which was significantly worse than those with PLC-negative findings (72% and 71%, p ≪ 0.0001), the survival rates of PLC-positive patients with squamous cell carcinoma were 53% and 34%, respectively, in comparison with PLC-negative patients (59% and 48%, p = 0.24) (Fig. 2A and B ).
Overall survival curves according to intraoperative PLC status: stage-based analysis. (A) All tested patients, (B) patients with stage I (IA + IB) disease, (C) patients with stage III (IIIA + IIIB) disease. The overall survival curves of all tested patients and those with stage I disease are shown in parts A and B. PLC-positive patients showed significantly poorer prognosis than those with PLC-negative findings (all patients: p ≪ 0.0001, those with stage I disease: p ≪ 0.0001); however, such prognostic significance disappeared in patients with stage III disease (C).
Univariate and multivariate analysis results for prognosis-associated factors.
Overall survival curves according to intraoperative PLC status: Histology-based analysis. (A): Adenocarcinoma, (B) squamous cell carcinoma. Whereas PLC findings for patients with adenocarcinoma had a significantly strong impact on survival (p ≪ 0.0001), those for squamous cell carcinoma lacked prognostic value (p = 0.24).
Regarding the tumor recurrence pattern among PLC-positive patients, distant metastases (32/89, 36.0%) were more commonly observed rather than local recurrence (24/89, 27.0%); however, it was important that 21 (23.5%) of the PLC-positive patients also showed postoperative pleural recurrence, which were observed in only 14 (2.4%) of PLC-negative patients. In addition, based on Kaplan–Meier analysis only regarding pleural recurrence as the event, the difference was also significant (Fig. 3 , p ≪ 0.0001, hazard ratio = 0.08, 95% CI = 0.04–0.15). It was noted that, whereas the rate of pleural recurrence more than 5 years after operation was only 0.3% (2/590 patients) among PLC-negative patients, such a late recurrence occurred in five (5.6%) of the PLC-positive patients, all of whom had stage I disease, and surprisingly, two of whom showed a very late recurrence after more than 10 years of follow-up. Among seven patients undergoing PICT, three showed local pleural recurrence, as shown by arrows in Fig. 3. Of five patients with late pleural recurrence, two patients had been treated with PICT.
![Pleural recurrence-free survival curves according to PLC status. Patients with PLC-positive findings showed pleural recurrence more frequently than those with PLC-negative findings (p ≪ 0.0001, hazard ratio = 0.08, 95% CI = 0.04–0.15). Late pleural recurrence 5 years after surgery was observed in five patients (5.6%) among PLC-positive patients. Arrows show pleural recurrence among PLC-positive patients undergoing postoperative intrathoracic chemothermotherapy (PICT) [13,14].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ejcts/35/2/10.1016_j.ejcts.2008.10.013/1/m_337.S1010794008010324.gr3.gif?Expires=1749430555&Signature=fDrc-NvaHmvSuDekAjyWH-u~0uykO2ftu4Cvj8YkyyEb4Xy8yy10f0fwGCEpgFn-i-IH5dHi6XmwSqjUxGdZb7GMYToV~FDOLLjIhK11x3kkQuVta7UqAFrviaX1wuJYQzT7vk4TgGFFlNEM3DUzqYQBWlBtqhgJUfMTSSdy9njnNehTKCEXQ-veKnujYCxge9xMPPK11prKQks~YtPmOmVQB6LYcLsx2LrRCjZOajDZZrMRmQktf3NMjys-Tg8J70eOTauyqfSu257MBBPxF9BByxtYVI1dZGTLksKrKmovAan5aJH6s8-W2m2xyy7vXnsnoqBPCC8CStkvEICccw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Pleural recurrence-free survival curves according to PLC status. Patients with PLC-positive findings showed pleural recurrence more frequently than those with PLC-negative findings (p ≪ 0.0001, hazard ratio = 0.08, 95% CI = 0.04–0.15). Late pleural recurrence 5 years after surgery was observed in five patients (5.6%) among PLC-positive patients. Arrows show pleural recurrence among PLC-positive patients undergoing postoperative intrathoracic chemothermotherapy (PICT) [13,14].
4 Comments
In the present study, we conducted postoperative follow-up for a long period, at least for more than 5 years, and obtained clinical information about recurrence and survival regarding the loco-regional pleural cavity. As a result, postoperative follow-up could be performed for more than 80% of surviving patients. In contrast, follow-up periods in many previously published reports were only 5–7 years [1–6,9], and such data may be insufficient to conclude the clinical significance of PLC. In fact, it is important that late pleural recurrence more than 5 years after surgery occurred in a minority of PLC-positive patients.
PLC status in patients undergoing surgical treatment for lung cancer is widely accepted to be a powerful prognosis-associated factor, as reported previously by many investigators [1–9], some who showed that it was an independent prognostic indicator by multivariate analyses [2–4,8]. In contrast, according to our preliminary report [9], PLC status immediately after thoracotomy was statistically lacking in prognostic value, although it univariately influenced postoperative survival. In the present study using many patients for long-term follow-up, its prognostic value was clearly demonstrated.
Thus, it can surely be concluded that PLC status has an important value in prognosis, at least for surgically treated patients, but interestingly, it was noted that its prognostic significance was limited in patients with rather early stage diseases. Namely, a difference in the PLC status effect on survival in patients with stage I disease was statistically observed, while it was not seen in those with stage III disease. In patients with stage II disease, the p value was marginally 0.098. Similar data have been previously reported by several investigators [1,2,6,7], but this study may be the first to describe clearly that the prognostic implication of PLC was found only among patients with early stage (stage I) disease. In contrast, considering that the PLC-positive rate is closely correlated with tumor progression-associated markers such as T factor, N factor, tumor size, pleural involvement, lymphatic and vessel invasion, the prognostic significance of the PLC status gradually disappeared in more advanced stage disease.
In addition, it is interesting that the prognostic significance of PLC was observed in adenocarcinoma, not in squamous cell carcinoma. Also, it was noted that the PLC-positive rate was marginally different between these histological types. The difference in PLC significance by histology has been pointed out by several investigators [1,2,4,6], but the reason is still unknown. We speculate that such a difference is owing to some differences in the biological and oncological characteristics between these two representative histological types. In our previous report [9], the number of cell clusters in the PLC solution was important for the postoperative clinical course, and there were generally fewer in the PLC solution in squamous cell carcinoma than in adenocarcinoma. Thus, PLC had little clinical significance in patients with squamous cell carcinoma, but the histological difference of prognostic significance should be re-evaluated using a larger group of patients.
When examining the postoperative recurrence site, distant metastases were commonly observed even among PLC-positive patients (36%). Considering the clinical findings of the PLC status in relation to tumor progression and poor prognosis, distant metastases were easily regarded as the major pattern of tumor recurrence among PLC-positive patients [2], in particular those with advanced stage disease. On the other hand, positive PLC findings in patients have been described as reliable information for potential carcinomatous pleuritis by many investigators [2,6,9,10]. The rate of pleural recurrence among such PLC-positive patients was considered to be 15–35%. We also reported preliminarily that pleural failure occurred in 17 (18%) of 97 PLC-positive patients and, in this series, the rate was as high as 23.5%. Moreover, we should pay attention to the timing of pleural recurrence. In the present study, it was noted that 5.6% of PLC-positive patients showed late pleural failure more than 5 years after operation and, to our surprise, very late pleural recurrence after more than 10 years occurred in two patients. Importantly, all patients showing such late or very late local recurrence in the pleural cavity had stage I disease. Therefore, for PLC-positive patients with stage I disease, long-term follow-up may be strongly required, especially focusing on local pleural recurrence. In other words, while systemic therapy should be planned for PLC-positive patients with advanced stage disease, local control therapy is also required for those with early stage disease. In our institute, PICT has been aggressively performed for selected patients with pleural dissemination [13,14] and, recently, its application was preliminarily expanded for PLC-positive patients with stage I or II disease. In the present series, only seven patients underwent this therapeutic modality, but the clinical effect has been not evaluated because of the small number. Several investigators have reported the possible usefulness of intrapleural hypotonic cisplatin therapy during operation [6,15,16]. Satoh et al. [6] emphasized that such local therapy may not be sufficient for prognosis improvement, but we think that a local therapeutic modality should be established as the first step for selected PLC-positive patients.
In conclusion, according to the present analysis of long-term follow-up after surgery, PLC immediately after thoracotomy for lung cancer may be an independent prognostic factor, especially for patients with stage I disease or adenocarcinoma. PLC-positive findings may indicate a high risk of pleural recurrence. Importantly, for PLC-positive patients with stage I disease, serial careful follow-up for more than 5 years is necessary while paying attention to late pleural recurrence.
References
Author notes
Presented at the 16th European Conference on General Thoracic Surgery, Bologna, Italy, June 8–11, 2008.




