-
PDF
- Split View
-
Views
-
Cite
Cite
Abdullah Kaya, Robin H. Heijmen, Hervé Rousseau, Christoph A. Nienaber, Marek Ehrlich, Philippe Amabile, Jean-Paul Beregi, Rossella Fattori, Emergency treatment of the thoracic aorta: results in 113 consecutive acute patients (the Talent Thoracic Retrospective Registry), European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 2, February 2009, Pages 276–281, https://doi.org/10.1016/j.ejcts.2008.10.052
Close - Share Icon Share
Abstract
Background: Elective thoracic endovascular aortic repair in selected patients with suitable aortic anatomy is associated with low morbidity and mortality, and is gaining widespread acceptance. Its benefit in acute thoracic aorta diseases, however, has not yet been demonstrated in high-numbered studies. This report presents data of the Talent Thoracic Retrospective Registry (TTR) of all patients who underwent endovascular stent grafting for acute thoracic aorta pathology. Methods: Between December 1996 and July 2004 data were collected regarding 113 consecutive patients who underwent emergent endovascular stent grafting of the thoracic aorta using the Talent thoracic stent graft (Medtronic, Inc., Santa Rosa, California) in 7 European referral centers. Acute thoracic aorta pathology consisted of 41 (36.3 %) traumatic aortic injuries, 37 (32.7 %) Stanford type B dissections, 5 (4.4 %) intramural hematomas, 18 (15.9 %) thoracic aorta aneurysms, 4 (3.5 %) pseudoaneurysms and 8 (7.1 %) penetrating ulcers. Results: In all patients the stent graft system could be introduced via the common femoral artery. Conversion to open surgical repair was necessary in 2 patients, one in the early phase due to persistent bleeding via backflow in the false lumen from a distal entry tear, and another patient in a late phase due to retrograde dissection. Intraoperative mortality was 1.8%, one patient suffered a massive myocardial infarction, and another died of tamponade secondary to retrograde dissection. Overall hospital mortality was 8.0% (9 patients). In only 2 of them, it was considered a stent graft procedure related death. New neurological symptoms were seen in 6.2% (7 patients), with complete recovery in 5 patients. Mean follow-up was 15 months (range 1–69 months). Late mortality was 8.7% (9 patients). Only one late death was considered aorta related. Overall re-intervention rate was 8.9% (n = 10) and was mainly for type I endoleak or persistent false lumen perfusion. Conclusion: Sub-analysis of the Talent Thoracic Retrospective Registry for endovascular stent grafting of acute thoracic aorta pathology in over 100 consecutive patients demonstrated its feasibility, with low morbidity and acceptable low mortality rates.
1 Introduction
Open surgical repair of the descending thoracic aorta in emergency settings carries a very high surgical risk. Reported mortality reaches 45%, paraplegia, pulmonary complications or renal failure may be observed in 12–45% of patients [1–6]. Thoracic endovascular aortic repair is associated with reduced morbidity and mortality when compared to open surgical repair [7–12]. In addition, patients considered unsuitable for surgery because of severe comorbid conditions may benefit from this less-invasive technique.
Currently, however, the benefit of endovascular stent grafting for acute thoracic aorta pathology has only been reported in small-numbered, single-center experiences. The Talent Thoracic Retrospective Registry (TTR) contains outcome data from patients who underwent endovascular stent grafting using the Medtronic Talent thoracic stent graft (Medtronic, Inc., Santa Rosa, California) in 7 European referral centers [13]. This report presents data of a sub-analysis of the TTR of all patients who underwent endovascular stent grafting for acute thoracic aorta pathology.
2 Patients and methods
The Registry has been described before [13]. In summary, 7 European referral centers (Nieuwegein, Bologna, Lille, Marseille, Rostock, Toulouse and Vienna) delivered data from patients treated with the Talent thoracic stent graft, with a minimum of 1-month follow-up. From the entire cohort study, 457 patients, data were collected of 113 (24.7 %) consecutive patients who underwent acute endovascular repair of the thoracic aorta between December 1996 and July 2004. The TTR study was approved by the ethical committee of each institute. Patient and thoracic aorta pathology characteristics are listed in Table 1 . Clinical information and imaging findings at last visit date were gathered and analyzed for adverse events. Data were collected on case report forms and checked for inconsistencies by the last author (R.F.).
2.1 Patient characteristics
There were 88 male and 25 female patients with a mean age of 54.3 years (range 19–91 years). Aortic pathology treated by acute endovascular stent grafting consisted of 41 (36.3 %) traumatic aortic injuries, 37 (32.7 %) Stanford type B dissections, 5 (4.4 %) intramural hematomas, 18 (15.9 %) thoracic aorta aneurysms, 4 (3.5 %) pseudoaneurysms and 8 (7.1 %) penetrating ulcers. All cases had one or more of the following symptoms: signs of rupture, impending rupture, distal organ malperfusion or persistent pain in spite of adequate antihypertensive therapy. Comorbid medical conditions consisted of hypertension (n = 58, 51.3%), previous cardiac event such as myocardial infarction (n = 21, 18.6%), chronic obstructive pulmonary disease (n = 17, 15.0%), renal insufficiency (n = 16, 14.2%; serum creatinine > 120 μmol/l), diabetes (n = 15, 13.3 %) or previous cerebrovascular accident (n = 7, 6.2%). Previous aortic surgery was reported in 16 (14.2%) patients. One patient had undergone cardiac surgery previously. None of the patients had received prior endovascular repair of the abdominal aorta. American Society of Anesthesiologists (ASA) classification > III was present in 62 (54.9%) patients. Mean aortic diameter for atherosclerotic aneurysms was 63.6 ± 18.4 mm (range 35–100 mm).
2.2 Imaging
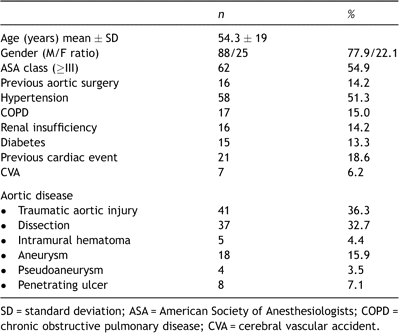
Diagnostic imaging in the emergency setting was most frequently by computed tomography scan alone (59.0%, Fig. 1 ) or in association with angiography (25.7%). Follow-up imaging frequency was according to the set protocol of each center, generally consisting of 1–6 months and yearly thereafter and comprised mainly of a computed tomography scan (77.3%) alone, combined with angiography (8.9%), magnetic resonance imaging alone (5.9%) or a combination of above with or without transesophageal echocardiography (7.9%). Data on exclusion of the pathology, the size of expansion or reduction of the aneurysm, endoleak, aneurysm sac or false lumen thrombosis, or stent-graft material alteration were collected.
(A) Computed tomography scan shows a ruptured post-dissection thoracic aorta aneurysm. (B) The patient survived the acute period after endovascular stent grafting (28 mm). (C) Result after 18 months follow-up.
2.3 Procedure
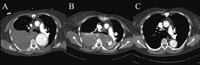
All procedures were performed using general anesthesia. The technique of stent graft insertion has been described before [12]. Total number of stent grafts used was 143 (mean 1.27 stent graft per patient). Lesions could be excluded by one stent graft in 91 patients (80.5%, Fig. 2 ), by 2 stent grafts in 16 patients (14.2 %) and more than 2 stent grafts were needed in 6 patients (5.3%). Mean length of covered aorta was 127 mm, ranging from 28 to 380 mm. Oversizing of the stent graft diameter by 10–20% compared with the diameter of the aortic neck for adequate fixation was used in chronically dilated thoracic aortas only.
(A) Aortic angiogram demonstrates a ruptured type B aortic dissection with multiple entry tears. (B) Angiographic result after placement of a Talent stent graft.
3 Results
In all patients the common femoral artery could be used for introduction of the stent graft system. The stent grafts could all be deployed at the intended position, which was predominantly localized in the distal aortic arch or proximal descending aorta, necessitating complete coverage of the ostium of the left subclavian artery in 14 of 113 patients (12.4%). In one of them, the left subclavian artery was transposed to the common carotid artery prior to stent grafting.
Conversion to open surgery 6 h after the stent graft procedure was necessary in 1 patient (0.9%), because of persistent bleeding following endovascular stent grafting for a ruptured aortic dissection, conceivably through backflow in the false lumen from a distal entry tear.
An additional intervention was indicated in 8 patients (7.1%), consisting of percutaneous drainage of hematothorax (n = 3), renal artery stenting for malperfusion (n = 1), abdominal aorta fenestration for distal malperfusion (n = 1), both following aortic dissection, and surgical reconstruction of the iliac artery because of iatrogenic dissection or rupture due to stent graft introduction (n = 3).
3.1 Mortality
The majority of patients were admitted to the intensive care unit following the stent graft procedure (92%). Median length of hospital stay was 13 days (range 1–90 days). Overall hospital and 30-day mortality was 8.0% (n = 9). Two of them (1.8%) died intraoperatively (procedural mortality). One patient was in severe shock preoperatively and died during stent graft implantation for a ruptured dissection. The other patient died of a massive myocardial infarction directly following successful stent graft implantation for a ruptured dissection (confirmed by autopsy). Two patients died during their hospital stay due to stent graft procedure related causes; one patient deceased due to recurrent bleeding caused by a massive type II endoleak from the left subclavian artery into the ruptured aneurysm sac (confirmed by autopsy), and the other patient developed a retrograde type A dissection postoperatively and died subsequently due to pericardial tamponade. Five of 9 patients have died due to non-stent graft related causes, such as cardiorespiratory failure (n = 4) and multiple organ failure (n = 1). This is outlined in Table 2 .

3.2 Morbidity
Incomplete exclusion of the thoracic aorta pathology following the procedure, i.e. endoleak, was observed in 15 patients (13.3 %). Predominantly type I endoleak (‘attachment leak’) was noted (13 of 15), with a type III endoleak (‘modular leak’) in 2 patients. This gives a technical success rate of 86.7% and a clinical success rate of 82.3% as defined in the reporting standards [14]. In 1 patient, the type I endoleak increased as noted on follow-up angiography and an endovascular re-intervention was successfully performed by balloon dilating the proximal fixation zone. All other endoleaks were small, and were closely followed up by computed tomography scanning. One patient underwent successful endovascular stent graft extension 4 days postoperatively because of persistent bleeding after endovascular repair of a ruptured dissection.
Other interventions performed during hospital stay were surgical evacuation of hematothorax by thoracoscopy (n = 2), transposition of the left subclavian artery to the common carotid artery for malperfusion of the left upper extremity (n = 1), and a femorofemoral crossover bypass for right lower limb ischemia after successful stent grafting of a ruptured thoracic aorta aneurysm.
Postoperatively, 7 patients (6.2 %) developed new neurological symptoms. Ischemic stroke occurred in 5 patients, subarachnoid hemorrhage in one patient, and transient paraparesis in one patient. The anatomic distribution of the ischemic stroke was various. Three patients had a right hemispheric stroke of which 2 intentionally had overstenting of the left subclavian artery ostium. One patient had a left hemispheric stroke and in another patient it was located in the brainstem, both without overstenting of the left subclavian artery ostium. In only 2 patients, the stroke resulted in permanent neurological damage. Three patients experienced an extensive post-traumatic respiratory distress syndrome.
3.3 Follow-up
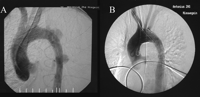
Mean follow-up was 15 months (range 1–69 months) and was complete for all hospital survivors treated with the Talent thoracic stent graft (n = 103). A follow-up for more than 2 years was available in 29 patients (28.1 %). There were 9 deaths (8.7 %) occurring following hospital discharge. The interval ranged from 1 to 19 months. Causes of death included pneumonia, respiratory insufficiency, neoplasm, myocardial infarction, and cerebrovascular accident in 2 patients. In 3 patients the cause of death was unknown (Table 3 ). Among the late deaths, there were 2 patients being followed closely for an endoleak (type I and III). One succumbed due to a stroke, and the other died of an unknown cause, 2 and 1 month after the stent graft procedure, respectively. Two of 9 patients that died during follow-up are therefore considered aorta related. The overall survival at 1 year is 84.1% (95% confidence interval; 75.2–90.0%) and at 2 years is 81.7% (95% confidence interval; 71.4–88.6%). This is illustrated in Fig. 3 .

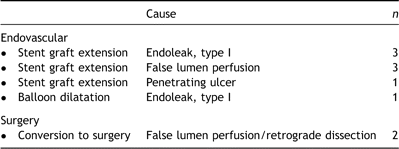
During follow-up, 3 patients developed a late occurring endoleak. Two patients, initially treated for aortic dissection and penetrating aortic ulcer respectively, developed a type I endoleak 3 and 6 months postoperatively, which was treated successfully by stent graft extension. One other patient, treated for aortic dissection, developed residual false lumen flow due to intercostal artery backbleeding (type II), which had resolved spontaneously at 6 months.
Six out of the initial 15 endoleaks had sealed spontaneously during follow-up (5 type I and 1 type III endoleak). Three patients needed a re-intervention: 1 patient, treated for degenerative aneurysm, had persistent type I endoleak with aortic diameter expansion, for which proximal stent graft extension was performed successfully at 19 months postoperatively. Another patient experienced retrograde dissection to the proximal aorta at 1 month, and was successfully converted to open repair. The third patient developed a penetrating ulcer at the proximal fixation site of the endoprosthesis at 4 months, which was treated by stent graft extension. The other 6 of 15 patients were kept conservatively. At the end of the follow-up period, however, aneurysm diameter has increased in 2 patients, and endovascular re-intervention may be indicated.
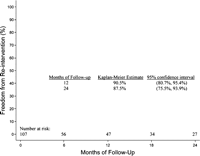
In the group of patients without initial endoleak, 3 re-interventions were necessary during follow-up. One patient, treated endovascularly for type B aortic dissection developed an unrelated type A dissection, with the intimal tear in the aortic root, during follow-up and was successfully operated. Two other patients had distal entry tears with persistent false lumen perfusion for which stent graft extension was performed distally at 5 and 7 months postoperatively. Overall re-intervention rate was 8.9% (n = 10). Eight of 10 were managed successfully by endovascular means (Table 4 ). The freedom from re-intervention at 1 year is 90.5% (95% confidence interval; 80.7–95.4%) and at 2 years is 87.5% (95% confidence interval; 75.5–93.9%), as presented in Fig. 4 .
4 Discussion
In elective patients with suitable aortic anatomy thoracic endovascular aortic repair has demonstrated its feasibility as an alternative treatment to open surgical repair [7–9]. This less-invasive approach may have its impact on the observed high mortality rate, associated with emergent surgical repair of the descending thoracic aorta [1–6]. So far, results of emergent stent grafting for acute descending aortic pathology have been reported in case reports or small-numbered studies [15–20].
The current analysis of the Talent Thoracic Retrospective Registry of 113 patients treated endovascularly for acute pathology demonstrates a hospital mortality of only 8.0%. Only 2 of 9 in-hospital mortalities were stent graft related (recurrent bleeding from massive type II endoleak and retrograde type A dissection). The majority have succumbed as a result of their poor preoperative clinical state, which, however, is probably also the reason of most deaths after open surgery. During a mean follow-up of 15 months, mortality was 8.7%. Only 2 deaths were considered aorta related. So, despite a low procedural risk in these high-risk patients with hospital mortality comparing favorably to open surgery, additional mortality is observed during early follow-up reducing long-term survival (Fig. 3).
In this study, new neurological symptoms were noted in 6.2% of patients, which is comparable to other small-numbered emergency stent graft reports [15–20]. A vast majority, however, resolved completely during hospital stay. Paraparesis occurred in 1 patient who received a single stent graft in the proximal descending aorta to exclude the entry tear of a ruptured type B aorta dissection. In contrast, 5 patients have received 3 stent grafts and 1 patient had 5 stent grafts for an elongated, tortuous thoracic aorta aneurysm and none of these patients experienced spinal ischemia. It appears that in the acute setting, covering an extensive section of the descending aorta may not be a risk factor for spinal ischemia.
Given the fact that in the acute setting details on arterial access are scarce, access difficulties may be expected. In the current study, however, in all patients the stent graft could be introduced through the common femoral artery. In literature, access failure is reported to be 1.5–7.7% [15–20]. Nonetheless, 3 patients needed surgical reconstruction of the iliacofemoral artery postoperatively, and 1 patient needed femorofemoral bypass grafting because of right lower limb ischemia. Potentially, the high number of acute dissection cases often lacking severe atherosclerotic peripheral arterial disease, may have reduced the number of observed access failure in the current report.
In addition, important information concerning the carotid and vertebral arteries is also scarce in the acute setting. Previous analysis of the total group of patients in the current TTR Registry demonstrated that overstenting the left subclavian artery to lengthen the proximal landing zone was a risk factor for stroke [13]. In the current report 2 of 13 patients with overstenting of the left subclavian artery without prior transposition developed transient stroke. The anatomic distribution of the stroke, however, was on the right hemisphere in both patients. Moreover it may constitute a cause of endoleak: one patient has died due to a massive type II endoleak through retrograde flow in the left subclavian artery into the aneurysm sac. Autopsy revealed a connection between the left subclavian artery and the aneurysm sac despite adequate proximal and distal sealing of the stent graft in the aorta. One patient experienced malperfusion of the left upper extremity and underwent transposition of the left subclavian artery, whereas another patient underwent this surgical procedure immediate prior to stent grafting.
Although in-hospital mortality was low in this high-risk category of patients, close monitoring postoperatively is mandatory to detect stent graft procedure related complications, which may prompt endovascular or open surgical re-intervention. Overall re-intervention rate was 8.9%, during a mean follow-up of 15 months. In the majority of cases, a type I endoleak or persistent false lumen perfusion indicated treatment. The majority was treated successfully by endovascular means. In addition, 2 patients followed closely for an initial type I endoleak experienced diameter expansion, and may need re-intervention in the short term. Conversion to open surgery was only necessary in 2 patients (inadequate false lumen exclusion with persistent bleeding, and retrograde aortic dissection).
Although observed hospital mortality compares favorably to earlier reported mortality rates of open surgical repair for emergent/ruptured descending thoracic aneurysms from 20.9% up to 45.2% [1,2], for acute aortic dissections of 22.4% [3], and for traumatic aortic lesions of 20–23.5% [4–6], a true comparison is not possible as prospective, randomized trials are still lacking. Additionally, the obtained results of open surgery for similar emergency conditions in the participating centers of this registry are not available for comparison. One should take these limitations into account when interpreting reported data on either technique.
In conclusion, sub-analysis of the Talent Thoracic Retrospective Registry for endovascular stent grafting of acute thoracic aorta pathology in 113 consecutive patients demonstrated its feasibility, with low morbidity and acceptable low mortality rates. Long-term results are not known yet and continued close surveillance of endovascularly treated patients is important since stent graft related problems may arise during follow-up. This is particularly true for young patients treated for traumatic aortic rupture.
References
Author notes
This is a company registry study of the Medtronic Talent Thoracic stent graft. Authors Rossella Fattori, Christoph A. Nienaber, Hervé Rousseau, Jean-Paul Beregi, and Robin H. Heijmen each report receiving consulting and/or lecture fees from Medtronic.
- aorta
- myocardial infarction
- stents
- pseudoaneurysm
- aortic aneurysm, thoracic
- patient referral
- hemorrhage
- hematoma
- ulcer
- descending thoracic aorta
- tissue dissection
- emergency treatment
- femoral artery
- follow-up
- hospital mortality
- intraoperative care
- neurologic manifestations
- perfusion
- surgical procedures, operative
- morbidity
- mortality
- pathology
- transplantation
- persistence
- aortic injuries
- endoluminal grafts
- thoracic aorta diseases
- endoleak
- thoracic endovascular aortic repair




