-
PDF
- Split View
-
Views
-
Cite
Cite
Nawid Khaladj, Malakh Shrestha, Sven Peterss, Ingo Kutschka, Martin Strueber, Ludwig Hoy, Axel Haverich, Christian Hagl, Isolated surgical aortic valve replacement after previous coronary artery bypass grafting with patent grafts: is this old-fashioned technique obsolete?, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 2, February 2009, Pages 260–264, https://doi.org/10.1016/j.ejcts.2008.09.051
Close - Share Icon Share
Abstract
Aim: High-risk patients are currently being evaluated for various catheter-based aortic valve replacement (AVR) techniques. To identify an individual patient’s risk, scores such as the EuroSCORE or STS risk calculator (RC) are used. The aim of the present study was to evaluate the surgical results in patients who underwent isolated AVR via a median re-sternotomy after prior CABG. Patients and methods: Between 01/96 and 01/08, 349 patients underwent AVR as a redo procedure. One hundred and thirty patients had undergone previous CABG; in 39 patients (29 male, median age 75 (60–84)) preoperative coronary angiography revealed open grafts with no need for additional revascularization (30 had LIMA grafts). These patients underwent isolated AVR. Operative mortality was calculated using the standard and logistic EuroSCORE, and the STS RC. Results: Operative (30-day mortality) was 5% (2 patients). Mean calculated predicted mortality rates for the cohort were: 12 ± 3% for the standard, and 32 ± 21% for the logistic EuroSCORE, and 10 ± 4% according to the STS RC. Receiver operated characteristics (ROC) analysis revealed 100% specificity for standard EuroSCOREs up to 12.5%, logistic EuroSCOREs up to 39.7% and up to 17.45% for STS RC, with a sensitivity of 69.5%, 75% and 97.2%, respectively. The STS RC showed significant better prediction of mortality than the EuroSCOREs (p = 0.006). Conclusions: Conventional AVR as a redo procedure after CABG with patent grafts can be performed with excellent results and lower mortality than estimated. Results of newer catheter-based AVR approaches should not to be compared with artificial scores to justify high morbidity rates.
1 Introduction
Conventional aortic valve replacement (AVR) is the therapy of choice in patients with aortic valve disease [1]. However, increasing patient age and comorbidities may increase operative mortality [2]. Therefore, less invasive approaches, such as transfemoral or transapical techniques, have been developed [3,4]. In this context, risk stratification is performed using different risk calculators such as the EuroSCORE or STS risk calculator (RC). Although these scores may be useful in estimating an individual patient’s risk, special sub-cohorts may not be represented properly, resulting in refusal to consider conventional surgery in these patients. This study was performed to compare the operative outcome in a defined subset of high-risk patients with various different calculated scores.
2 Patients and methods
This retrospective study was approved by the institutional review board; no individual patient consent was required.
From 01/96–01/08, 349 patients underwent aortic valve replacement as a redo procedure at our institution. One hundred and thirty had undergone previous coronary artery bypass grafting (CABG): in 39 patients, preoperative coronary angiography revealed open grafts (30 LIMA grafts) with no need for additional revascularization. Median age was 75 years (range 60–84 years); 29 were male. Further preoperative data are shown in Table 1 .
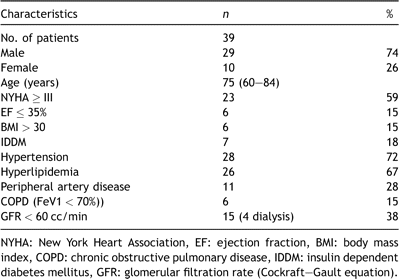
2.1 Preoperative management
All patients underwent standard preoperative examinations (chest X-ray, electrocardiogram (ECG) and blood tests), evaluation of their extracranial vessels (Doppler ultrasound), as well as pulmonary function tests for detection of chronic obstructive pulmonary disease (COPD) (forced expiratory volume in 1 s ≪ 70%). Coronary angiography (with special attention to the course of the LIMA graft in relation to the sternal wires), and echocardiography, were also routinely performed: a reduced ejection fraction was defined as ≤35%. Glomerular filtration rate (GFR) was estimated using the Cockraft–Gault equation; impaired renal function was defined as a GFR ≪ 60 cc/min. Computed tomographic (CT) scans of the chest were performed for visualization of bypass grafts, and distances of the brachiocephalic vein, the aorta and the right ventricle from the sternum. Furthermore, gross evaluation or Doppler examination of the femoral vessels was performed in anticipation of possible groin cannulation.
2.2 Surgical technique
Ventilation was discontinued immediately prior to re-sternotomy. Patients were placed in Trendelenburg position to decrease the right ventricular preload. Care was taken to avoid hypertension. The skin above one femoral artery was marked with a clamp for optional emergency cannulation.
After careful re-sternotomy, mediastinal structures were dissected, and bypass grafts identified if necessary. After systemic heparinization, in most cases, the ascending aorta was cannulated. Alternatively, in cases of major adhesions of cardiac structures to the sternum, one femoral artery was cannulated electively prior to sternotomy (n = 3). In the majority of cases, venous return was established with a two-stage cannula placed in the right atrium. CBP was initiated, and the patient cooled to a target temperature of 32 °C. A vent was placed in the left heart through the right superior pulmonary vein. Myocardial protection was achieved by administration of cold blood cardioplegia (6–8 °C) into the aortic root. In selected cases, retrograde cardioplegia was given. The LIMA graft was clamped selectively. After cardioplegic cardiac arrest, aortic valve replacement was performed in a standard fashion using a biological or mechanical prosthesis, dictated by the patient’s age and preference. Intraoperative data are shown in Table 2 .
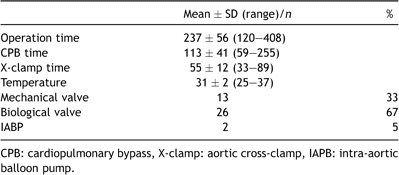
2.3 Score calculation
All patient data were entered by an independent party into the EuroSCORE version available online (standard/logistic) and the STS risk calculator, and predicted mortality was calculated for all three scores.
2.4 Statistical analysis
Results were expressed as mean ± standard deviation, median plus range, or percentage, respectively. Statistical analysis was performed using Mann–Whitney test, or Fisher’s exact test for categorical variables. Receiver operating characteristics (ROC) were plotted: the area under the curve as well as sensitivity and specificity were calculated. Exact p values and exact confidence intervals were calculated using the Clopper–Pearson method. A p value less than 0.05 was considered to be significant. Statistical analysis was performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA) and StatXact 6 software (Cytel Inc., Cambridge, MA, USA) in co-operation with the Department of Biometrics of Hannover Medical School.
3 Results
Overall mortality was 5% (2 patients). One patient died following ventricular fibrillation of unknown etiology. Another patient died from multi organ failure after sepsis. All other patients were discharged home or into a rehabilitation facility. Observed mortality for the entire group, as well as the calculated scores, is plotted in Fig. 1 .
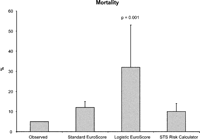
Mortality of the study population versus calculated EuroSCOREs and STS Risk calculator, data are expressed as mean ± standard deviation.
All three scores overestimated mortality with an observed versus expected mortality ratio of 0.42 for the EuroSCORE (0.16 logistic) and 0.5 for the STS RC.
Statistical analysis revealed significantly more accurate prediction of mortality for the STS RC (p = 0.006) compared with both EuroSCOREs.
Exact p values and the appropriate confidence interval for the mortality are shown in Table 3 .

ROC curves were plotted for all three scores (Figs. 2–4 ), with the following sensitivity and specificity:
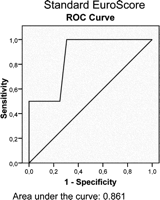
Receiver operating characteristics (ROC) curve for the standard EuroSCORE.
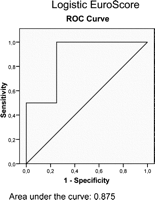
Receiver operating characteristics (ROC) curve for the logistic EuroSCORE.
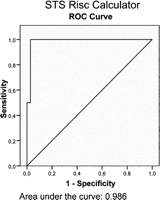
Receiver operating characteristics (ROC) curve for the STS risk calculator.
Standard EuroSCORE: sensitivity 100% (for an estimated risk up to12.5%); specificity 69.45%;
Logistic EuroSCORE: sensitivity 100% (for an estimated risk up to 39.7%); specificity 75%;
STS RC: sensitivity 100% (for an estimated risk up to 17.45%); specificity 97.2%.
Patients who died were significantly older (82 ± 2.88 years vs 72 ± 5.78 years, p = 0.01). There were also significantly more patients requiring preoperative dialysis who died following surgery (p ≪ 0.008).
4 Discussion
Aortic valve replacement as a redo procedure can be performed with excellent results and lower mortality than predicted from standard formulae [5–7]. The majority of studies have had a focus on isolated redo aortic valve procedures; only a few papers have been written on AVR with redo myocardial revascularization after previous CABG [8–10].
In this context, a recent study of more than 2600 patients revealed that re-operation itself is not an independent predictor of mortality during AVR [5]. Predictors of mortality were active endocarditis; preoperative NYHA class, and the amount of comorbidity. Comparable results have been published by Potter and co-workers [7].
Several studies have documented that the EuroSCORE is an accurate model for risk stratification in cardiac surgery [11,12]. However, in high-risk populations, the value of the EuroSCORE is questionable, especially for elderly patients undergoing AVR [2,13]. This may be due to the fact that the EuroSCORE has not been calibrated for isolated AVR [14]. Nevertheless, the score is frequently used to justify non-standard or experimental procedures [3,4]. This may cause a selection bias with regard to surgical treatment of patients classified by the EuroSCORE as high risk.
The STS RC is considered a valuable alternative. A very recent study by Dewey and co-workers concluded that the STS predicted risk of mortality most accurately predicted perioperative and long-term mortality for the highest risk patients having aortic valve replacement [15]. Similar results were found in the current study. Receiver operated characteristics also revealed much better predictive values for the STS score.
Our data show that even so-called high-risk patients can be operated with a lower operative risk than generally anticipated. A multi-center study dealing with how to develop a risk model for in-hospital mortality following AVR was published in 2007 [16]. Such individual well-defined models may help to identify specific patient risks for various different underlying pathologies in the future [17].
In addition to preoperative risk calculations, careful patient preparation is essential for a positive outcome following reoperative AVR with previous CABG. Preoperative examination and diagnostic tests, especially high resolution CT scanning of the chest, is the most important tool for proper operative planning. This can avoid injury to bypass grafts, native coronary vessels, the right ventricle, or the brachiocephalic vein during re-sternotomy [18].
Nevertheless, increased patient age as well as significant comorbidity, such as preoperative dialysis, were significant predictors for mortality in our study. This special subset of patients may profit from innovative techniques [19].
The impact of off-pump and percutaneous valve implantation for so-called ‘higher risk’ patients in this context has been discussed controversially. Beside limited experience with these new techniques, durability, periprosthetic regurgitation, high incidence of A-V block and incorrect implantation are the major drawbacks compared to conventional AVR [20,21].
5 Conclusion
Conventional AVR as a redo procedure after CABG with patent grafts can be performed with excellent results and a lower mortality than estimated. Results of newer catheter-based AVR approaches should not be compared with standardized anticipated mortality scores for conventional surgery in order to justify high morbidity rates for these alternative techniques. Thus far, most of the scores are not well calibrated for AVR in high-risk patients. Currently, the old-fashioned surgical approach, in experienced hands, should still be considered the gold standard of treatment for high-risk AVR.
Appendix A Conference discussion
Dr T. Walther (Leipzig, Germany): I think you have shown, in contradiction to your title, that it is not an ‘old-fashioned’ method but that conventional surgery is a standard we should all apply to suitable patients. Your patients are 75 years old, on average, and interestingly, those who died were 82, and that is more the target population we target with transcatheter techniques at present. So they may still be slightly different. Maybe you can comment on the overall risk. Of course, your EuroSCORE was high, but I think some of those you are treating with transcatheter techniques are even sicker and definitely slightly older than the 75-year mean age you mentioned.
How would you act with a patient with a porcelain aorta, for example, in these days? Of course, in a retrospective analysis, transcatheter techniques were not available.
And you pointed out the need, I think, for a good scoring system. At present we don’t have a new one. There may be one which is going to be developed for aortic valve stenosis. However, I personally feel the logistic EuroSCORE is very easy to get. You just need to divide it by two or three, that number, because we now overestimate the risk, and we have a good guideline if you then go to a patient and talk to him and decide what optimal approach to choose for him to treat him effectively. So what is your suggestion? What kind of scoring system should we use at present?
Dr Khaladj: The aim of this study was not to blame the transapical or transfemoral approaches but to keep in mind that patients, even at high surgical risk, can be operated conventionally. Furthermore, it is important to recognize that risk scores, which are commonly used, such as the EuroSCORE, are not calibrated for high-risk patients undergoing aortic valve surgery. We definitely agree that for patients who are very old, with a significant amount of comorbidities (e.g. requiring dialysis), these new techniques might be an intelligent option.
The other question?
Dr Walther: Well, just a question on porcelain aorta, how would you react at present in these days and which scoring system would you recommend using?
Dr Khaladj: If a porcelain aorta is present we have to implant a conduit or consider the patient for a transapical or transfemoral approach.
And the last question?
Dr Walther: Scoring systems, what would you recommend, a practical guide for surgeons?
Dr Khaladj: From our results, the STS risk calculator leads to reliable scores. But we also consider the patients conditions, the environment and discuss the procedure with the family. The primary goal must be the benefit for the patient and not the challenge for the surgeon.
Dr M. Antunes (Coimbra, Portugal): I just wanted to question you about some technical details. What technique did you use for protection of the myocardium? The reason why I ask this question is that I recently decided not to bother with the patent IMA graft, and perfuse cardioplegia into the aortic root, which involves the venous grafts. And we have found that the heart is half perfused by the IMA and half protected by the cardioplegia, with very good results. What is your preferred technique now?
Dr Khaladj: Fortunately three senior surgeons and co-authors of this paper are in the audience. If the chairmen allow, Dr Shrestha could comment on the technique of LIMA-dissection.
Dr Antunes: While he comes up, may I just make a short comment with regards to developing the scores. The Working Group on Valvular Heart Disease of the European Society of Cardiology is currently preparing a statement exactly on that subject. I think there is a pressing need for a new score specifically designed for aortic valve stenosis. None of these scores has been developed for valves, especially not for aortic stenosis.
Dr C. Campanella (Edinburgh, Scotland): My question was very much along the same line from the technical viewpoint. In other words, from your presentation I think most of the patients had a patent LIMA. How do you deal with the patient with two LIMAs and the patient with two LIMA grafts and two LIMA to graft, all patent? Do you have any other strategies, non-fibrillation, like perfusing all the time and just cross-clamp the aorta and perfuse the heart all the time?
Dr Khaladj: In this cohort there were no patients with two patent IMA grafts.
Dr Shrestha: First of all, the answer, in all our redo cases we do a preoperative CT scan to see how the LIMA and patent grafts lie. And most of the time if you can prepare the LIMA, we always clamp the LIMA and give blood cardioplegia antegrade, almost always antegrade, and, of course, you have to cool down the patients to 32 degrees. If in case that you cannot find the LIMA, sometimes it is very difficult, then the options would be to cool the patients down to 30 degrees, 28 degrees, and, in very rare cases, the extreme cases, you can do it under circulatory arrest, or you can also prepare the subclavian artery, the left subclavian artery, and just clamp it instead of trying to free the LIMA and then going forward for the organ protection. But the most important things are always blood cardioplegia antegrade. We had actually a double mammary, but I think it is the same. The most important thing is always cooling down the patients and doing it in a conventional way. But, of course, you have to be used to the techniques what you are doing every day.
Dr Campanella: May I make a comment, very brief? The alternative is why bother to cool the patient if you found the LIMA and give cardioplegia and all the rest of it? Let the LIMA perfuse the heart, keep normothermia, and do the valve replacement with a beating heart. We did present our results on Friday, 36 patients, mortality not over 5%, mortality of 0%. So I think it is a subject maybe many of us should look very critically to the old-fashioned technique of beating heart and normothermia.
References
Author notes
The manuscript was presented as an oral presentation at the forthcoming 22nd Annual meeting in Lisbon of the European Association for Cardio-Thoracic Surgery (EACTS).
This study was supported by the German Research Foundation (HA 2927/2-2).
These authors contributed equally to this work.
- coronary angiography
- coronary artery bypass surgery
- aortic valve replacement
- preoperative care
- surgical procedures, operative
- tissue transplants
- morbidity
- mortality
- catheters
- revascularization
- coronary artery bypass-left internal mammary artery (lima) graft
- patents
- surgical mortality
- european system for cardiac operative risk evaluation
- society of thoracic surgeons risk calculator




