-
PDF
- Split View
-
Views
-
Cite
Cite
Matthias E.W. Kirsch, Tomonori Ooka, Kostantinos Zannis, Jean-François Deux, Daniel Y. Loisance, Bioprosthetic replacement of the ascending thoracic aorta: what are the options?, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 1, January 2009, Pages 77–82, https://doi.org/10.1016/j.ejcts.2008.09.011
Close - Share Icon Share
Summary
Increasing patient age and improved durability of latest generation bioprostheses have stimulated the use of bioprosthetic devices in the setting of ascending aortic replacement as an alternative to mechanical valved conduits or aortic valve-sparing procedures. We performed an English literature review to assess different surgical options that have been described for bioprosthetic replacement of the ascending aorta. Reported options include: (1) composite valved conduits using a stented bioprosthesis; (2) composite valved conduits using a stentless bioprosthesis; (3) total xenopericardial valved conduits. Composite valved grafts using stented bioprostheses offer a safe and durable option for bioprosthetic replacement of the ascending aorta. Other options are of more recent use and await medium-term results.
1 Introduction
Since its first description by Bentall and De Bono in 1968 [1], the technique for composite aortic valve and root replacement using a mechanical valved conduit has evolved to become the ‘gold standard’ for the treatment of combined aortic valve and root disease [2]. Although this operation provides excellent long-term survival and durability of repair, it exposes the patients to the risks of valve- and anticoagulation-related complications such as thromboembolism and bleeding. Thus, in a highly selected low-risk patient population, we have observed a high linearized rate of minor thromboembolic events (10.3 per 100 patient-years; 95% CI: 7.29–13.31) after elective Bentall procedure using a mechanical valved conduit [3].
Consequently, valve-sparing procedures such as David’s reimplantation [4] or Yacoub’s remodeling procedures [5] have become an increasingly appealing alternative in patients for whom lifelong anticoagulation is contraindicated or undesirable. These procedures have been shown to provide encouraging results [6], even in clinical settings such as bicuspid aortic valve, Marfan syndrome or acute type A aortic dissection. However, these techniques are not always applicable in case of associated severe aortic valve disease. Furthermore, they are technically more challenging and require an undeniable learning curve. Other procedures designed to allow a biological replacement of the ascending aorta, such as aortic homografts or the Ross operation, cannot be considered as true alternatives owing to their limited availability and applicability [7].
Thus, several technical options have been devised to allow replacement of the aortic root and tubular ascending aorta using a bioprosthetic replacement device. This type of procedure would combine the benefits of avoiding lifelong anticoagulation to those of general applicability and low risk of technical failure. On the other hand, these techniques expose patients to structural bioprosthetic valve deterioration and its associated risk of reoperation.
In this article we will consider the currently described surgical options for bioprosthetic replacement of the ascending aorta. Three main technical approaches have been described and include: (1) composite valved conduit using a stented bioprosthesis; (2) composite valved conduit using a stentless bioprosthesis; (3) total xenopericardial valved conduit.
2 Composite valved conduit using a stented bioprosthesis
The intra-operative construction of a composite valved conduit using a stented bioprosthesis appears a straightforward approach considering the excellent reported long-term durability and low incidence of the thromboembolic events for both porcine and pericardial bioprostheses.
Classically, the stented bioprosthesis is implanted within a cylindrical graft [8,9]. The diameter of the graft is chosen 5 mm larger than that of the bioprosthesis to allow an easy fit and reduce the risk of physical contact between the aortic valve leaflets and the wall of the graft. The rinsed valve on its holder is placed inside the graft and the end of the graft is sutured to the sewing ring of the valve using a continuous polypropylene suture. The completed bioprosthetic valved conduit is then implanted using annular sutures that engage both the valvular sewing ring and the vascular graft. The construction of the composite graft in the operating room potentially lengthens both aortic cross-clamp and CPB times because of the absence of ready-for-use devices. However, the added time, reported in a few studies to be of 5–7 min, can hardly be expected to influence postoperative morbidity and mortality [8,9].
However, there are two concerns about this strategy. The first concern is about the durability of a stented bioprostheses implanted within a cylindrical graft. Indeed, in addition to the previously mentioned risk of physical contact between aortic valve leaflets and the wall of the graft, the reduced compliance of the prosthetic ascending aorta and the absence of sinuses of Valsalva are both known to increase the shear stress on the aortic valve leaflets [10,11]. All of these factors might contribute to leaflet injury and accelerated structural valve deterioration. Thus, some authors advocate the use of latest generation aortic grafts such as the Gelseal Valsalva graft (Sulzer Vascutek, Renfrewshire, Scotland) which provide pseudo-sinuses and a well-defined sinotubular junction [12]. This anatomical design might facilitate normal valve leaflet motion, reduce hemodynamic stress and thus have the potential for improved bioprosthetic valve durability [13]. Recently, however, Etz et al. [14] have reported excellent long-term durability of the stented, mostly bovine pericardial, bioprosthesis implanted within a straight cylindrical Dacron tube graft (Table 1 ). Thus, reoperation for structural valve dysfunction was required in only 1 of 275 patients (202 were male, mean age 69 ± 11 years) 12 years postoperatively. In addition, freedom from other valve-related complications such as thromboembolism, hemorrhage, and prosthetic valve endocarditis was satisfactory and similar to that observed after simple aortic valve replacement [14].
Summary of results after bioprosthetic aortic root replacement using different surgical techniques (see text for description of surgical techniques).
Another apprehension is the putative complexity of a reoperation in case of structural valve dysfunction. Indeed, since the bioprosthetic valved conduit is implanted using annular sutures that engage both the valvular sewing ring and the Dacron tube graft, replacement of the sole bioprosthesis is impossible and usually requires the re-replacement of the entire aortic root. Our [15] and other groups [16,17] have shown that aortic root replacement after previous cardiac surgery can be a high-risk procedure. Thus, two groups have described modified procedures principally aimed at simplifying reoperation [18,19]. In both techniques, the bioprosthetic valve is implanted within the Dacron tube graft in such a fashion that the proximal end of the graft forms a 2–20 mm long skirt below the valvular sewing ring. This sub-valvular skirt is then sutured directly to the patient’s aortic annulus using running polypropylene sutures (Fig. 1 ). Although these techniques require some attention to avoid coronary obstruction by the higher than usual seated bioprosthesis, they offer several theoretical advantages. First, the miniskirt is much more workable than the rigid sewing ring of a stented bioprosthesis and might facilitate the proximal suture line in case of a friable aortic annulus. Furthermore, the miniskirt can be bevelled in order to take off from the root in a more cranial direction than allowed by a straight Dacron tube graft. Second, the implantation of the bioprosthesis within the graft and not within the native aortic annulus allows its upsizing in case of small native aortic annulus [20]. Finally, in case of reoperation, the deteriorated bioprosthesis could be easily accessed by transection of the tube and explanted without interrupting the proximal suture line between the native aortic annulus and the miniskirt. A new prosthesis could then be sutured in its place, within the Dacron tube graft. However, such a reoperation has not yet been reported.
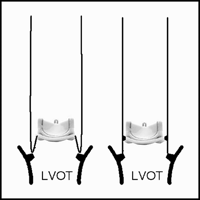
Schematic drawing of two technical options to construct a bioprosthetic valved conduit using a stented valve with creation of a sub-valvular Dacron skirt. LVOT, left ventricular outflow tract.
3 Composite valved conduit using a stentless bioprosthesis
In the setting of aortic valve replacement, implantation of a stentless bioprosthesis has repeatedly been associated with an improved level of left ventricular mass regression at 6 months, reduced transvalvular gradients and improved effective orifice area index [21]. However, whether these hemodynamic advantages translate into reduced postoperative morbi-mortality and improve late survival still remains controversial [22]. Nevertheless, these putative advantages have also prompted the use of stentless bioprostheses for the construction of composite valved conduits for bioprosthetic replacement of the ascending aorta.
Similar to the implantation techniques used for isolated aortic valve replacement, there are basically two techniques to combine a stentless bioprostheses with a Dacron tube graft in order to construct a composite valved conduit: (1) the sub-coronary technique and (2) the full-root technique with graft extension.
3.1 The subcoronary technique
This approach has been championed by Urbansky et al. who have been using a composite graft consisting of a stentless valve bioprosthesis incorporated within a sealed woven Dacron tube graft [23–25]. The composite graft is assembled intra-operatively after measuring the size of the aortic valve annulus. A stentless porcine valve (SPV Toronto; St. Jude Medical, Inc., St. Paul, MN) is placed inside a collagen-coated Dacron tube graft (Intergard; InterVascular, La Ciotat, France) leaving a proximal free margin of 3–5 mm in length. The proximal suture line of the stentless bioprosthesis is fixed to the Dacron tube graft with a running mattress suture. Doing this, the inevitable small difference in circumference between the Dacron tube graft and the stentless bioprosthesis has to be divided evenly to avoid tissue buckling. The authors report that they primarily oversized the stentless bioprosthesis in view of postoperative dilatation of the Dacron tube graft, but in some (non-specified) instances they preferred to undersize it. The free proximal end of the Dacron tube graft is then sutured to the native aortic annulus with pledgeted interrupted mattress sutures. Following this, the upper circumference of the stentless bioprosthesis is reimplanted within the tube graft using a second running mattress suture. As for conventional aortic valve replacement using a stentless substitute in sub-coronary position, great care has to be taken in order to avoid prosthetic valve distortion with subsequent regurgitation. The rest of the operation (coronary ostia implantation and distal aortic anastomosis) is completed in the usual fashion.
Urbansky et al. have recently reported their experience in 45 consecutive patients operated on between 1998 and 2001 with their homemade composite graft (Table 1) [25]. At a mean follow-up of 18 months, no patient required a valve-related reoperation and three thromboembolic events (two minor, one major) were recorded. Echocardiographic follow-up demonstrated favorable hemodynamics with mean transvalvular gradients of 8.0 ± 3.1 mmHg. Furthermore, they noted no regurgitation across the valve and no contact between the bioprosthetic cusps and the Dacron tube.
One of the emphasized advantages of this option, in addition to the absence of anticoagulation and low transvalvular gradients, is the theoretical ease of reoperation. Indeed, the stentless bioprosthesis is fixed on the tube and not on the native aortic annulus. Thus, the complete stentless valve or its leaflets can be resected and replaced without interrupting the proximal suture line between the native aortic annulus and the Dacron tube graft. However, such a reoperation has not been reported to date.
In order to avoid the potential drawbacks of a straight cylindrical tube, De Paulis et al. have reported a modification of the above described technique by using a stentless bioprosthesis in combination with a latest generation aortic graft with pseudo-sinuses [26]. Following the same concept, a composite bioprosthetic valved conduit has recently been commercialized by Sulzer Vascutek (Renfrewshire, Scotland, UK). The BioValsalva™ is constructed by suturing a stentless aortic valve (Elan, Kohler, Leeds, UK) inside a Triplex™ Valsalva graft, which can be stored in glutaraldehyde along with the bioprosthesis while maintaining a complete blood impermeability. As a result, the ready-to-use composite biological valved graft is currently available in different sizes (21–27 mm), which avoids the need of assembling it on the surgical table.
3.2 The full-root with graft extension technique
In order to reduce the risk of bioprosthetic valve distortion during implantation potentially leading to valve regurgitation [27] or accelerated deterioration [28], the stentless bioprosthesis can alternatively be implanted using the full-root method. Although this allows for easy and reproducible replacement of the aortic root [29,30], the commercially available stentless bioprostheses are usually too short to replace the ascending tubular aorta. Thus, when the whole of the ascending aorta needs to be replaced, additional strategies have to be employed. These may include: (1) the use of an extended stentless bioprosthesis; (2) direct anastomosis between the distal part of the bioprosthetic root and the proximal aortic arch after extensive mobilization of the heart and the aorta; (3) interposition of a Dacron tube graft (Fig. 2 ).
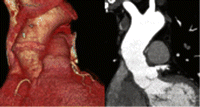
Late postoperative CT scan with three-dimensional reconstruction of a patient who had an ascending aortic replacement using Freestyle full-root plus Dacron graft extension technique.
An extended version of the stentless aortic root bioprosthesis Freestyle, with about a 2 cm longer aortic component than the normal bioprosthesis, can be supplied by the manufacturer Medtronic Inc. (Minneapolis, MN). Hemmer et al. have used this valve on six patients with aortic valve disease and dilated ascending aorta (mean diameter 49 ± 2.8 mm) [31]. In all patients, an oblique trimming of the xenograft conduit facilitated to compensate the mismatch at the level of the distal anastomosis after radical excision of the diseased aortic wall. However, the availability of this extended root xenograft is still extremely limited owing to cost and restriction of regulation and packaging.
Primary end-to-end anastomosis technique described by Massetti et al. might prevent the need for graft interposition [32]. Their technique consisted of extensive mobilization of the aortic arch, supra-aortic vessels, and inferior vena cava to approximate the distal part of the aortic root and the proximal end of the aortic arch to accomplish a tension-free anastomosis in spite of a more than 5 cm long aortic resection. They have applied this technique to 34 patients (mean age 65.2 years, range 25–78 years) with ascending aorta aneurysm. Operative mortality was 3% (1/34) and was related to aortic rupture after mediastinitis. Late death occurred in three patients and no patient required reoperation at a median follow-up of 72 months. However, this technique might leave some tension at the level of the distal anastomosis with its inherent risk of late dehiscence and false aneurysm development. Furthermore, in case of important size discrepancy between the aortic root bioprosthesis and the proximal aortic arch, distortion of the bioprosthetic sinotubular junction might induce aortic valve regurgitation [33].
Thus, the most practical technique appears to be the interposition of a short segment of Dacron tube graft between the bioprosthetic aortic root and the aortic arch, as first reported by Westaby et al. in the setting of aortic dissection [34], and Akpinar et al. in the setting of ascending aortic aneurysm [35]. However, long-term results with this technique are not available yet (Table 1). One potential limitation of this technique is that it adds one suture line between the aortic root bioprosthesis and the proximal end of the Dacron tube graft. This additional suture line obviously prolongs the aortic cross-clamp time for a few minutes. However, the most important concern is about the fate of this suture line considering the often friable structure of glutaraldehyde treated tissues and the difference in compliance between the bioprosthetic root and the Dacron graft. This suture might be at risk for early bleeding or late dehiscence with false aneurysm formation. Thus, some authors advocate the use of strips of felt or pericardium to reinforce this suture line. Alternatively, surgical glues can also be applied to this suture line for additional reinforcement. In our experience, this suture line can be performed safely and without additional reinforcement techniques when one takes care to invaginate the distal end of the bioprosthetic root into the proximal Dacron tube graft.
The choice of the diameter of the Dacron tube graft is often reported in relation to the size of the bioprosthetic root. Thus, Akpinar et al. preferred using a Dacron graft one size smaller than the Freestyle valve [36]. In contrast, we and others [37,38] prefer to oversize it, typically in a range from 1 to 3 mm. However, choosing the diameter of the Dacron graft in respect to the labeled diameter of the valve might be confusing and beside the point. Indeed, the manufacturer’s size label corresponds to the diameter of the proximal Dacron reinforced rim of the bioprosthesis and not to its distal diameter. We feel that the size of the Dacron interposition graft should be determined intra-operatively by (1) the outer diameter of the distal end of the bioprosthetic root and (2) the diameter of the proximal aortic arch. The diameter of the interposition graft can then be chosen to correct, at least in part, any size discrepancies between the bioprosthetic aortic root and the proximal aortic arch, allowing to reduce the beveling of the distal suture line. This two-step reconstruction of the ascending aorta allows a tailored approach to each patient’s anatomy.
Whether this operative procedure will facilitate an eventual reoperation remains to be determined [39]. As for reoperations after previous homograft implantation [40], Byrne et al. have suggested the possibility of implanting a new stented valve inside a previous Freestyle valve after resecting its damaged cusps [37]. However, the new prosthesis will have to be undersized in respect to the previous one. Furthermore, late bioprosthetic aortic wall calcification might complicate this type of procedure. Indeed, Akpinar and Guden have reported two reoperations with Edwards Prima valve [41]. They had to perform the entire root re-replacement because the extremely calcified suture line between the valve and the left ventricular outflow tract made it impossible to put stitches through it. Even though the Freestyle™ (Medtronic Inc., Minneapolis, MN) may be more resistant to calcification than homografts and other stentless bioprostheses due to its anticalcification treatment [42], some calcification of its aortic wall has to be expected. Thus, if the patient is seen as high-risk for reoperation, percutaneous aortic valve implantation might be a future endovascular alternative to surgery.
4 Total xenopericardial valved conduit
The Shelhigh™ BioConduit™ NR-2000C is a totally new bioprosthetic conduit, constructed using individual non-coronary porcine cups, which are fitted on a scalloped shaped tubular bovine pericardium. The conduit and valve are glutaraldehyde cross-linked, detoxified and heparin-treated with No-React. This proprietary detoxification process eliminates residual glutaraldehyde and ensures stable cross-linking, and leads to less calcification and tissue deterioration in the long term. This valved conduit is available in sizes from 21 to 31 mm. The 15 cm long pericardial cuff is long enough to facilitate the anastomosis between the conduit and the remaining distal aorta, being trimmed appropriately in patients who need extended Bentall operation. Carrel et al. have employed it in 35 patients with various aortic root diseases (aortic valve stenosis with poststenotic aortic dilatation, annuloaortic ectasia, acute type A aortic dissection) and demonstrated satisfying clinical and echocardiographic results at 1-year follow-up [43]. Siniawski et al. have also reported good early results similar to those of homografts in 25 patients with active infective endocarditis including six with aortoventricular dehiscence [44]. The ease of implantation, favorable hemodynamic profile, and its versatility will make this option an attractive alternative to homograft or other biological materials, if mid- or long-term results will confirm the short-term results. Unfortunately, the US Food and Drug Administration announced on April 17, 2007 the seizure of all medical devices manufactured by Shelhigh, Inc. of Union, NJ. Shelhigh’s products were seized because they were manufactured under conditions that may have led to their contamination. Furthermore, Carrel et al. have very recently reported several cases of emergency reoperations and unexplained deaths occurring after implantation of the Shelhigh™ BioConduit™ NR-2000C [45,46]. Reoperations revealed complete disintegration of the graft and destruction of the aortic root. Although the patients presented with a septic-like syndrome, extensive microbiologic examinations did not identify any causative organism. Thus, at the moment of writing, the use of the Shelhigh™ BioConduit™ NR-2000C cannot be recommended.
5 Conclusion
The increasing age of patients undergoing aortic root surgery has increased the need for a composite bioprosthetic valved conduit. Thus, several surgical teams have devised strategies to construct a homemade device intra-operatively using either stented or stentless bioprostheses. Each of the reported techniques has its own theoretical advantages and drawbacks. At the moment, the largest and longest published experience is that gained with composite valved grafts using stented bioprostheses. The other options are of more recent use and no medium-term results have been published yet. The concept of bioprosthetic root replacement has also been taken up by the industry and some composite bioprosthetic valved conduits are already commercially available in different sizes. In the near future one has to expect major improvements and a large diffusion of these techniques of bioprosthetic replacement of the ascending aorta.





