-
PDF
- Split View
-
Views
-
Cite
Cite
Erdal Okur, Volkan Baysungur, Cagatay Tezel, Gokcen Sevilgen, Gokhan Ergene, Mertol Gokce, Semih Halezeroglu, Comparison of the single or double chest tube applications after pulmonary lobectomies, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 1, January 2009, Pages 32–36, https://doi.org/10.1016/j.ejcts.2008.09.009
Close - Share Icon Share
Abstract
Objective: Draining of the chest cavity with two chest tubes after pulmonary lobectomy is a common practice. This study aimed to investigate whether using two tubes after a pulmonary lobectomy is more effective than using a single tube. Method: This prospective randomised study included 100 consecutive patients who underwent lobectomy or bilobectomy for any pathological condition between May 2006 and November 2007. In the 50 patients in the ‘single-tube group’, only one 32 F chest tube was inserted, and in the 50 patients in the ‘double-tube group’, two 32 F chest tubes were inserted. Pre-, intra- and postoperative variables in both the groups were compared. Results: The pre- and intraoperative characteristics of the patients were similar in both groups. The mean amount of drainage from chest tubes was 600 ± 43.2 cc in the single-tube group and 896 ± 56.2 cc in the double-tube group (p ≪ 0.001). The mean values of postoperative pain assessed on the visual analogue scale (VAS) in the early (second day) period were 4.28 ± 0.21 in the single-tube group and 5.10 ± 0.23 in the double-tube group (p = 0.014). The VAS scores in the late (second week) period were 1.48 ± 0.13 in the single-tube group and 2.00 ± 0.17 in the double-tube group (p = 0.01). All other related parameters were similar in both groups. Conclusions: Insertion of two chest tubes is not more effective than the insertion of a single chest tube after pulmonary lobectomy. Moreover, using a single tube is in fact more effective than using two tubes in that it causes less postoperative pain and less pleural fluid loss.
1 Introduction
Drainage of the pleural space after lobar resection of the lung is generally performed by using two chest tubes: one is placed into the posterior and basilar region to drain fluid, while the other is directed towards the apex for removing the air from the chest. It has been assumed that a complete control on fluid and air in the pleural space could only be maintained by inserting two chest tubes [1,2]. The use of a single chest tube after lobar resections has been reported in a few studies in the literature [3–5].
Theoretically, using a single chest tube causes less pain and discomfort during the postoperative period and is more economical. Less pain in the postoperative period may improve the patient’s capability to perform respiratory exercises, and thereby, facilitate better lung expansion and decrease the rate of complication. It can also result in an earlier discharge from the hospital.
This randomised controlled study aims to compare the early postoperative results of using single and double chest tubes following lobar resections of the lung.
2 Patients and methods
This prospective randomised study includes the patients who had undergone pulmonary lobectomy or bilobectomy operation at our clinic, beginning from May 2006. The exclusion criteria included (a) lobectomies along with chest wall resections, (b) intrapericardial resections, (c) thoracoscopic lobectomies and (d) patients in whom the surgeon expected increased postoperative haemorrhage. Prolonged intubation of more than 24 h was required in two patients, and one patient required mechanical ventilation the day after extubation. There was one mortality on the third postoperative day due to cardiac arrest. All these patients were also excluded from the study.
The patients were randomised into two groups: in one group, a single chest tube was used after operation, and in the other, two chest tubes were used. Primary outcomes investigated were differences in degree of postoperative pain and the rate of postoperative complications. The secondary outcomes investigated were differences in the amount and the duration of chest tube drainage and the length of hospital stay. Primary outcomes were planned to be assessed after every 50 consecutive patients and the study would be stopped if statistically significant differences were found.
2.1 Randomisation of patients
Either one or two chest tubes were placed in turns, i.e., if a single chest tube was placed in one patient, two tubes were inserted in the next one. Patients’ records were kept by the chief resident and the same nurse in operating room. The surgeon, who was performing the lobar resection most of the time, was not aware that the patient was in single-tube group or double-tube group until completion of the resection and haemostasis. The study could not be designed as ‘blind’ because the same surgical team that performed the operations had to manage the patients postoperatively.
2.2 Surgical method
All patients were operated via lateral thoracotomy without dissecting the serratus anterior muscle and by entering the chest through the fifth intercostal space. After ligation of the vessels, the bronchus was closed mostly by hand suturing, or anastomosis was performed by polyglycolic acid sutures in cases of sleeve lobectomies. Incomplete fissures were divided by using mechanical staplers. Air leaks from the lung parenchyma and bronchial stump were checked by instilling water to the hemithorax and inflating the lung at 30 mmHg positive pressure. Any tear in the lung causing air leak was covered by a fibrin sealant material (TachoSil® Nycomed, Denmark). Systematic lymph node sampling was done in all patients with lung cancer. After haemostasis, we inserted two 32 F polyethylene chest tubes (Bicakcilar®, Istanbul, Turkey) in the control group, one tube in the midaxillary line in the most dependent side of the hemithorax, and the second tube was placed through the anterior axillary line towards the apex. In the single-tube group, chest tubes of the same size were inserted in the midaxillary line in all patients at the dependent side of the hemithorax and directed to the apex. A new hole was created in the lowest part of the tube that stayed inside the chest. No prophylactic space-reducing procedures such as pleural tenting or pneumoperitoneum were performed in any patients in both groups.
2.3 Postoperative management
All patients were managed for at least one night at the postoperative intensive care unit. The chest tubes were connected to an underwater seal. We used two separate chest drainage bottles for the chest tubes in the control group. Negative intermittent suction (15–20 cm H2O) was applied to the chest tubes on the day of operation only. The patients were encouraged to cough and exercise on an incentive spirometer. The patients were returned to the thoracic surgical unit on the next day if there was no contraindication. Air leak and the amount of drainage from the chest tubes were recorded twice a day. Daily chest X-rays were obtained before the removal of the chest tube(s) and on the day of discharge. In the single-tube group, the chest tubes were removed when the air leak stopped and the amount of pleural drainage decreased to less than 250 cc/day. In the control group, the posterior tube was removed when the amount of pleural drainage decreased to less than 250 cc/day. The indication for removal of the second chest tube in this group was the same as that in the single-tube group. A residual pleural space was not a contraindication for the removal of the tube if there was no air leak. A control chest X-ray was obtained for all patients after the removal of a chest tube. The patients were discharged from the hospital 1 day after the removal of the chest tubes if there were no complications.
2.4 Pain management
We used either patient-controlled narcotic analgesia (5 mg loading dose, followed by 2 mg bolus with 10 min lock-out time) or continuous infusion (5 mg loading dose followed by 1–1.5 mg/h) of morphine sulphate during the first day after the thoracotomy in the postoperative intensive care unit. When the patients returned to the ward, they received intravenous tramadol (three or four times, 50–100 mg/day) together with the nonsteroidal anti-inflammatory drug, tenoxicam (two times, 20 mg/day) and paracetamol (three or four times, 500 mg/day). On the morning of the second postoperative day, the degree of pain was recorded by using the visual analogue scale (VAS) before administering the analgesia. The VAS is a 10 cm horizontal line that is labelled ‘no pain’ at one end and ‘worst pain imaginable’ at the other. The patients were asked to mark the intensity of their pain on this line. The patients were educated about VAS and the scoring system before the operation.
They were discharged with a prescription for NSAIDs only and were seen at our clinic 1 week after discharge from the hospital. Their degree of pain at that time was recorded together with their chest X-ray findings.
2.5 Parameters measured and recorded
(a) Preoperative parameters: age, sex, primary pathology, percentage of forced expiratory volume in the first second (%FEV1), and forced vital capacity (%FVC), (b) intraoperative parameters: thoracotomy side, resected lobe, the length of parenchyma stapler used as cm, presence of pleural adhesions, and use of sealing material, (c) postoperative parameters: duration of chest tube drainage, amount of drainage, prolonged air leaks (>5 days), need for additional chest tube insertion, residual pleural space, other complications, pain score on the second day and in the second postoperative week, and duration of hospital stay.
Statistical analyses were performed using the computer-based, SPSS V.11 (SPSS Inc., Chicago, IL, USA) program. The patients’ characteristics and results were represented as mean ± standard deviation or mean ± standard error (SE). Student’s t-test or Mann–Whitney U test was used for numerical variables, and the Pearson chi-square (χ2) test or Fisher’s exact test were used for categorical variables, where appropriate. A p value of less than 0.05 was considered significant.
3 Results
The study was stopped after assessment of 100 consecutive cases (50 patients in each group) in November 2007 when statistically significant differences on pain score were observed between the groups.
Non-small cell carcinoma was the most common pathology and constitutes 86% of all patients in two groups. Other pathologies were bronchiectasis 5, multi drug resistant tuberculosis (MDR-TB) 2, carcinoid 1, and sequestration 1 in single-tube group and bronchiectasis 3, carcinoid 1, MDR-TB 1 in double-tube group.
There were no significant differences between two groups compared considering pre- and intraoperative characteristics (Table 1 ). On comparison of the postoperative characteristics, the single-tube group was found to have a considerably lesser amount of total pleural drainage than the double-tube group [600 ± 43.24 cc (±SE) vs 896 ± 56.23 cc (±SE), respectively; p ≪ 0.001]. Patients in the single-tube group experienced slightly less pain both in the ‘early’ and ‘late’ postoperative periods. The mean VAS score on the second postoperative day was 4.28 ± 0.21 (±SE) in the single-tube group and 5.10 ± 0.23 (±SE) in the double-tube group (p = 0.014). The mean VAS score in the second postoperative week was 1.48 ± 0.13 (±SE) in the single-tube group and 2.00 ± 0.17 (±SE) in the double-tube group (p = 0.01). Although the durations of chest tube drainage and hospital stay were slightly shorter in the single-tube group, the difference was not significant. We found no difference between the two groups considering complication rates (Table 2 ).
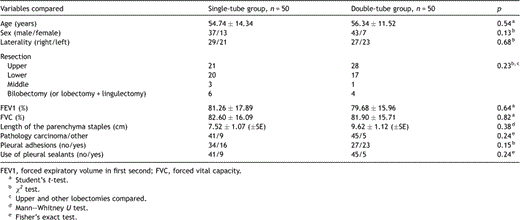
Preoperative and operative characteristics of patients in both groups.
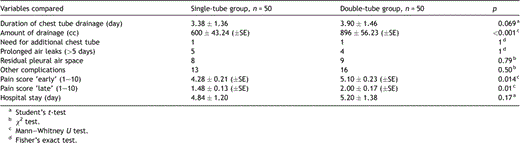
Comparison of the postoperative drainage characteristics, pain scores and complications.
Another apical chest tube insertion was needed in one patient in both groups because of prolonged air leaks and apical pleural space. These two patients were discharged from the hospital with a Hemlich® valve, and the chest tubes were removed on the tenth and eleventh postoperative day.
4 Discussion
The use of two chest tubes after pulmonary lobectomy is a common practice and it is advocated in the textbooks [1,2]. One tube, which is placed anteriorly and directed to the apex, drains the air and the other, which is placed more posteriorly and inferiorly, drains the fluid. Why can a single tube not achieve the same purpose if it is used to drain both the air and the fluid? In addition, it is well known that patients complain of pain at the chest tube insertion site more than at the thoracotomy site. Therefore, theoretically, if we do not use two chest tubes, the patient may feel less pain during the postoperative period.
In a nonrandomised study, Alex et al. first used a single tube in a group of 60 lobectomy patients and compared the results with those observed in another group with two chest tubes [4]. They found no differences with regard to the postoperative outcomes between the groups; however, less pain was observed in the single-tube group. In a randomised study, Gomez-Caro et al. found that a single-tube group required a lesser amount of analgesics than the double-tube group [3]. The amount of drainage and the duration of the chest tube drainage were found to be similar in both groups. Further, Pawelczyk et al. in their retrospective nonrandomised series found that placing a single chest tube after lobectomy reduces the hospitalisation time and treatment cost [5].
Our prospective randomised study differs from other similar studies in its method and the results. In the single-tube group, we created a new hole at the most dependent side of the chest tube for better drainage of fluid collected above the diaphragm. Another difference between our study and the other studies is that we used VAS scores, which assess pain on a wider scale (10-point scale), and we recorded the pain intensity twice (second postoperative day and second postoperative week). The amount of chest tube drainage was lesser in the single-tube group than in the double-tube group (600 cc vs 896 cc, p ≪ 0.001). We think that there might be few possible reasons for the decreased fluid drainage in the single-tube group. First, the duration of drainage in the single-tube group was shorter than that in the double-tube group. Increased pleural secretion in double-tube group may be explained also by greater pleural fluid secretion from the pleura due to higher irritation caused by two tubes. However, is it really necessary to drain all the fluid in pleural space by chest tubes or can the pleura absorb this excess fluid physiologically? We did not note any pleural fluid collection after removing the chest tubes unless there was a residual space. In our opinion, the pleura have the capability to absorb a greater amount of fluid, and it is advisable not to drain an excess amount of pleural fluid, which means ‘unnecessary fluid losses’. This opinion is supported by Cerfolio and Bryant [6]. They reported that after pulmonary resections chest tubes can be removed even if there is a high amount of fluid still being drained (up to 450 cc in a day).
We used the VAS for assessing of the degree of pain in this study. The VAS is one of the most commonly used and very effective pain measurement tools in clinical practice [7–9]. The VAS is a 10-degree scale, from 1 (no pain) to 10 (worst pain imaginable). The patients were asked to quantify the intensity of their pain on this scale. However, the VAS scoring has certain limitations since it is subjective and expresses the intensity but not the quality of pain [6]. The aim in the postoperative period is to maintain the pain intensity below 3 on the VAS, i.e., the patients do not complain about pain unless they are asked. In our study, the mean VAS score on the second postoperative day was 4.28 in the single-tube group, while it was 5.10 in the double-tube group. These scores may appear slightly high, but these are the scores before the usual analgesic dose was administered. They decreased to 1.48 and 2.0, respectively, during the second postoperative week and were accepted as reasonable.
Presently, technologically superior instruments are being used in lung resection operations, such as linear stapler for incomplete fissures, sealing materials for parenchymal air leaks or other advanced materials for haemostasis. Air leaks and bleeding are observed to a lesser extent after lung resections than they were two or three decades ago. This may also be another reason why only one chest tube should be used after pulmonary lobectomy.
In conclusion, insertion of two chest tubes is not more effective than insertion of a single chest tube after standard pulmonary lobectomy. Moreover, using a single tube is, in fact, more effective than using two tubes in that it causes less postoperative pain and less pleural fluid loss without a change in the postoperative complication rate. However, we still recommend using two tubes in patients with lobectomy and chest wall resection or in patients in whom a higher incidence of haemorrhage is expected.
Appendix A Conference discussion
Dr M. Mueller (Vienna, Austria): It is a very convincing concept, and it is surprising that it has not been studied in the past more intensively. It is easy to understand what you are doing. You use just one chest tube, you reduce the local trauma, you reduce pain and by that, you reduce hospital stay and costs. You have done a proper search of the literature, and, as you said correctly, there is only little printed information available.
I have two questions for you.
You state in your results that you found a significant difference between both groups regarding the drainage volumes, and you like to explain that by two different concepts.
Your first hypothesis is that you say if you use two tubes, you drain better, so you have more meticulous, more precise drainage of the chest cavity, and you empty the pleural space more precisely compared to one tube. This would imply that you leave some residual fluid in the chest when you use only one tube, but this does not seem to be clinically relevant since you don’t see any residual fluid in the chest X-ray in the follow-up of your patients with a single tube.
Your second hypothesis is that you say this might be kind of biased because you have removed tubes earlier in your single tube group and therefore you have less fluid collection in this group. I would like to state that it could be the other way around – you remove tubes earlier and therefore you have statistically less drainage volume.
Maybe you could comment on that, especially whether the reason why you have less drainage with one tube might be that one tube simply causes less pleural irritation.
The second, for me surprising fact was that you are using drainages with 32 French. So even if you use two tubes in your patients, you use two 32 French tubes, and this is to me a surprising fact, because you state that the site of the chest tubes would be the source of the maximum pain load of the patients. So I would understand if you said you would try to reduce this pain by also reducing the size of your tubes. Maybe you can comment on that as well.
Dr Okur: First I will answer your second question about using No. 32 French tubes. You may be right about this. This was our practice in the past. We used to use 32 French at that time. This may be due to our fear of tube blockage due to blood coagulation. Actually now we use No. 28 chest tubes, but in the literature there are studies showing that even No. 24 French tubes can be as effective as larger tubes. You may be right about this criticism.
Concerning your first question, our observation is that the basal tubes can drain much more fluid than the apical tubes, but we haven’t seen any fluid accumulation in the single tube group unless there is a residual pleural space.
Concerning less irritation by a single tube, this may be right. With a single tube there may be less irritation and this may cause a less amount of drainage from the tubes.
Dr T. Grodzki (Sczcecin, Poland): I have listened with great interest to your lecture, however, it is a little bit something not new for us, because since 30 years in my department, before even I started my career, we used only a single drain after lobectomy. The important thing is the placement and production of additional holes going down just before the drain leaves the chest. So our experience is in thousands, and I fully agree it is enough to use one drain. However, I am not pretty sure about your study regarding pain, because the pain around the drain, it depends on many factors: if the drain is silicone, flat, or rounded, or the drain more rigid, it is a difference; the way how we place the drain, how we fix it, with a stitch or with two stitches, et cetera, et cetera. So the conclusion regarding less pain is from one side obvious that one drain is better than two drains, however, there are a lot of additional factors affecting the pain after drain placement.
Dr Okur: Our method of chest tube insertion was standard during this study. We put only one stitch to fix the tube in place. You are right that there are a lot of factors affecting the pain at chest tube sites, and I congratulate you because you discovered that using one tube was better than using two tubes much earlier, but you know that even today in most textbooks, authors recommend using two chest tubes after lobectomies.
Dr M. Al-Abdullatief (Riyadh, Saudi Arabia): Maybe I missed it, but you didn’t mention the chest tube drain to be in suction or no suction, and that can affect the status of drainage of fluid mainly. And the other part, we have a lot of company products on the market, and they are different in the number of holes and also the size of hole. I don’t know which one you used.
Dr Okur: We only used suction in the postoperative ICU on the day of operation, not in the thoracic unit. We don’t like to use suction too much, actually. We use polyethylene tubes, which have five or six holes on it. We use these tubes every time. We don’t have experience with other kind of tubes, actually.
References
Author notes
Presented at the 16th European Conference on General Thoracic Surgery, Bologna, Italy, June 8–11, 2008.




