-
PDF
- Split View
-
Views
-
Cite
Cite
Edward J. Hickey, Gruschen Veldtman, Timothy J. Bradley, Aungkana Gengsakul, Cedric Manlhiot, William G. Williams, Gary D. Webb, Brian W. McCrindle, Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 1, January 2009, Pages 156–164, https://doi.org/10.1016/j.ejcts.2008.06.050
Close - Share Icon Share
Abstract
Background: Adult survivors with tetralogy of Fallot constitute a growing population with congenital heart disease. We investigated an inception cohort who underwent surgical repair. We aimed to characterize late hazard or risk for death, and determine the time-related risk of late re-operation and pulmonary valve replacement (PVR). Methods: All children (n = 1181) with tetralogy of Fallot born before 1984 who underwent surgical repair at our institution were included. Follow-up (median 20 years after repair) was obtained from 2003 to 2006 via chart review, clinic consultation and telephone interview. Outcomes were analyzed using parametric and competing risks techniques with bagging. Results: Corrective repair performed from 1960 to 1998 included transannular patch (n = 370), right ventricular outflow tract patch (n = 326), no patch (n = 333) and right ventricular–pulmonary artery conduit (n = 54). Overall, 85 ± 1% survived to adulthood (age >18 years). Thirty years after repair, survival was 80 ± 1%, instantaneous hazard or risk of death was 0.5 ± 0.07% per year and half of survivors had undergone surgical re-operation. Surgical era of repair did not influence late risk of death. Therefore, with early surgical mortality ≪2% since 1985, 20-year survival has improved to 94 ± 1%. If trends in late risks match those of the earliest repairs, 40-year survival will be ≈90% for children repaired in the modern era. Pulmonary atresia variant (n = 88) was associated with three-fold higher late risk of death than classic tetralogy of Fallot (n = 1069). Presence of associated branch pulmonary artery stenosis or atrioventricular septal defects conferred a less optimal late prognosis. Risk of PVR was low, constant (0.8% per year) and independent of surgical era. Both pulmonary atresia and absent pulmonary valve (n = 15) variants were associated with higher risk of late re-operation or PVR. Survival after re-operation or PVR (88 ± 3% and 94 ± 3% at 20 years, respectively) was excellent. Conclusions: Surgical progress has not influenced late risks for death, re-operation or PVR in adults with repaired tetralogy of Fallot. Instead, reduction of early surgical mortality to ≪2% is responsible for excellent late survival >90% overall. The constant risk of PVR is low and independent of repair type. Baseline morphologic features are important determinants of late outcome.
1 Introduction
Despite the risk of re-operations, late outcomes for tetralogy of Fallot survivors are reportedly excellent following even the earliest repairs [1–3]. Amongst the first 106 patients repaired by Lillehei in the 1950s, 91% of those patients discharged alive were still surviving 30 years later [4], although patient selection may have been favorable. Such successes with this and other complex defects have therefore resulted in a new and growing population of adults with repaired or palliated congenital heart disease. This population is believed to number approximately 800,000 adults in the United States alone [5], and their late prognosis needs to be clarified.
In addition, total surgical correction of tetralogy of Fallot erroneously implies curative repair. Although anatomical and physiological correction may be achieved, an important risk of re-operation appears to persist among late survivors [2]. In particular, techniques for right ventricular outflow tract (RVOT) reconstruction frequently distort pulmonary valvular and RVOT function causing pulmonary regurgitation and late risk of pulmonary valve replacement (PVR).
For the large population of adults presently living with repaired tetralogy of Fallot, a detailed appreciation of the time-related instantaneous hazard or risks of death, re-operations and PVR would be of considerable interest. These late hazards are multifactorial, but given the considerable progress in surgical strategies, it is likely that era of repair is important in addition to original morphology and strategy of correction. We therefore investigated an inception cohort of patients who underwent surgical repair of tetralogy of Fallot and would now be of adult age. The cohort spans a wide surgical era, extending from the earliest introduction of surgical repair to the mid-1990s. We aimed to characterize late hazard or risk for death, and determine the time-related risk of late re-operation and PVR.
2 Methods
2.1 Patients and follow-up
The study population consisted of an all-inclusive inception cohort of all 1181 children who underwent corrective surgical repair of tetralogy of Fallot at our institution and were born prior to 1984. All patients would therefore be of adult age (>18 years) at the time of follow-up if all had survived. Institutional ethics board approval was sought and a waiver of consent was obtained because of the historical nature of the study. Tetralogy of Fallot was diagnosed using recognized morphological criteria (anterior deviation of the infundibular septum with ventricular septal defect and obstruction to right ventricular outflow [6]). The majority was determined to have typical morphology and were therefore considered to have classic tetralogy of Fallot (n = 1069). Alternatively, a minority were classified as having pulmonary atresia (n = 88) or absent pulmonary valve (absent-PV) (n = 15) subtypes, or otherwise unknown morphologic classification (n = 9). Patients with pulmonary atresia/ventricular septal defect lacking typical features of tetralogy of Fallot were not included. Of the total cohort, 46 children (4%) had an aorta overriding the ventricular septal defect such that >50% was judged to arise from the right ventricle (double outlet right ventricle variant).
Patients were followed up between 2003 and 2006 through a combination of chart review, direct clinic consultation (n = 343) and telephone interview (n = 683). Follow-up of survivors was obtained to a median of 20 years (ranging up to 48) from the time of corrective surgery (median age at last point of contact 25 years, ranging up to 70).
2.2 Analysis of time-related outcomes
Time-related transitions to defined events were modeled parametrically using multi-phase hazard domain techniques [7]. Several outcomes other than death were investigated, including: (1) any open surgical re-intervention using cardiopulmonary bypass, (2) PVR with a prosthetic valve device or (3) other defined surgical procedure (Table 1 ). For outcomes other than death, competing risks methodology [8] was used in order to account for patients who died and were therefore no longer at risk of the event occurring. Separate models of the rate of transition (hazard function) from the point of corrective surgery to each competing outcome (surgical re-intervention or otherwise death without re-intervention) were created. These separate time-related hazard functions were then combined to yield the proportion of children reaching the defined transitional states at any given point in time.
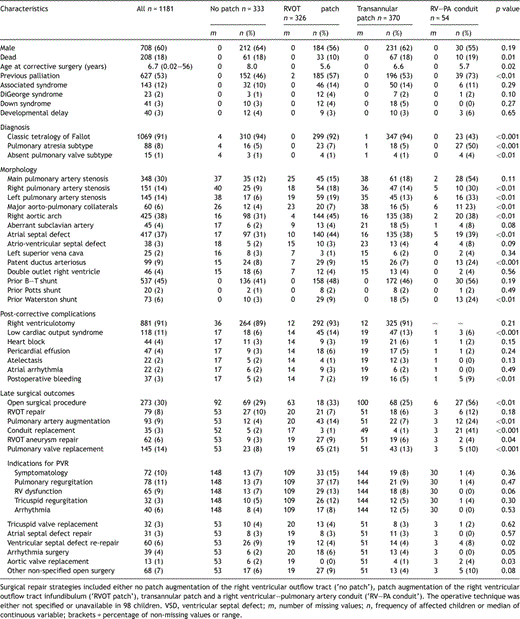
Cohort characteristics and baseline morphological features in all children (n = 1181) born between 1927 and 1983 who underwent corrective surgery for tetralogy of Fallot at the Hospital for Sick Children
2.3 Risk-hazard analysis and statistical analysis techniques
Selected patient-specific characteristics (Table 1) were determined and used in risk-hazard analysis of parametric models, using forward stepwise multi-variable regression. Morphologic variables corresponded to the time of diagnosis. Continuous variables were examined by decile analysis to determine possible transformations to improve linear calibration. Variables were initially tested by univariate regression analysis and subsequently by multivariable analysis, examining each defined hazard-phase separately. Correlations were explored between variables and appropriate interaction terms between colinear variables were tested in regression equations. In all regression models, the influences of surgical era were adjusted for by including a variable representing the date of surgical correction. In addition, interactions between era and procedural variables were explored to account for time-related changes in practice. Missing values were imputed with the mean for that variable and a general missing value indicator created. This general missing value indicator was subsequently tested as a parameter in the regression analysis to refute the possibility that patients with missing data may be different in terms of characteristics or risk from those in whom the data is not missing. Variables with excessive (>75%) missing values or associated with fewer than five events were suppressed during multivariable analysis to avoid the risk of over-determination. Reliability of variables was guided by bootstrap re-sampling [9] (n = 1000, threshold for inclusion p ≪ 0.1). The percentage of re-samples in which variables are included indicates the percentage reliability of the p value derived for that particular variable. Variables appearing in ≪50% bootstrap re-samples were therefore considered unreliable and were not included in the final models.
All analysis was performed using SAS statistical software version 9.1 (Cary, NC). Data are described as mean with standard deviations or medians with ranges. Differences between groups were compared using chi-squared statistics, analysis of variance or t-tests as appropriate. Significance was considered p ≪ 0.05.
3 Results
3.1 Cohort characteristics
The date of corrective surgery ranged from 1960 to 1998. The age at corrective surgery progressively decreased with time (r = −0.14, p ≪ 0.001), from a median of 7.6 years in the 1960s to 4.2 years in the 1980s. Prior palliation with a systemic-pulmonary artery shunt had been undertaken in 627 (53%) patients. Overall, prior palliation became slightly more frequent as the surgical era progressed (r = 0.06, p = 0.05). In those who received a palliative shunt, a Blalock–Taussig or modified Blalock–Taussig shunt was the predominant type (86%). However, use of an aortic-pulmonary artery shunt (Waterston (12%) or Potts (3%) shunt) was slightly more common in later corrective eras (r = 0.07, p = 0.01).
Surgical repair (sub-pulmonary resection and ventricular septal defect closure) was undertaken without the need for RVOT patch augmentation in 333 patients (‘no patch repair’). Otherwise, a transannular patch was used (n = 370), or patch augmentation of the RVOT (‘RVOT patch’, n = 326). A remaining 54 patients received a right ventricular–pulmonary artery conduit as part of the corrective procedure and for 98 patients the specific type of original repair could not be determined (Table 1).
Differences in patient characteristics between the repair types are shown in Table 1. Prior palliation was more common in children who underwent repair using a right ventricular–pulmonary artery conduit. The use of a conduit was more common in children with absent-PV and especially pulmonary atresia subtypes. In addition, baseline branch pulmonary artery stenosis (and consequently major aorto-pulmonary collateral arteries and patent arterial ducts) was more frequent in children who received conduits. Baseline patient-specific characteristics did not otherwise differ between the three categories of surgical repair that did not use a conduit.
3.2 Overall survival
The proportion of children who reached adulthood (18 years of age) was 85 ± 1%. Survival following corrective surgery incorporates both early and late hazard phases for death. The large early hazard phase was strongly associated with an earlier era of surgery (Table 2 ). Estimated mortality 1 year after correction was 27 ± 2% for 1965 compared with 2 ± 1% for 1985 (Fig. 1a ). Worse early mortality was also associated with morphological variants of tetralogy of Fallot and the presence of co-existing cardiovascular anomalies (particularly an atrioventricular septal defect, Table 2). In addition, prior palliation with either a Potts or Waterston systemic-pulmonary artery shunt prior to corrective surgery was associated with significantly greater early mortality (independent of underlying morphology).
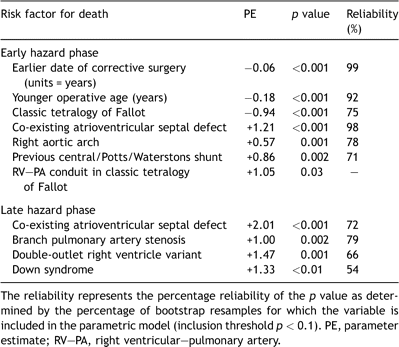
Incremental risk factors for time-related death following corrective repair of tetralogy of Fallot (n = 1181)
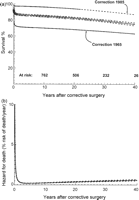
(a) Time-related survival for all 1181 study patients (black). Predicted survival stratified by era of corrective repair is shown for 1965 (red) and 1985 (blue). (b) Hazard function for all-cause mortality. Circles represent Kaplan–Meier estimates at events (deaths). Solid lines represent parametric continuous point estimates. Overall survival was 85 ± 1%, 82 ± 1%, 80 ± 1% and 77 ± 2% at 10, 20, 30 and 40 years respectively. Two hazard phases exist: a large early phase resolving within 2 years of corrective surgery, followed by a slow-rising late hazard phase. 75% of all deaths occurred within 3 years of corrective surgery. Instantaneous hazard for death was 0.25 ± 0.03%, 0.37 ± 0.05%, 0.49 ± 0.07% and 0.59 ± 0.09% per year at 10, 20, 30 and 40 years. For patients repaired in 1965, survival was 72 ± 2% and 63 ± 3% at 1 and 40 years. For patients repaired in 1985, 1-year survival was 97 ± 2%, 20-year survival was 94 ± 1% and their 40-year survival is predicted to be 87 ± 2%. For stratified plots, remaining variables are set at their mean value. Dashed lines and bars enclose 70% confidence intervals.
The late hazard phase for death was low and slow-rising. The instantaneous risk of death was 0.5 ± 0.07% per year at 30 years after correction, and the rate of hazard increase was approximately 0.1% per decade (Fig. 1b). However, this late hazard for death was independent of the era of correction. Therefore, improvements in overall late survival instead predominantly reflect improvements in early survival. In 1965, for example, estimated survival 1 year after correction was 72 ± 2%, whereas by 1985, 1-year survival had improved to 97 ± 2%. Because differences in corrective surgical era do not influence the late hazard for death in this cohort, these improvements in early survival translate into predicted 40-year survival of 64 ± 3% and 88 ± 2% for patients repaired in 1965 and 1985, respectively (Fig. 1a). Late hazard for death was also independent of the type of surgical repair, including the use of a trans-annular patch (p = 0.43) or right ventricular–pulmonary artery conduit (p = 0.38).
Three categories of risk factors were found to influence the late hazard for death: tetralogy of Fallot subtype, co-existing cardiovascular defects and Down syndrome (Table 2). Pulmonary atresia subtypes exhibited a three-fold higher late hazard for death compared with either classic tetralogy of Fallot or absent-PV variants. Only half of the original cohort with pulmonary atresia subtype were predicted to be alive 40 years following correction (Fig. 2a ), representing an attrition of almost 40% of those who survived the early risks of corrective surgery.

Risk-adjusted survival for all patients (n = 1181), stratified by: (a) pulmonary atresia subtype versus either classic tetralogy of Fallot or absent PV subtype, (b) the presence of branch pulmonary artery stenosis at the time of diagnosis, (c) the presence of double-outlet right ventricle variant morphology and (d) the presence of an atrioventricular septal defect. For each stratified plot, the remaining variables are set at the mean value for the cohort. The late hazard for death at 30 years was 1.4 ± 0.5% per year for pulmonary atresia subtype versus 0.30 ± 0.06% per year for other subtypes. The presence of an atrioventricular septal defect conferred particularly poor early and late survival: 38 ± 10% (hazard 3.5 ± 1.6%) at 30 years. Lines represent the parametric determination of the continuous point estimates of survival. Dashed lines enclose 70% confidence intervals.
Branch pulmonary artery stenosis (at the time of diagnosis, Fig. 2b) or double outlet right ventricle variant morphology (Fig. 2c) was both associated with elevated late hazard for death. However, an atrioventricular septal defect conferred an especially strong influence on late survival: the hazard for death was seven-fold higher at 30 years (Fig. 2d).
3.3 Re-operation
We explored the time-related risk of undergoing surgical re-operations late following corrective surgery (Fig. 3a ). Re-operations were common: 30 years after correction, half of survivors had undergone a re-operation (Fig. 3). However, the instantaneous risk of re-operation gradually decreased (2% per year at 10 years, gradually decreasing to 1.6% per year at 40 years). The era of corrective surgery did not reach the threshold for significance as an influential risk factor (p = 0.07).

Time-related risk of reaching the mutually exclusive competing outcomes of either (1) undergoing surgical re-operation, (2) death without re-operation, or otherwise, (3) remaining alive without re-operation. (a) Thirty years after correction, 40 ± 1% had undergone re-operations, 18 ± 1% had died without re-operations, 42 ± 2% remained alive without re-operations and the instantaneous hazard for re-operation was 1.7 ± 0.1% per year. (b) For a patient with classic tetralogy of Fallot in combination with branch pulmonary artery stenosis, Down syndrome, a right aortic arch and having received a central systemic pulmonary artery shunt prior to repair in the 1980s, fewer than 10% remain alive and free from re-operation after 30 years. (c) For a patient with classic tetralogy of Fallot, but no associated cardiovascular anomalies or genetic syndromes repaired in 1985, the early hazard for death was diminished but the risk of re-operation remained. If the late hazard phase continues as predicted, more than half of survivors remained alive and free from re-operation after 40 years. Lines represent parametric continuous point estimates.
Classic tetralogy of Fallot was associated with significantly reduced risk of re-operation (parameter estimate −0.93, p ≪ 0.001) compared with either pulmonary atresia or absent-PV variants. Branch pulmonary artery stenosis (at the time of diagnosis) (p = 0.01) and particularly a co-existing atrioventricular septal defect (p = 0.01) were both associated with higher risk of re-operation. Therefore, fewer than 10% of children with a constellation of unfavorable risk factors may be alive and free from repeat surgery 30 years after repair (Fig. 3b). In contrast, for children with favorable baseline morphological features who underwent correction in 1985, more than 50% may remain alive and free from repeat surgery 40 years later if late hazard phases match those of earlier eras (Fig. 3c). Note that while more recent surgical era has diminished the early hazard for death, a significant risk of re-operation persists (even in the presence of favorable morphology).
The use of a right ventricle–pulmonary artery conduit at the time of correction was associated with a higher risk of re-operation (parameter estimate (PE) +0.71, p = 0.001), although this repair type constituted only the minority (5%) of repair types. For the bulk (95%) of the cohort who underwent repair without use of a right ventricle–pulmonary artery conduit, re-operation was independent of the particular corrective surgical repair type (no patch, RVOT patch or trans-annular patch).
3.3.1 Survival after re-operation
Survival after re-operation was 88 ± 3% at 20 years and was independent of the original corrective surgical repair technique (including the use of a conduit). The only independent risk factors for death following surgical re-operation were pulmonary atresia subtype (PE +0.84, p = 0.05) and especially the presence of a co-existing atrioventricular septal defect (PE +1.8, p = 0.001) at the time of initial repair. Because the type of initial corrective repair was not an important determinant of risk of re-operation, it is likely that features intrinsic to the pulmonary atresia and atrioventricular septal defect morphology in some way influence time-related longevity of initial repair strategy.
3.4 Pulmonary valve replacement
We chose to specifically investigate the late risk of PVR because of the increasing interest surrounding this procedure in order to limit sequelae of chronic pulmonary regurgitation. PVR was undertaken in 145 patients; as the first re-operation in 54, otherwise after other surgical re-operations in 91 patients. For all 1181 patients, the hazard for PVR was constant, remaining at 0.8 ± 0.07% per year for the duration of the study period. Classic tetralogy of Fallot was associated with reduced risk of undergoing PVR (PE −0.84, p ≪ 0.001) compared with either pulmonary atresia or absent-PV variants. Surprisingly, however, although the use of a right ventricular–pulmonary artery conduit intuitively mandates PVR at some stage, it was not associated with an increased time-related need for PVR (p = 0.39) compared with other repair types. Transannular patch in particular has been implicated with increased risk of late PVR [10]. In this cohort, trans-annular patch was independent of the time-related risk of undergoing PVR (p = 0.95).
In the 956 patients who all had classic tetralogy of Fallot and did not receive a conduit at the time of corrective surgery the late risk of undergoing PVR was associated with branch pulmonary artery stenosis at the time of diagnosis, older age at the time of corrective surgery, the presence of a right aortic arch and presence of an atrioventricular septal defect (Table 3 ). In addition, the late hazard for PVR was influenced by the competing risk of death (and its associated risk factors). A patient with classic tetralogy of Fallot with branch pulmonary artery stenosis, Down syndrome and right aortic arch who underwent surgical correction at the age of 5 years in 1980 was predicted to have a 40% chance of being alive without a PVR 30 years later (Fig. 4a ). In contrast, a favorable patient with classic tetralogy of Fallot with none of these associated risk factors who underwent corrective surgery at the age of 2 years in 1985 would be predicted to have more than 80% likelihood of being alive without PVR 30 years later if late hazard phases match those of earlier eras (Fig. 4b). The relevance of a right aortic arch was explored by examining its correlation with other variables. A right aortic arch correlated with presence of several other morphological variables including atrial septal defects (r = 0.93, p ≪ 0.001), atrioventricular septal defects (r = 0.07, p = 0.02), and the presence of associated syndromes (r = 0.07, p = 0.02) and is therefore likely a robust surrogate for unfavorable co-existing morphology.
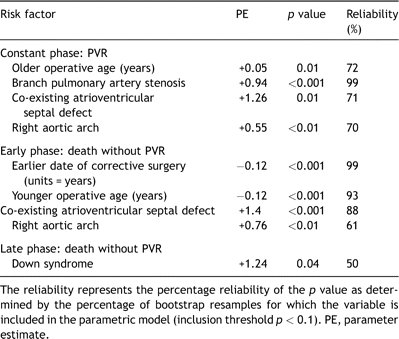
Incremental risk factors for time-related risk of undergoing pulmonary valve replacement (PVR) following corrective repair of tetralogy of Fallot (n = 1181)
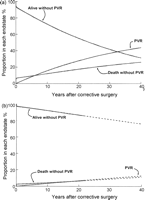
Time-related risk of undergoing pulmonary valve replacement (PVR) in all patients with classic tetralogy of Fallot who did not receive a right ventricular–pulmonary artery conduit (n = 956). The model includes the competing risks of either (1) undergoing PVR, (2) death without PVR, or otherwise (3) remaining alive without PVR. (a) Risk of PVR stratified for a child with Down syndrome, a right aortic arch and branch pulmonary artery stenosis who underwent corrective repair in 1980 at the age of 5 years. (b) Risk of PVR stratified for a child with classic tetralogy of Fallot, but no associated cardiovascular anomalies or genetic syndromes repaired in 1985 at age 2 years. Lines represent parametric continuous point estimates.
3.4.1 Survival after PVR
Seven patients died after undergoing PVR (n = 145). The parametric survival estimates were therefore 96 ± 2% and 94 ± 3% at 10 years (63 remaining at risk) and 20 years (17 remaining at risk) after PVR respectively. No features (including baseline morphology) other than younger age at the time of PVR (PE −0.12, p = 0.04) were associated with worse survival following PVR by univariate or multivariable analysis.
4 Conclusions
The late risk of death for adults with repaired tetralogy of Fallot is very low (≈0.5% per year 30 years after correction), although it does gradually increase with age. In addition, although early surgical mortality has seen major improvements during the four decades spanned by this study, the late hazards for death, surgical re-intervention and PVR have remained unchanged. Instead, the specific tetralogy of Fallot subtype and presence of associated cardiovascular lesions are the more important determinants of late outcome. Interestingly, although long-term consequences of using a transannular patch have received considerable attention [2,11,12], we did not observe any association between type of initial surgical correction and either late death, risk of re-operation or risk of PVR.
The proportion of children reaching adulthood is predominantly determined by the magnitude of the early surgical mortality. In the early years following the introduction of corrective techniques, early mortality was as high as 25%. However, by the mid-1980s, this had reduced to as little as 2%, both in this series and those of others [13]. Because late survival appears independent of the era of corrective surgery, these large improvements in early surgical mortality should translate into excellent long-term prognoses for infants undergoing repair in the more recent eras. Our models predict that if trends in late hazards match those of the earliest surgical repairs, approximately 90% of all children who underwent repair of tetralogy of Fallot in the late 1980s and early 1990s are likely to survive into the fifth decades of life (Fig. 1a).
The nature of corrective repair strategy has received considerable interest, in particular use of transannular patching. The small proportion of patients who received a right ventricular–pulmonary artery conduit experienced a higher risk of surgical re-operation. Importantly, however, for the majority (95%) of patients in whom the use of a conduit is avoided, the nature of original corrective procedure did not influence either the late risk of death or risk of PVR.
This study consistently identifies certain baseline morphological features as important determinants of late risk of re-intervention and death. Morphologic tetralogy of Fallot subtypes, in particular pulmonary atresia, confer worse late prognoses. Pulmonary atresia is more frequently associated with branch pulmonary artery stenosis which is itself an important independent risk factor for late death, re-operation and PVR. The mechanisms for elevated late mortality associated with pulmonary atresia are not clear from this investigation. However, pulmonary artery stenoses are likely to be associated with pulmonary regurgitation, right ventricular dilatation and risk of dangerous arrhythmias [14,15].
Pulmonary regurgitation is a frequent late consequence of RVOT reconstruction in tetralogy of Fallot. Transannular patching has received particular attention as a possible cause of pulmonary regurgitation and long-term morbidity [2,11,12]. However, others have not demonstrated worse pulmonary regurgitation or ventricular dilatation [16], and in our investigation transannular patching was not associated with increased risk of PVR or re-operation in this cohort compared with other repair types. We believe this finding to be attributed to the fact that late pulmonary regurgitation is a consequence of all repair types and not specific only to transannular patches. Consequently, efforts to avoid transannular patching may not translate into improved freedom from late PVR. Instead, a greater understanding of the natural history of pulmonary regurgitation and the development of deleterious sequelae is likely to be of value; particularly with regard to the nature and timing of intervention. Whether the profile of late pulmonary regurgitation is similar in more modern surgical management paradigms is not known and will be an area for future study.
Pulmonary regurgitation may progress insidiously and can be well tolerated even when severe. However, right ventricular volume-overload leads to gradual dilatation and decline in function, exacerbated by secondary tricuspid regurgitation. Ventricular rhythm disturbances can result and premature ventricular contractions and widening QRS complexes are understood to herald a threat of sudden death [14]. Atrial arrhythmias are also more common [17]. PVR, often in combination with ventricular or atrial cryoablation [15,18], has therefore been adopted to address these sequelae of severe pulmonary regurgitation, although the precise indications and optimal timing for PVR have not been fully clarified.
Optimal timing of PVR has been the focus of considerable debate. PVR improves functional status attributable to pulmonary regurgitation [17], aids right ventricular re-modeling [19] and stabilizes QRS duration [15,20]. Recently, several investigators have advocated earlier PVR in order to preserve right ventricular function and pre-empt the onset of dangerous arrhythmias [21,22]. On the other hand, bioprosthetic pulmonary devices have a limited life-span, and PVR in young adults will mandate (perhaps several) repeat devices. Therefore, any advantages in earlier PVR must outweigh the risks associated with additional subsequent re-operations. In this cohort, the hazard for PVR has been low, constant, and independent of era. Therefore, their clinical management changed little, despite increasing concern over the last 10–15 years surrounding the risks of chronic untreated pulmonary regurgitation. It will be interesting to see whether the time-related risk of undergoing PVR is different in contemporary cohorts of children.
Co-existing cardiovascular anomalies significantly influence late prognosis. Especially unfavorable was the presence of an atrioventricular septal defect. Surgical correction of tetralogy of Fallot with atrioventricular septal defects has historically been more hazardous [23], although improved with technical refinement [24]. However, the risk of re-operation and late death was elevated several-fold in this cohort and others [23], indicating that this abnormality confers survival disadvantages that persist long after initial repair. Interestingly, the risk of undergoing late PVR was also increased in children with atrioventricular septal defects, which may relate to a greater propensity for right atrioventricular valve regurgitation as a consequence of pulmonary regurgitation. Double outlet right ventricle variant morphology and the presence of a right aortic arch were also associated with late risk of death and PVR respectively. It is difficult to interpret their precise causal associations; it is likely that they represent surrogates for other important prognostic factors.
Important alterations in surgical management strategies have emerged in the intervening period between this inception cohort and present day. In particular, there has been a shift away from the use of palliative system-pulmonary artery shunts and single-stage repair early in infancy is now the norm in most institutions. This change in strategy has not been detrimental to early survival [25], but the consequences on late outcomes are unknown. At our institution, we did not consistently adopt single-stage corrective repair until late in the 1990s, and the findings of this study are therefore likely to be applicable to patients until that point.
The advantages of studying a large and historical population denominator paradoxically introduces limitations. It has not been possible to accrue detailed morphological baseline indices which might be useful clinical prognostic markers. In addition, technical and intra-operative information has not been available and might provide insight into specific factors that have resulted in improvements in outcome. Lastly, determining optimal timing of PVR will require repeated assessment of cardiac function over numerous time-points and examining the impact of PVR on subsequent echocardiographic progression and functional outcome. We have not attempted to undertake such an analysis in this study and therefore can draw no conclusions regarding causality.
In summary, this study provides unique insight into the burgeoning population of adult survivors with tetralogy of Fallot. Despite important advances in clinical care and decision-management, differences in surgical era do not influence late hazards associated with surgical correction of tetralogy of Fallot. Many patients require surgical re-intervention, regardless of underlying morphology. However, tetralogy of Fallot subtype and presence of specific co-existing lesions are important determinants of late survival and risk of PVR. Now that early mortality associated with corrective repair is ≪2%, overall survival rates approaching 90% at 40 years are anticipated for infants repaired in the late 1980s and early 1990s.
Appendix A Conference discussion
Dr J. Stark (London, United Kingdom): I would just like to ask one question. The type of repair in your study was not determining pulmonary valve replacement. How would you explain that the transannular patch did not influence late insertion of the pulmonary valve?
Dr Hickey: The use of a transannular patch did not in our cohort influence the risk of pulmonary valve replacement, and that may be because patients can tolerate even severe pulmonary regurgitation for some considerable length of time before they decompensate. It may largely be dependent on the particular practices and indications for pulmonary valve replacement within institutions, and certainly in the more contemporary era, some institutions are adopting aggressive pre-emptive pulmonary valve replacement early on, whereas that wasn’t the case in our institution in the 80s and 90s.
Dr M.T. Kayali (Aleppo, Syria): My concern is, when you do tetralogy repair do you accept a mild to moderate PA gradient with saving the PA ring or do you accept a transannular patch with huge pulmonary regurgitation? Most of the time we face this in the OR.
Dr Hickey: Yes, that’s right. The purpose of this study was not to dictate management strategies. That’s an important point of this analysis. Our emphasis in terms of progressing the surgical strategies of tetralogy repair centers largely around earlier outcomes. The purpose of this cohort was to pick an inception cohort, a historical one, who are currently adults and look back to where they originated from regardless of what they actually had and whether it was right or wrong. They are a real cohort of adults who we’re having to manage in contemporary practice now and we want to characterize where they are now from a historical perspective.
Dr Kayali: It could be more helpful if you did a subanalysis on the procedure. Were you saving the ring or not? I don’t know.
Dr Hickey: Given the size of the cohort, which this part of the cohort is 1100, and there are several others who died without repair, up to 500, a cohort of that size on one hand gives you a huge patient denominator to work with. The flip side of that is that we can only have a parsimonious set of variables with which to investigate, and we haven’t been able to explore surgical details of the original repair, just crude management strategies; a patch, where the patch was, etc.
Dr R. Cesnjevar (Hamburg, Germany): I missed the influence of the BT shunt on your cohort a bit. If one looks at the reoperation rate, the least of it was because of pulmonary valve replacement. So what were the other kind of reoperations? Was it residual VSD closure or PA enlargement? What was it?
Dr Hickey: Unfortunately we haven’t got an exact breakdown of all of the other types of reoperation. They were classified as open reoperations through the sternum requiring going on cardiopulmonary bypass. That was the definition we used. As far as BT shunts are concerned, it’s an interesting point, because in our institution we were quite late in switching to a single-stage repair, and, in fact, the incidence and type of BT shunt did not change over the length of our cohort and also did not influence outcomes, other than a central shunt which was associated with greater early mortality.
Dr D. Sidi (Paris, France): To go back to Dr Stark’s question, you said it was a difference in practice from the transannular patch, but could you show that there was much more pulmonary regurgitation when there was a transannular patch? Do you have data to show that the amount of pulmonary regurgitation was higher in the patients with a transannular patch?
Dr Hickey: No, we don’t have that data.
Dr Sidi: It’s just an assumption.
References
Author notes
Presented at the 21st Annual Meeting of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 16–19, 2007.
- pulmonary artery stenosis
- tetralogy of fallot
- consultation
- congenital absence of pulmonic valve
- pulmonary artery
- conduit implant
- atrioventricular septal defect
- congenital heart disease
- adult
- child
- follow-up
- heart ventricle
- pulmonary atresia
- repeat surgery
- surgical procedures, operative
- survivors
- telephone
- replacement of pulmonary valve
- medical records review
- right ventricular outflow tract
- surgical mortality




