-
PDF
- Split View
-
Views
-
Cite
Cite
Edward J. Hickey, Gordon Jung, Cedric Manlhiot, Andreas G. Sakopoulos, Christopher A. Caldarone, John G. Coles, Glen S. Van Arsdell, Brian W. McCrindle, Infective endocarditis in children: native valve preservation is frequently possible despite advanced clinical disease, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 1, January 2009, Pages 130–135, https://doi.org/10.1016/j.ejcts.2008.08.020
Close - Share Icon Share
Abstract
Background: Recent reports describing surgical experiences with childhood IE are sparse. We sought to determine patient-specific characteristics and their impact on outcome for children with infective endocarditis (IE) undergoing surgical intervention. We therefore reviewed all cases of culture-proven IE referred for surgical intervention at our institution over the last three decades. Methods: Of 15,124 cardiovascular surgical procedures performed between 1978 and 2007 at our institution on children under the age of 18, only 30 (0.2%) were undertaken for a primary diagnosis of IE. All 30 children underwent chart review and retrospective risk-hazard analysis. Results: Median patient age was 9.8 years (range 10 weeks to 17.5 years). Underlying congenital cardiac lesions were present in 22 (77%) and previous intra-cardiac repair in 9 (30%). Septic emboli occurred in 13 (46%), causing permanent strokes in 4 (14%). Streptococcus viridans and Staphylococcus aureus were the predominant organisms. S. viridans was associated with underlying congenital lesions (p ≪ 0.01). S. aureus was associated with abscess formation (p ≪ 0.03), clinical sepsis (p ≪ 0.04), acute deterioration (p ≪ 0.01), prolonged hospitalization (p ≪ 0.01) and death (p ≪ 0.01). Aortic, mitral and tricuspid valves were involved with equal frequency, more than the right ventricular outflow tract. Two valves were involved in 30%. The native valve was preserved at operation in 22 (73% cases). Univariate predictors for valve replacement included increased leaflet thickening (p ≪ 0.01) and occurrence of septic embolization (p = 0.02), whereas moderate/severe valvular regurgitation was not significant. Five-year freedom from IE-related death and re-intervention was 84% and 80%, respectively. At latest follow-up 96% of patients are NHYA I. Conclusions: Children undergoing surgery for infective endocarditis frequently have advanced disease with embolic complications and double valve involvement. However, preservation of the native valve is frequently possible. Need for valve replacement is suggested by leaflet thickening and embolization. Despite the advanced pathology, survival and functional outcomes are favorable.
1 Introduction
Improvements in the diagnosis and management of infective endocarditis (IE) have been offset by shifts in both the adult and pediatric at-risk populations and causative organisms [1]. Childhood IE was previously only a rare complication of rheumatic heart disease. However, the growing number of patients living with surgical repairs of congenital lesions now represent the majority of patients developing IE in childhood [2]. Staphylococci have also become increasingly prevalent due to their association with invasive procedures and a predilection for prosthetic material [1]. Infective endocarditis in childhood is therefore likely to become an increasingly common and difficult surgical problem in cardiovascular units.
Recent reports describing surgical experiences with childhood IE are sparse [3–6]. We therefore decided to review all cases of culture-proven IE referred for surgical intervention at our institution over the last three decades. In particular we sought to characterize the contemporary profile of IE requiring surgical intervention and identify patient-specific features that influence surgical management, need for valve replacement and subsequent outcome.
2 Patients and methods
All 30 consecutive children (under the age of 18) who were referred for surgical intervention with culture-proven IE between 1978 and 2006 at our institution were analyzed via retrospective chart review. These cases represent 0.2% of the total 15,124 cardiovascular surgical procedures performed at our institution during this period. Institutional ethics board approval was sought, but individual patient consent was not obtained because of the historical nature and patients were not directly contacted and a waiver of consent was therefore obtained. Follow-up was available to a mean of 5.1 years after surgery providing 130 patient-years.
Data were accrued from institutional medical records detailing patient demographics, pre-intervention echocardiography and angiography, operative procedures, subsequent clinic attendances and autopsy reports in the advent of death. Data were entered and analyzed using SAS statistical software, version 9.1 (SAS Institute, Cary, NC). Data are described as frequencies, medians with ranges, means with standard deviations or odds/hazard ratios with 95% confidence intervals (CI) as appropriate. Actuarial time-related outcomes were calculated using Kaplan–Meier principles. Univariate risk-hazard analysis was undertaken to identify predictors for outcome. In the case of missing data, mean values were imputed and the frequency of the missing data was indicated. Significance was considered p ≪ 0.05.
3 Results
3.1 Clinicopathology
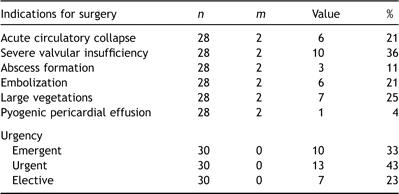
Median age at the time of surgery was 9.8 years (range 10 weeks to 17.5 years). Associated congenital heart defects were present in the majority of cases (Table 1 ) although in seven of these (23%) the congenital heart defects were an incidental finding revealed during the investigation into IE. An additional child with a structurally normal heart was immunosuppressed having undergone a recent bone marrow transplant. Six children (20%) therefore had no known predisposing factors.
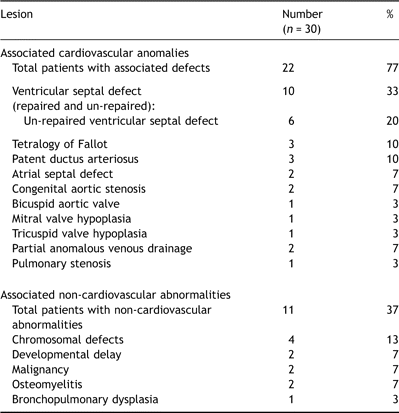
Incidence of associated cardiovascular and non-cardiovascular abnormalities
Previous sternotomy had been performed in nine (30%) and an additional three had undergone patent ductus ligation or coarctation repair via thoracotomy (Table 2 ). Prosthetic material (other than suture) was present in eight (27%) at the time of diagnosis.
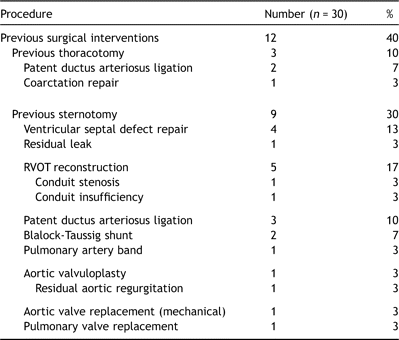
The predominant clinical features at presentation were constitutional (lethargy, 80%; malaise, 79%) and non-specific features of systemic sepsis (fever, 90%). Physical signs associated with adult IE were instead uncommon (splinter hemorrhages, 20%; nail clubbing, 4%; no child had Osler’s nodes or Janeway lesions). More than 90% had elevated inflammatory markers or leukocytosis, and anemia was present in 79%. Of particular relevance to surgical intervention using cardiopulmonary bypass, 19% exhibited an inflammatory thrombocytopenia on presentation.
The causative organism was invariably either Streptococcus viridans (37%), Staphylococcus aureus (40%) or Staphylococcus epidermidis (17%). S. viridans was particularly associated with the presence of underlying congenital heart defects (odds ratio 12.8 (1.1–139.5), p ≪ 0.01) and shorter overall hospitalization (odds ratio 0.87 (95% CI 0.78–0.98), p ≪ 0.01). By contrast, S. aureus was a predictor of more aggressive clinicopathological disease, more frequent postoperative complications, delayed recovery and death (Table 3 ). However, neither organism was predictive of the type of surgical procedure subsequently undertaken, including the need for valve or root replacement.
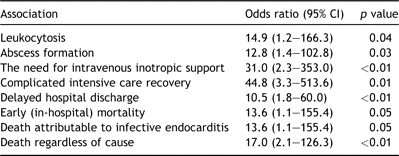
Clinical embolization had occurred preoperatively in approximately half (46%) the patients in this series. Cerebral embolization occurred preoperatively in four (14%) children, all of whom suffered permanent neurological deficit. Additional sites of systemic embolization included the popliteal artery, bilateral superficial femoral artery, spleen liver and bilateral renal infarctions. Four (14%) suffered pulmonary embolization. Two patients underwent formal surgical embolectomy. The first of these had sustained bilateral superficial femoral artery embolization. Although thrombus was retrieved, pulses could not be restored and the patient eventually died of multi-organ failure. The second patient sustained a significant pulmonary embolus from a large tricuspid valve vegetation and underwent uncomplicated pulmonary embolectomy as the solitary surgical procedure.
The aortic, mitral and tricuspid valves were the primary site of infection with equal frequency (Table 4 ). The pulmonary valve or right ventricular outflow tract conduit was less frequently involved. Two or more valves were involved in as many as a third of patients and the preoperative sensitivity of echocardiography in identifying these cases was 90% (9 in 10 cases). A solitary case involved infected ventricular septal defect (VSD) patch material (Dacron®) and spared valvular apparatus. The sensitivity and specificity of transthoracic echocardiography for evaluating abscess formation was poor: five were diagnosed, of which two were false-positive. An additional two abscesses were discovered at operation in children who had undergone preoperative trans-thoracic echocardiography.
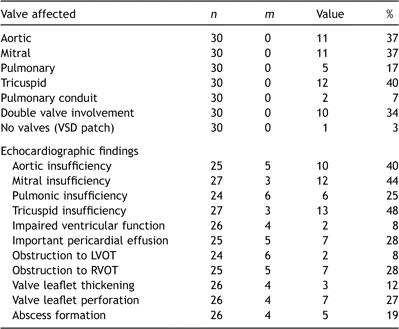
Pre-operative evaluation of cardiac involvement with endocarditis
Ventricular function was invariably preserved, although important (moderate or severe) valvular insufficiency was common. Consequently, severe valvular insufficiency with or without acute cardiogenic shock was the most common indication for surgical intervention (Table 5 ). Interestingly, the presence of a septal defect was protective against acute hemodynamic collapse (p ≪ 0.01), perhaps by off-loading the ventricle in the face of severe valvular regurgitation.
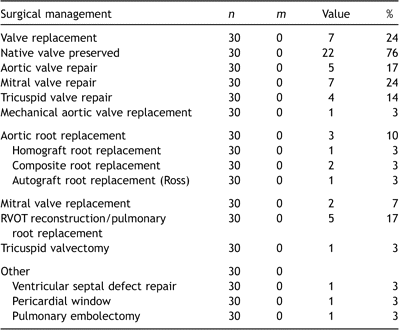
3.2 Surgery
One patient with S. aureus tricuspid valve IE developed a large pyogenic (culture-positive) pericardial effusion and underwent open drainage as an isolated procedure and recovered uneventfully. All the remaining patients underwent an intra-cardiac operation (Table 6 ).
In the four children who had suffered preoperative cerebrovascular accidents (CVA), the decision to operate was driven by on-going systemic embolization. The mean interval from CVA to surgical intervention using cardiopulmonary bypass was 6 days (range 2 days to 2 weeks). All had undergone preoperative computerized tomography to confirm the cerebral infarct. One child had a confirmed hemorrhagic infarct of a large enough size to dissuade preoperative anticoagulation. An additional fifth child underwent surgery for endocarditis 6 weeks after subarachnoid hemorrhage from prior to mycotic basilar aneurysm repair. None of these five children sustained progression of cerebral injury attributable to cardiopulmonary bypass, but all exhibited permanent neurological sequelae at last follow-up.
The native valve was preserved in three-quarters of children, and all of those under the age of 11 years. In the seven patients in whom valve replacement was necessary (mean age 14 years), a mechanical device was implanted in four. Preoperative features that were associated with the need for valve replacement included increased leaflet thickening (odds ratio 7.1 (1.1–45.9), p ≪ 0.01) and the occurrence of embolization (odds ratio 12.7 (1.8–91.1), p = 0.02). The severity of valvular regurgitation was not itself a significant predictor in this series.
3.3 Recovery and survival
3.3.1 Early mortality
All patients were placed on postoperative antibiotic regimens, ranging from 2 to 24 weeks. The median intensive care stay was 4 days (range 1–88) and median post-intensive care hospitalization was 6 days (range 2–36). Important postoperative complications occurred in 13 (43%) children, and were especially prevalent in S. aureus infections (Table 3). Complications included rhythm disturbances (3), bleeding (2), lung abscess (1), valvular regurgitation (2) and multi-organ failure (5).
There were three (10%) early deaths attributable to the episode of IE. The first case was a 13-year-old with a long history of myelodysplasia who had undergone bone marrow transplant the previous year, and was consequently immunocompromised. She developed staphylococcus aortic valve IE with root abscess for which a composite aortic root replacement was performed. After a protracted stay in intensive care her recovery was complicated by fungal infection of her prosthetic graft and she died as a consequence.
In the second case, a 17-year-old boy with developmental delay but structurally normal heart developed S. aureus IE of his mitral valve. Despite mitral valve replacement, his systemic sepsis persisted and he developed signs of tricuspid valve vegetations, pneumonia and peripheral septic emboli. His condition continued to deteriorate and he died from multi-organ failure.
The third early death involved a 9-year-old girl with Down syndrome who had a previous repair of ventricular septal defect. She re-presented with florid sepsis and septic pulmonary emboli. At operation the tricuspid valve septal leaflet required repair with bovine pericardium. Unfortunately she developed severe postoperative tricuspid regurgitation and required re-operation for tricuspid valve replacement 3 days later. She did not recover from her established multi-organ failure.
3.3.2 Late mortality
An additional four patients have subsequently died (Fig. 1a ), none directly attributable to the episode of IE (freedom from endocarditis-related death 84% ± 6 at 5 years). Univariate risk factors for all-cause mortality included established preoperative hemodynamic shock, mitral valve involvement and double valve IE (Table 7 ). Of the survivors, 22 (96%) are known to be physically active, free from cardiac symptoms and reported to be in NYHA I subjective functional classification.
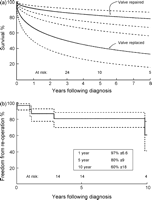
(a) Survival after surgical intervention (n = 30) for infective endocarditis stratified by whether the child underwent valve replacement or not. Five-year survival was estimated to be 82% ± 8 or 41% ± 19 according to whether the valve was replaced or not respectively (p = 0.05). The solid line represents parametric determination of continuous point estimates, and the dashed lines enclose 70% confidence intervals. (b) Freedom from re-operation (percentage) after surgical intervention for infective endocarditis. The solid line represents Kaplan–Meier estimates, and the dashed lines enclose 70% confidence intervals.

Re-operation has subsequently been performed in five children, and was directly attributable to the episode of IE in four (Fig. 1b). Three re-operations were to replace a regurgitant repaired valve, and one was to implant a prosthesis after earlier tricuspid valvectomy.
4 Discussion
Overall, outcomes following IE have not reflected the improvements in surgical, anti-microbial and critical care over the past 50 years [7]. The reasons for this stem from gradual changes in the vulnerable patient populations and offending organisms. In adults, the burden of rheumatic heart disease has been replaced by an aging population with rising rates of prosthetic valve implants and trans-venous pacemakers. In addition, advances in anti-microbial therapy have been offset by the increasing prevalence of staphylococcal infections complicating interventional procedures. Streptococcal species historically accounted for 60–80% of all cases in which modern antibiotics are expected to offer cure in over 90% [1]. By the 1990s, however, S. aureus was reported as the single most common pathogen causing IE across all ages [1].
The at-risk population of children has also experienced important changes [6,8]. Little over 50 years ago, a palliative systemic-pulmonary shunt was the only management option for most congenital heart defects. By contrast, almost all children can now be offered long-term corrective or palliative surgical therapeutic options. Frequently these strategies involve reconstruction using prosthetic material and repeated interventions. Although the current reported rate of IE in patients with congenital heart disease is as low as 1% [2], the overall frequency will presumably increase in parallel with the growing pediatric and adult population with repaired congenital heart defects. In this series, 77% of cases occurred in association with underlying congenital heart disease, whereas this rate may be as high as 90% [4].
Similar to the situation in adults, etiologic organisms are also likely to shift towards those with a predilection for prosthetic material (S. epidermidis) and repeated interventions (S. aureus). At least 40% of cases of IE occurring in repaired congenital heart disease are now caused by staphylococcal species [2]. However, it is noteworthy that as many as 20% of our series occurred in children with no known predisposing factors and that in five of these six children the organism was S. aureus. This emphasizes the potential threat of this organism even in patients with structurally normal hearts.
The risk of IE in congenital heart disease is closely linked to abnormal intra-cardiac shunts and flow accelerations. Prior to surgical intervention for congenital heart disease, IE is most common in those with ventricular septal defects [2]. Following intervention, congenital aortic stenosis, pulmonary atresia or tetralogy of Fallot with a systemic-pulmonary artery shunt are associated with the highest reported risk of developing IE. Right ventricular-pulmonary artery conduits are also associated with a moderately high risk. By contrast, anatomically corrected lesions, successfully treated transposition of the great arteries, atrial septal defects, ventricular septal defects (with no residual defect) and patent ductus arteriosus, are associated with low risk. Infective endocarditis occurring after ventricular septal defect repair is reported to be universally associated with a residual leak. This was our experience in the single child with infected ventricular septal defect patch material.
Our experience includes high rates of double valve involvement, embolization, clinical features of severe systemic sepsis, and severe valvular insufficiency. These features suggest advanced disease. It is impossible to determine whether this is predominantly due to organism virulence in a vulnerable population or otherwise due to late referral to surgical services. It is also difficult to draw conclusions regarding management practices without understanding the population denominator of all children diagnosed with IE over the same period. Given that this surgical series re-presented 0.2% of the caseload of our service, 3–4 times as many children may have been managed medically [2,6,9], if the rates of pediatric IE reported by others are accurate. The advanced disease state at the time of surgery may be a result of the insidious presentation and lack of defining features of IE in children. For example, the clinical manifestations were predominantly vague and constitutional, rather than signs that might specifically be associated with IE. Especially alarming is the very high rate of systemic embolization compared with other series [3–5], which might be avoided by greater clinical suspicion or earlier intervention.
Few other centers have reported their recent surgical experience with infective IE in children. Over a 27-year period, the experience of Monro’s group totaled only 16 children [3]. They reported a slightly higher rate of prosthetic tissue IE (19%), similar anatomical distribution of infected valves, similar rate of etiologic organisms and associated congenital heart defects in 69%. Interestingly, they described a slightly higher rate of cusp perforation, abscess formation or chordal rupture which may explain their need for valve replacement in as many as 63% [3].
A group from Uruguay instead reported a series of 26 infants and children in whom the native valve was preserved in 72% [5], therefore more closely reflecting our experience herein. Although their in-hospital mortality was higher (19%), the majority of deaths were attributable to systemic sepsis and not the surgical management strategy. Finally, Horvath and colleagues in 1989 reported a small series of 11 children in whom the native valve was replaced in 45% [4]. They reserved surgery only for intractable congestive heart failure, systemic emboli or persistent sepsis and experienced early mortality similar (9%) to that reported here.
A key conclusion from our experience is that the native valve can be preserved in the face of several unfavorable clinical and pathological features. The advantages of avoiding valve replacement in adult IE have been clearly demonstrated [10], but preserving the native valve is especially important in children. In particular, mitral valve replacement confers a high risk of repeat replacement (>70% at 15 years [11]) and elevated mortality [12], especially in younger children [13]. We have previously demonstrated age less than 2 years and a mitral prosthesis smaller than 20 mm both to be independent predictors for earlier valve re-replacement [13], and the risks are even higher for younger children with annular dimensions ≪16 mm [14]. By contrast, mitral valve debridement and repair using Carpentier’s techniques offer excellent late survival, freedom from re-operation and late functional status [15]. If mitral valve replacement is necessary it is somewhat reassuring that late function is reportedly favorable and that somatic growth continues such that larger prostheses can usually be implanted at re-operation [13]. Outcomes are also acceptable for mechanical valves implanted in other positions [16,17].
It is likely that larger study populations might identify leaflet perforation and abscess formation as independent risk factors for valve replacement. However in our experience neither preoperative echocardiographic evidence nor the intra-operative finding of an abscess were strongly associated with the need for valve replacement. This is partly because several valve replacements were necessary for reasons other than an abscess. In addition, in one confirmed aortic root abscess, the native valve was preserved through debridement and irrigation. Root abscesses in children have previously been associated with mortality as high as 27% [18]. In contrast, all our patients with confirmed abscesses survived the early period, although one died late and one required a late re-operation.
In previously reported reviews, root abscesses in children have invariably been attributed to staphylococcal infections [18]. The fact that three of the five confirmed abscesses in our series were secondary to S. viridans emphasizes the aggressive nature of non-staphylococcal infections. Nevertheless, S. aureus was associated with more aggressive clinical disease, greater morbidity and mortality. None of this series had primary fungal IE, although one child developed fungal septicemia postoperatively and died. Reported survival following fungal IE remains poor (little over 40% compared with ≪20% in previous eras) with high rates of recurrence [19].
The timing of surgery in children who have confirmed CVA is a source of considerable anxiety. Intuitively, a longer interval is advisable especially in the context of confirmed intra-parenchymal hemorrhage. However, on-going systemic embolization usually mandates early intervention. It is reassuring that none of the patients in this series showed progression of their cerebral injury during the operative window.
This report is limited by the small number of subjects and the unknown population denominator of children diagnosed with IE at our institution during the study period. Nevertheless, it serves to emphasize the rare nature of the condition and the fact that its prevalence is likely to increase with the burgeoning population of children and young adults living with repaired or palliated congenital heart disease. Higher clinical suspicion and improved imaging techniques should aim to implement surgical intervention before the onset of systemic embolization and irreparable damage to the valvular apparatus. Valve replacement can be avoided in the majority of circumstances, sometimes even in the presence of an annular abscess. Preserving the native valve should translate into improved late survival, freedom from re-operation and functional outcomes.




