-
PDF
- Split View
-
Views
-
Cite
Cite
Georges Decker, Willy Coosemans, Paul De Leyn, Herbert Decaluwé, Philippe Nafteux, Dirk Van Raemdonck, Toni Lerut, Minimally invasive esophagectomy for cancer, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 1, January 2009, Pages 13–21, https://doi.org/10.1016/j.ejcts.2008.09.024
Close - Share Icon Share
Summary
Since 1992, various combinations of thoracoscopy (VATS), laparoscopy or hand-assisted thoracolaparoscopy have been used for ‘minimally invasive’ cancer esophagectomy (MIE). Despite widespread current use, indications and potential benefits of the many technical approaches remain controversial. A systematic literature search was conducted until June 2007. Out of 128 publications, 46 original series (1932 patients) met the inclusion criteria and were analyzed for surgical and oncological outcome. No prospective controlled study has compared any MIE technique to another or to open surgery. Most publications are retrospective series of highly selected patients, mostly excluding high-risk patients and locally advanced (T3) tumors. Altogether, the overall conversion rate was 5.9%, mortality 2.9% and morbidity 46%, many papers reporting only major complications. Overall, rates for pulmonary complications were 22%, leakage 8.8% and vocal cord palsy 7.1%. Fifteen tracheo-bronchial injuries or fistulas (1% of all VATS cases) were reported. Laparoscopy and VATS were combined in 11 series (609 patients, 4.7% conversions, 2.4% mortality). VATS combined with (mini)-laparotomy was reported in 14 papers (743 patients, 6.3% conversions, 2.4% mortality). Laparoscopy combined with right thoracotomy was reported in four papers (147 patients, 5.4% conversions, 2% mortality). Laparoscopic transhiatal resections were reported in 17 papers (433 patients, 7% conversions, 4.6% mortality). Overall morbidity rates for these four approaches were 43%, 47.6%, 51.6% and 46%, respectively. Data on oncological outcome are scarce. Lymph node retrieval (median of all series: 14 nodes, range 5–62) was mostly inferior to open surgery standards and follow-up too short to draw definitive conclusions regarding long-term survival. Based on the available literature, the morbidity and mortality of MIE is substantial and not inferior to radical open esophagectomy in experienced centers. Many different operative techniques for MIE have been reported without obvious superiority for any of them. The term ‘minimally invasive’ is not supported by hitherto reported results. Selection bias and huge variability in extent of resection and lymphadenectomy impair comparisons of different MIE techniques. Oncological outcome of MIE remains largely unknown by lack of good quality data and selection bias. MIE remains an investigational and still evolving treatment for invasive cancer.
1 Introduction
Radical surgery currently offers the most realistic chance of cure from cancer of the esophagus or gastroesophageal junction when spread beyond the most superficial epithelial layers but not extending beyond locoregional lymph nodes [1,2]. Nowadays, experienced centers can consistently perform such aggressive ‘conventional’ surgery with mortality rates of 3–5% and consistently obtain overall 5-year survival rates of at least 35–40% [1–3]. Few other oncological operations however are as heavily influenced by experience than esophagectomy for cancer and experienced centers not only achieve lower mortality rates but also much higher cure rates than low-volume centers [4,5].
However, despite considerable improvements in cancer staging, patient selection and surgical results during the last decades, overall and pulmonary complication rates have remained sufficiently high to encourage the search for alternative operative techniques that could potentially achieve similar cure rates but with less morbidity and hence probably a better postoperative quality of life.
With this objective in mind, less invasive surgical approaches have been introduced since the eighties under the form of transhiatal resections (e.g. without any opening of the chest), either with or without the use of (video)-mediastinoscopes [6–8]. Benefits over transthoracic resections in terms of reduced pulmonary and overall complications were however limited and could only recently be formally proven by a large randomized study [3,9]. However, transhiatal esophagectomy (THE) did not reduce mortality compared to transthoracic approaches and the drawback of THE is clearly the inability to perform a radical lymph node dissection of the mediastinum, thus compromising the chance for long-term survival in any patient with cancer of the thoracic esophagus, including those of the distal third [3,10].
The use of thoracoscopy and/or laparoscopy for esophageal resection was introduced in 1992 by Cushieri et al., hoping that it would further reduce pulmonary morbidity while potentially improving the oncological quality of the resection by enhancing visual control during the mediastinal dissection [11]. Several small series subsequently demonstrated the feasibility of this new approach but pulmonary morbidity was severe and the early enthusiasm quickly faded [12–14]. Despite considerable morbidity, the terminology of ‘minimally invasive’ esophagectomy (MIE) was born and would continue to be applied [7]. During the late nineties, general advances in laparoscopic skills and equipment encouraged many centers to restart using laparoscopy, thoracoscopy, video-assisted ‘mini’-laparotomy or ‘mini’-thoracotomy (VATS) as well as various forms of hand-assisted, robot-assisted or hybrid techniques for esophageal resections. A few large series have since reported reasonable outcomes with some of these approaches, leading to a more frequent use of MIE [15–17].
However, 15 years after the introduction of MIE, evidence from controlled randomized studies is still lacking to confirm any benefits of MIE in terms of surgical outcome and safety nor its oncological adequacy as compared to standard ‘open’ resections. Further problems such as those inherent to the learning phase of MIE have not been formally addressed. The specific results of the various currently available technical approaches for MIE thus need to be evaluated before any appropriate sample size calculations for future randomized studies could be made. We thus thought it appropriate to systematically review the available literature on MIE.
2 Methods and materials
The literature until June 2007 was electronically searched via MEDLINE (Pubmed®) using various combinations of the terms: *esophagectomy, laparosc*, thoracosc*, VATS, minimally invasive *esophagectomy, MIE. Only full-text papers in English were included. Publications in non-peer reviewed journals, review papers without original data and all studies not reporting a minimum set of surgical outcome data were excluded. All series not specifically dealing with cancer of the esophagus or GEJ were excluded, as were all series of less than 10 cases, in order to avoid a potentially negative impact of early learning curves. In case of redundant publications by a center, only the largest and most recent publication was included unless previous studies covered different time periods or different operative techniques; e.g. one center has published 19 papers on MIE of which three met the inclusion criteria. Many technical variations of MIE were found with many of them being hybrid techniques. Papers on robotic MIE techniques were included if general inclusion criteria were met.
As traditionally done for open esophagectomy techniques, MIE papers were divided into two groups of either transhiatal or transthoracic resection techniques [9]. THE by definition does not use any opening of the chest and the anastomosis is performed either in the neck or mediastinum. MIE-THE thus implies the use of laparoscopy for the abdominal stage either with or without use of a mini-laparotomy for parts of the operation such as specimen retrieval or creation of the gastric tube (17 studies included).
Any other technique combining an abdominal stage and a thoracic stage was defined as transthoracic esophagectomy (TTE), whether anastomosis was to be performed in the neck or mediastinum. The 29 analyzed TTE studies were further subdivided into three categories:
Thoracoscopic and laparoscopic esophagectomy (with or without a mini-laparotomy).
Thoracoscopy and laparotomy (either ‘mini’- or standard laparotomy).
Laparoscopy and thoracotomy (either ‘mini’- or standard thoracotomy).
Some papers reported on more than one of these technical approaches [16,18]. Whenever results were reported with sufficient details, the different patient populations were treated as separate publications.
All surgical outcome data (operative times, duration of hospital and intensive care stay, conversion rates, complications, blood loss, transfusion rates), as well as oncological outcome data (tumor stages, lymph node retrieval, rates of complete resection, recurrence rates, survival data) were retrieved and introduced in a computer database for subsequent analysis. Overall, we noted a wide variability in definitions of mortality (e.g. 30-day vs in-hospital) and morbidity (often pulmonary morbidity was reported only if severe, e.g. reintubation or tracheostomy).
Simple descriptive statistics were performed. If some data were available only for a subset of the included studies, the number of patients and studies on which the statistic was performed was reported. Due to reporting bias and very considerable variations in selection criteria and extent of surgical resections performed within the different technical approaches, we felt it was inappropriate to perform any statistical significance tests for comparison of outcome between the various MIE techniques.
3 Results
One hundred and twenty-eight papers were found of which 82 were excluded for one or several exclusion criteria. The remaining 46 studies included 1932 patients (median 24 patients, range 10–319) and reported a variety of technical approaches for MIE:
17 series performed transhiatal resections including 433 patients (median 20 patients; 10–68).
29 series performed various transthoracic approaches for MIE including 1499 patients (median 25 patients; 10–319).
Transhiatal techniques mostly described laparoscopic abdominal techniques with transhiatal mobilization of the esophagus and cervical anastomosis. Altogether only a handful series were strictly laparoscopic as many used a ‘mini’-‘laparotomy’ during some time of the operation, either for hand-assisted dissection, specimen removal, creation of the gastroplasty or oversewing of the gastric tube [16,17,19–22]. Two laparoscopic series were strictly ‘transabdominal’ using transhiatal mediastinal esophagogastrostomy after limited segmentary esophageal resection with low intrathoracic anastomosis [23,24].
The group of transthoracic MIE is also a very heterogeneous group of publications; few series exclusively used endoscopic techniques (combined thoracoscopy and laparoscopy with or without cervical anastomosis) [15,25–27]. Most series are hybrid combinations of ‘minimally-invasive’ techniques for some part of the operation with conventional open approaches for another part of the operation (e.g. laparoscopy combined with thoracotomy or laparotomy combined with thoracoscopy) again with either a cervical or intrathoracic anastomosis. Others used hand-ports [28–30], mediastinoscopes [7] or robotic assistance for parts of the procedures [31–33].
Within each type of surgical approaches, the extent of esophageal resection also varied from segmental to subtotal or total resection of the thoracic esophagus and the extent of lymph node dissection ranged from the lack of any formal lymphadenectomy (majority of papers) to an extended mediastinal and abdominal lymph node dissection in a few others [18,34–39].
Overall results for the 46 series: median operative time was 330 min (range of medians 200–550 min) with a 5.9% conversion rate to open procedures (range 0–36%). Median ICU stay was 1 day (range 0.5–5) in 37 series reporting this outcome. Median hospital stay was 11.3 days (5.5–29) in 36 series reporting this outcome. Blood losses were reported in 27 studies. Median blood loss was 331 ml (53–1500 ml). Transfusion rates were reported in only 11 studies with a median transfusion rate of 13.5% (0–46%).
Fifty-five cases (2.9%) of postoperative mortality (30-day mortality in the majority of papers) were reported out of 1914 exploitable cases.
The overall complication rate was 48% (969 of 1809 cases, range of series: 18.5–100%). Pulmonary complication rate was 22% (391 of 1800 cases (median 20%; range of series 0–76%). Anastomotic leaks were reported in 8.8% (163 of 1850 cases; 0–25%).
Vocal cord paralysis occurred in 7.1% (129 of 1803 cases; median of series 12%; range 0–55%). Reoperations were reported in 6.1% (63 of 1073 patients for whom this information is available).
Other reported complication rates of MIE were: chylothorax 2.4% (45 of 1856), 0.8% tracheo-bronchial tears or necrosis (15 out of 1856 patients evaluable), 1.3% (massive) bleedings (24 of 1856 patients). The reported incidences of splenectomies (six cases or 0.3%) and other visceral injuries (pancreas, colon) were low (five cases or 0.3%). As many studies stated that they reported only ‘major’ complications, the incidences of common surgical complications such as surgical site infections, cardiac (arrhythmias) or thrombo-embolic complications were not mentioned by most series and thus no reliable incidences could be calculated.
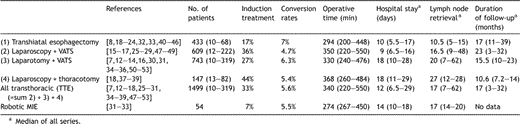
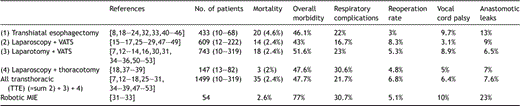
The specific results of the different technical approaches are listed in Table 1 . Morbidity and mortality is reported in Table 2 . Tumor stages are listed in Table 3 . Four THE studies and six studies on TTE techniques did not report any precise tumor stages [7,12,23,24,29,33,42,50,51,53]. Thus, data on tumor stages were available for 81% of all THE cases (352 of 433 patients) respectively 90% of all TTE cases (1357 of 1499 patients).
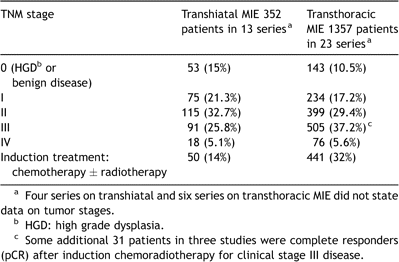
Pathologic staging of cancer patients operated by transthoracic and transhiatal MIE techniques.
Oncological outcome of MIE:
Twenty-six papers (56%) reported no oncological outcome data or follow-up whatsoever. Rates of complete tumor resection (R0) were reported in 15 studies (33%). R1/2 rates ranged from 0 to 24% (median 12%).
Lymph nodes retrieval was reported in 29 papers (63%): median of all series was 14 nodes (5–62). Lower yields were reported after transhiatal than after transthoracic MIE (Table 1).
Twenty-one papers (46%) reported no description or data on any type of lymph node dissection (LND). Only six papers reported removal of any regional abdominal LND (e.g. celiac nodes) or any nodes beyond those of the lesser gastric curve.
Only 15 studies reported performance of mediastinal LND beyond immediately peri-esophageal nodes (e.g. removal of subcarinal or any upper mediastinum lymph node stations).
Twenty-four papers (52%) reported some follow-up results. Median follow-up of all MIE papers was 17 months (3–39). Six cases of thoracoscopic or laparoscopic port site metastasis have been reported in four series [7,19,34,52]. Several other cases however were reported in papers not included in our analysis and since most series have only short follow-up and did not specifically report on this complication, the resulting 0.3% incidence found in this review might underestimate the real risk [54].
Overall, only 12 papers (26%) reported 1-year survival rates being a median of 75% (33–91%). Reported 3-year survival was 42% (19–59% range in eight papers) and only three centers reported overall 5-year survival rates of 42% median (22–52%) [16,28,34].
Stage specific survivals were available in some studies. Five-year survival rates for stage I was 86% (62–100%; median of 5 series), stage II, 45% (35–69%); stage III, five papers reported 16% median 5-year survival (0–27%) [16,17,23,34,55].
4 Discussion
The terminology of minimally invasive esophagectomy suggests less morbidity after MIE than after conventional esophagectomy. In order to verify this hypothesis, our literature review pooled all relevant series on MIE published until mid 2007. No controlled comparative trial was found and the few retrospective comparative cohort studies are limited by small numbers of patients [21,63,55] or major selection bias making both study groups incomparable [16]. Nguyen et al. compared 18 thoracoscopic and laparoscopic resections with their historical experience with open resections [63]. Operative times, blood loss, transfusion requirements, ICU and hospital stays were shorter after MIE but without any difference in fistula or respiratory complication rates. Bresadola et al. retrospectively compared 14 laparoscopic transhiatal resections to 14 open transhiatal resections and found no difference in morbidity, mortality, transfusions or time in ICU [55]. The only significant differences were longer operative times and a shorter hospital stay after MIE. Bernabe et al. reported 17 patients (all stage 0 and I cancers) with hand-assisted laparoscopic THE, compared to a historic group of open THE with similar indications [21]. The only differences were reduced blood loss (difference of 210 ml) and a 2 days shorter hospital stay after MIE.
Finally Smithers et al. recently reported the largest available series of MIE, comparing 309 thoracoscopic-assisted esophagectomies with 23 totally MIE (laparoscopic and thoracoscopic) and 114 open esophagectomies during the same time period [16]. This study was biased by the fact that 37 converted or unresectable cases were excluded from outcome analysis and that patients undergoing an open resection had different tumor types and more advanced tumors; e.g. more cancers of the cardia and subcardia and more stage III tumors underwent a total gastrectomy and less frequently had received preoperative chemoradiotherapy. This study was an update of this center’s earlier series [64] and some selection bias was likely as in-hospital mortality in the earlier paper was eight patients (5.3%) but only seven patients (2.3%) in the updated series [16,64]. The authors concluded that totally endoscopic MIE did not offer any benefit over the combination of thoracoscopy and laparotomy and they have since abandoned laparoscopy for the abdominal part of the operation. Their thoracoscopic resections were found to have marginal benefits over open resections such as reduced blood loss (400 ml vs 600 ml), transfusion rates (27% vs 37%) and a 1 day shorter hospital stay (13 days vs 14 days). The morbidity profile was similar for all three approaches except for a much higher stricture rate of the anastomosis after MIE (22% vs 6%). Overall 62% of MIE patients had any type of complication and 26% had a respiratory infection. This largest available study concluded that the added benefit of MIE was minimal but found no detrimental effect on long-term survival since the overall 5-year survival was 41% (16% for stage III). Using a policy of standard mediastinal LND (including peri-esophageal and subcarinal but not upper mediastinal nodes), Smithers et al. retrieved a median of 17 lymph nodes (and thus above the average of this review) [16]. Others have show that even more extended lymph node dissections can be performed by MIE and lead to excellent 5-year survival rates above 50% [34]. Such studies suggest that not the type of access but rather the radicality of the mediastinal dissection is important for good outcome.
Considering the lack of clear benefit for MIE over conventional esophagectomy in these comparative studies, any additional validation of MIE could only be based on cumulative assessment of available retrospective case series. Acknowledging the vast variability in patient selection, operative techniques for MIE as well as the various criteria for outcome measurement used in individual studies, we are aware of the methodological limits of this review. However, there is currently no better evidence available.
Esophagectomy for cancer is a formidable undertaking and its inherent risks for morbidity and mortality remain reliable criteria for outcome assessment. Pulmonary complications are the most frequent source of complications and mortality after an esophagectomy [1,2]. Their reduction seems to be the primary aim of any MIE technique. Considerable variations in the very definition of a pulmonary complication, may however explain the unusually wide range of pulmonary complication rates found by this review (0–76% of patients; median 20%). Some centers defined pulmonary complications as any unexpected pulmonary event (atelectasis, need for bronchoscopy) and reported pulmonary complications in 30–76% of patients [13,16,48]. Others reported only the most severe pulmonary complications (e.g. reintubation, ARDS, need for tracheostomy) and consequently found much lower rates of 0–6% [28,30,34]. Considering such reporting bias, the overall respiratory morbidity rate of 22% after MIE remains high and not obviously inferior to open surgery [1–3,9]. Some of the reasons for this might be long operative times and inherent need for prolonged single lung ventilation during thoracoscopic esophageal mobilization.
Furthermore, reported rates of vocal cord palsy seem high after MIE (9%) and impairment of swallowing with subsequent inhalation may play a major role in the development of respiratory infections after MIE [59,60].
As the group of transthoracic MIE resections included a fair number of series without any dissection in the neck or upper mediastinum, it is not surprising that vocal cord paralysis was reported less frequently after TTE than after THE (6.4% vs 9.7%). Previous literature reviews on conventional esophagectomy reported an almost three-fold higher incidence of vocal nerve palsy after transhiatal resections (9.5–11.2%) as opposed to transthoracic resections (3.5–4.8%). Comparison with such data would suggest that MIE with thoracoscopic mediastinal dissection might carry a slightly higher risk of laryngeal nerve injury compared to a conventional transthoracic esophagectomy [9,60,61].
Comparing pulmonary complication rates between this review and literature reviews on open esophagectomy may again be hampered by variations in the very definition of a pulmonary complication [9,61]. Reviewing the literature of open esophagectomy between 1986 and 1996, Rindani et al. found 25% of respiratory complications in 1904 patients undergoing an Ivor-Lewis esophagectomy versus 24% in 2329 patients operated transhiatally [61]. Another systematic literature review in the nineties found 19% of pulmonary complications after TTE and 13% after THE [9]. In a recent prospective population-based study on conventional esophagectomy in Scotland, overall and pulmonary complication rates were 44% and 20.4%, respectively [62]. Our study found very similar overall and pulmonary complications rates for transhiatal and transthoracic MIE without apparent advantage for any of the various technical approaches for MIE. More powerful comparisons could only be made using data from controlled studies using rigorous definitions of the different complications.
Other than respiratory complications, the classical complications of esophagectomy such as anastomotic leaks and vocal cord palsy also remain a significant problem in MIE. Overall, 6% of all MIE required conversion to open surgery and another 6% required reoperations for complications. Anastomotic leakage rates were 6.5–13% for the various techniques for MIE and vocal cord palsy was reported in 3.1–9.7% of patients with the highest nerve injury rates after transhiatal and robotic MIE techniques (Table 2).
MIE further harbors the risk of additional uncommon but dramatic complications such as tracheo-bronchial injuries or necrosis. Fifteen such cases were reported in the papers we included in our review but several more were found in small series that were excluded. All 15 cases occurred after thoracoscopic esophageal mobilization (1% of all VATS cases). The risk of severe tracheo-bronchial injuries thus seems to be increased compared to open resections. During the nineties, no single case of tracheal necrosis was reported after open TTE and 0.6% after transhiatal resection [9]. Time will tell if this highly lethal complication is a specific risk of thoracoscopic MIE or rather a ‘collateral damage’ in a learning curve of a complex technique.
If MIE cannot be shown to be less invasive, some studies at least suggest that postoperative ventilation times, blood loss, transfusion rates, length of ICU and hospital stays could be favorably influenced by MIE [18,35,41,52]. Other series of MIE however still rely on systematic postoperative ventilation [8,30,31,39,40,47] and prolonged ICU stays after MIE [20,39,40,44]. All these parameters however are influenced by many factors other than the mere operative approach, e.g. anesthesia protocol, routine use of perioperative epidural analgesia or the availability of intermediate care units. Previous literature reviews have studied all these perioperative outcome parameters after conventional open esophagectomies during the nineties [9]. During that era, the duration of postoperative ventilation and ICU stays were shorter after open transhiatal compared to transthoracic resections [3,9]. Since then, anesthesiological perioperative management has evolved and the feasibility of routine immediate extubation after radical transthoracic esophageal resections has been shown [56,57]. Today, when medium-care units and epidural anesthesia are available, most patients should not need to stay in an ICU independently of whether the esophagectomy is performed by conventional transthoracic, transhiatal or MIE approach.
Similarly, hospital stays are influenced by many factors other than surgical technique, e.g. preoperative nutritional status, use of neoadjuvant therapies, or access to rehabilitation facilities. Total median hospital stays of 15–19 days have been reported for conventional esophagectomy in the setting of controlled trials as well as population-based studies [3,58]. As for many other surgical procedures, fast-track esophagectomy programs have been shown to further reduce hospital stays to the range of 8–11 days [56,57]. The present review on MIE found a very wide range of median hospital stays ranging from 6 to 29 days. This suggests that health care practices vary considerably between different countries and that the operative technique itself may play only a minor role in the determination of hospital stays. With an overall median stay of 11.5 days, MIE does not seem to be much faster than what can be expected today after conventional open esophagectomy in high volume centers [56,57].
Other than shorter hospital stays, potential areas of superiority of MIE over conventional esophagectomy might be reduced blood loss. Transfusion rates were too inconsistently reported to perform any analysis. The reported median blood loss of 330 ml however compares favorably to a median of more than 800 ml after open resections [61] and severe bleeding (1.3% of all cases) also rarely occurs after MIE and may compare favorably to conventional esophagectomy where this was reported in about 1.4–5% of all resections [9,61].
4.1 Learning curve
Undoubtedly MIE is technically complex surgery and therefore issues related to training and learning curves should be addressed before any widespread application. Table 4 analyzed the influence of surgical experience on outcome. Centers reporting more than 50 cases had lower mortality and morbidity rates than those with lesser experience while apparently performing a more extensive lymph node dissection that could potentially allow higher cure rates. No specific survival data are currently available on MIE to confirm this supposition but for conventional esophageal cancer surgery, the influence of caseload has well been shown. In the Surveillance Epidemiology and End Results (SEER) database, centers performing less than four esophagectomies annually had 5-year survival rates of 17% compared to 34% in centers performing more than 14 esophagectomies per year [5]. We thus have to be aware that the results of this literature review on MIE may be heavily influenced by the data of four single centers who contributed 833 patients (43%) of all 1932 reported cases [15–17,34]. Our study eliminated small series of less than 10 cases to avoid the potentially negative impact of early learning curves. Thus any center contemplating the start of a MIE protocol should consider that their early results are most likely to be worse than the average outcome in this review [39].
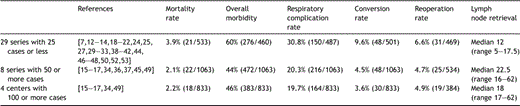
Effect of the learning curve: outcome of series with 25 or less cases compared to series with more than 50 respectively more than 100 cases.
4.2 Oncologic outcome
Literature reviews on conventional esophagectomy in the eighties and nineties reported overall 5-year survival rates of 22–26% [9,61]. Nowadays most high volume centers report overall 5-year survivals of 40% or more. Even in the setting of controlled trials and national databases, 40% 5-year survival appears to be an adequate benchmark for esophageal cancer surgery [1–3,62].
According to a meta-analysis on transhiatal versus transthoracic open esophagectomies during the nineties, both techniques were then evenly used for all esophageal carcinomas and stage distribution was similar: 45% of patients in THE series were stages III to IV versus 51% in TTE series [9]. In the present review on MIE this does not seem to be the case (Table 3). Transhiatal MIE series included considerably more early stage patients than series on transthoracic MIE (36% stage 0 or I versus 28% for transthoracic MIE). Conversely, transthoracic MIE series included more stage III and IV patients than transhiatal series (43% vs 31%) and induction chemo- or chemoradiotherapy was given more frequently before transthoracic MIE resections (32%) than before transhiatal MIE (14%) again reflecting major bias with more advanced stages in studies on TTE. Overall 5-year survival rates of both approaches thus are not directly comparable and if both approaches were to have similar oncologic efficiency, THE series should theoretically be able to report much higher 5-year survival rates due to this more favorable case-mix.
From the few available follow-up data, this however does not seem to be the case. Currently no single center has yet reported overall 5-year results for THE. Palanivelu et al. reported 3-year survival rates of 62% for stage I respectively 44% for stage II [23]. This is far below the 80% 5-year survivals that should be expected for stage I after open transthoracic resection and standard two-field LND [1–3,65]. Within the limits of the very limited survival data available, transhiatal MIE techniques seem to achieve suboptimal oncological results compared to transthoracic techniques.
For transthoracic MIE, overall 4–5-year survival rates of 22–52% have been reported [16,28,34,48]. Noteworthy, these were reported in series with median numbers of resected lymph nodes (17, 17.5 respectively 62) above the median of this review (Table 1) [16,34,48]. More mature survival data from other centers are certainly needed before MIE may be accepted as an oncologically valid approach for patients with advanced invasive cancer.
For patients with stage I disease, 3–5-year survival rates of 70–100% have been reported with MIE [15,16,26,34]. This seems similar to the results of conventional esophagectomy. MIE thus may offer an attractive option for those patients with intramucosal stage I tumors.
For stage I carcinomas and high grade dysplasia, MIE is currently in direct competition with other less invasive surgical techniques such as limited vagal-sparing esophagectomy [65] or jejunal interposition (Merendino procedure) [66]. These non-laparoscopic procedures can achieve 5-year survival rates above 80–90% while aiming at improved functional outcome in what should be expected to be long-term survivors. An increasing body of evidence also suggests that many tumors confined to the mucosa (T1a) or very superficial submucosa might be adequately treated by non-resectional endoluminal techniques, which by definition are less invasive than MIE [2,67,68]. For T1b tumors (tumors extending into the submucosa) lymph-node involvement is encountered in at least 30% of patients and radical LND seems to be most beneficial for such patients [65,69]. If at all, MIE in such patients should thus only be performed in settings where an adequate LND is possible.
5 Conclusions
Overall and similarly to previous less exhaustive literature reviews on MIE, the present review finds no evidence that MIE offers any real benefit for patients with esophageal cancer [70,71].
This systematic literature review confirms the feasibility of esophagectomy using ‘minimally invasive’ techniques such as thoracoscopy and/or laparoscopy. In experienced hands, MIE could be an attractive approach for patients with benign conditions requiring esophageal resection and for early cancers (pT1a) in which no need for aggressive lymphadenectomy or concerns on lateral resection margins exist. Various technical options for MIE exist and currently available results do not show a specific advantage of any of these techniques nor which could be specific indications for MIE compared to other, even less invasive, treatments such as endoluminal ablation techniques for high-grade dysplasia or early carcinomas.
The morbidity and mortality of MIE remains substantial and not inferior to conventional esophagectomy, at least when performed by experienced surgeons. The results of this review clearly do not support the term ‘minimally invasive’ which should preferably be replaced by ‘minimal access’ esophagectomy. Controlled prospective studies seem urgently needed to prove if MIE is as safe as conventional esophagectomy.
From an oncological point of view, this review could not find enough evidence to support MIE as a valid oncological treatment option for invasive esophageal cancer and widespread use of MIE for this indication therefore should not yet be encouraged. In 2008 the best chance for a patient with invasive esophageal cancer still results from thorough staging, adequate patient selection, use of induction therapies when required and surgical management in centers with expertise and high annual volume of esophageal resections. Any added benefit through the use of video-assisted ‘minimally invasive’ techniques at this time remains purely speculative.
Appendix A Conference discussion
Dr S. Mattioli (Bologna, Italy): It is a lot of work just to review all the papers. I am not sure that you have even got much at the end of the story but I have a question for you: in the 80s or early 90s we discussed the validity of the transhiatal esophagectomy versus the open thoracotomic esophagectomy. Now, do you have an idea of the results comparing the minimally invasive esophagectomy with the open and the transhiatal esophagectomy? Because the philosophy of the operations, in my opinion, is the same. So what do we have to do to compare the open results of MIE with those achieved in both groups of operation?
Dr Decker: Minimally invasive transhiatal resections cannot really be compared to transthoracic resections because the patients are not the same. In fact, in the transhiatal series there were much more cases of high grade dysplasia and T1 tumors. Nowadays, also in open surgery, less patients with invasive carcinoma are operated by a transhiatal approach than would have been the case 10 years ago. So it might not be possible any more to do any randomized trial comparing the transhiatal minimal invasive resections. There are only a few studies available comparing open esophagectomy to MIE. These are not similar patients and no comparisons can really be made. In the two somewhat larger comparative trials, the study groups were very biased. In the largest study coming from Australia, all esophageal cancers were operated using thoracoscopy and laparotomy and all the open cases were in fact cancers of the gastric cardia. These cancers were often invading the whole stomach and a total gastrectomy had to be done. So the study groups were completely different and we should not make any conclusion based on these studies. There is no data for the moment to really answer your question and after 15 years of clinical activity with these operations we obviously need to do a serious study about the validity of minimally invasive esophagectomy.
Dr R. Berrisford (Exeter, U.K.): Thank you very much, I think that this overview is a very valuable addition to the literature to try to sort out what is a very confusing picture with so many different hybrid procedures being called MIE. Just a couple of comments. First of all, I think it is really valuable that you pointed out the potential new complication or additional danger of minimal access that you can’t feel the bronchus: airway injury is an important complication to avoid in minimal access esophagectomy.
Second, you quoted five papers as ‘MIE’ but the situation is even more confusing because at least some of those series involved a small laparotomy to make the gastric tube and really only two of these papers are totally minimal invasive esophagectomy. I think those who have been doing this operation realize that making a gastric tube purely laparoscopically does have its potential danger as well.
Dr Decker: Yes, I remember one of the first slides listing all the different techniques. Most of these techniques are some kind of hybrid technique and only a very few papers are on the strictly minimal invasive technique.
Dr Berrisford: You gave some comparative figures for open esophagectomy for some parameters but not all, where did you get those data from?
Dr Decker: As mentioned, most comparisons with open surgery used the data from the Dutch literature review on esophageal resections performed during the 90s (Ann Thorac Surg) and the Dutch trial comparing open transhiatal to open transthoracic resections for adenocarcinoma of the gastro-esophageal junction and esophagus (New Engl J Med).
Dr Berrisford: So you didn’t do a comparative study during the same period looking at papers on open esophagectomies to see what the comparison truly was?
Dr Decker: No, that study would have to cover from 1992 to 2007. We thought it was not necessary to repeat these literature reviews on open surgery as there are already two very complete papers on this topic. The Dutch paper covering the years 1990 to 1999, included more than 50 studies. There is another literature review from Australia comparing open Ivor-Lewis versus transhiatal resections and covering the years 1985 to 1995. Considering this, we thought that we would not change much by adding a few years of literature to these available data.
Dr Berrisford: But you are swimming against the tide in a way, you are going to stop the flow of minimal invasive techniques, so presumably you are working towards a study. What would be your comparison and what would be your end point?
Dr Decker: I also believe that the current tide is not going to be stopped and maybe it should not be stopped because MIE could have some good aspects too. It was difficult to show in a 10 min presentation, but over the last few years some centers seem really to have improved with MIE as can be seen for instance for lymph node retrieval. People doing these operations start to become aware that adequate lymph node removal is important. Another factor is, as for many other operations in minimally invasive surgery, that it might not be the same surgeons who will do these operations nowadays and many surgeons doing minimally invasive esophagectomies might not have much background in open esophageal cancer surgery. Some basic aspects about this surgery may just have to be learned by them. About studies that have to be done, there are a few studies ongoing. In France, they have just started a randomized controlled trial comparing laparoscopy versus laparotomy for the gastric tube construction and doing the thoracic part by thoracotomy.
References
Author notes
Presented at the 15th European Conference on General Thoracic Surgery, Leuven, Belgium, June 3–6, 2007.
- cancer
- esophagectomy
- laparoscopy
- laparotomy
- surgical procedures, operative
- thoracic surgery, video-assisted
- thoracoscopy
- thoracotomy
- vocal cord paralysis
- lymph nodes
- morbidity
- mortality
- neoplasms
- lymph node dissection
- esophagectomy, minimally invasive
- pulmonary complications
- distal intestinal obstruction syndrome




