-
PDF
- Split View
-
Views
-
Cite
Cite
Jiangang Wang, Xu Meng, Hui Li, Yongqiang Cui, Jie Han, Chunlei Xu, Prospective randomized comparison of left atrial and biatrial radiofrequency ablation in the treatment of atrial fibrillation, European Journal of Cardio-Thoracic Surgery, Volume 35, Issue 1, January 2009, Pages 116–122, https://doi.org/10.1016/j.ejcts.2008.09.014
Close - Share Icon Share
Abstract
Objective: The aim of this study was to compare, in patients with permanent atrial fibrillation (AF), the efficacy and safety of left atrial ablation with that of a biatrial procedure and to assess the risk factors for late failure of sinus rhythm restoration. Methods: Between January 2004 and January 2007, 299 consecutive patients underwent the radiofrequency ablation procedure for AF associated with concomitant cardiac surgery. According to a prospective, open, and randomized trial, 149 patients underwent left atrial plus cavotricuspid isthmus ablation (left atrial group), while 150 patients underwent biatrial ablation (biatrial group). The postoperative and mid-term follow-up results were compared between the two groups. Both univariate and multivariate analyses were used to assess the risk factors for late recurrence of AF. Results: There were seven in-hospital deaths (2.3%), including two in the left atrial group (1.3%) and five in the biatrial group (3.3%), and there were no differences in the incidence of the mortality and complications during the postoperative and follow-up periods between the groups. At discharge, sinus rhythm was maintained in 77.1% of the patients, including 78.2% of those in the left atrial group and 75.9% in the biatrial group (p = 0.68). Follow-up was completed in 97% of the patients, with a mean time of 28 ± 5 months. At the latest follow-up, two deaths occurred in the biatrial group. Sinus rhythm was documented in 237 (85.0%) out of all the patients, including 85.2% (121/142) in the left atrial group and 84.1% (116/138) in the biatrial group patients (p = 0.87). Using a multivariate analysis, a left atrial diameter of ≥80 mm (p = 0.02) was an independent predictor for a late recurrence of AF. Conclusions: Both the left atrial combined with cavotricuspid isthmus ablation and biatrial maze procedure is safe and effective in treating patients with AF, with an acceptable sinus conversion rate, mortality and morbidity. A left atrial dimension of ≥80 mm was a significant predictor for a late recurrence of AF.
1 Introduction
Atrial fibrillation (AF) is the most commonly sustained cardiac rhythm disturbance and its prevalence increases with age. AF may occur with or without structural heart disease. Significant morbidity, mortality, and health care costs are associated with the condition. The patient’s clinical condition often deteriorates owing to the hemodynamic compromise associated with the arrhythmia, and thromboembolic events directly related to the arrhythmia can be devastating.
The Maze III procedure, described by Cox et al., remains the gold standard for the surgical treatment of AF [1]. The Maze III procedure is highly effective; however, it is an extensive and time-consuming technique, which precludes its widespread application. A variety of other sources such as cryoablation, microwave, and radiofrequency have been used to create lesions similar to those used in the original ‘cut and sew’ technique [2–6].
The role of the pulmonary veins and posterior left atrium in the genesis of atrial fibrillation is well established. Some groups who have adopted an ablation pattern involving only the left atrium have reported favorable results, while others insist on the routine use of a biatrial approach. In this prospective and randomized study, we aimed to compare the efficacy and safety of left atrial ablation, combined with cavotricuspid isthmus ablation, with that of a biatrial procedure in eradicating AF in patients with a permanent condition
2 Patients and methods
Between January 2004 and January 2007, 299 consecutive patients underwent the radiofrequency ablation procedure for permanent AF associated with concomitant cardiac surgery. The patients in AF with no organic heart disease were excluded from the study. The study was planned as a prospective, open, and randomized trial with parallel groups. Eligible patients were assigned to one of the two study arms immediately before the ablation procedure according to a computer-generated randomization list. The ethical committee of the hospital and the CDA approved the radiofrequency ablation procedure as a standard clinical practice, and all patients were informed of the investigational nature of the procedure and provided written informed consent before being included in the study and randomized. According to randomization, 149 patients underwent left atrial plus cavotricuspid isthmus ablation (left atrial group), while 150 patients underwent biatrial ablation (biatrial group). The clinical characteristics of the patients in the two groups were similar (Table 1 ).

There were 116 men and 183 women, with a mean age of 53 years (range, 24–77 years). One hundred and sixty patients (53.5%) had a preoperative New York Heart Association functional class (NYHA) of III or IV. The mean left atrial diameter was 71 ± 20 mm (range, 28–163 mm) and the left ventricular ejection was 59 ± 9% (range 32–83%). Thrombi in the left atrium were observed in 63 patients (21.1%). Twenty patients (6.7%) had one or more previous embolic events. Fourteen patients (4.7%) had previous cardiac operations.
2.1 Surgical procedure
All procedures were performed using routine cardiopulmonary bypass with bicaval and aortic cannulation under moderate hypothermia. The procedure was performed using bipolar ablation system in all patients (Cardioblate BP surgical ablation device; Medtronic, Minneapolis, MN).
After cardioplegic arrest, left atrial incision was performed through the interatrial groove. The left atrial appendage was either amputated and sutured afterward, or a circumferential radiofrequency lesion was created around its base and the orifice over sewn from inside the atrium. In addition to the incision in the interatrial groove, isolation of the right pulmonary veins was completed by a circular ablation line. The left pulmonary veins were encircled and a connecting line was performed between both islands of pulmonary veins on the roof, as near to the left atrial roof as possible to avoid injury to the esophagus. An ablation line from the left pulmonary veins to the posterior mitral annulus was then performed with caution so as not to injure the circumflex coronary artery (Fig. 1 ). Cavotricuspid isthmus ablation was then performed to achieve a bidirectional conduction block. Division of the ligament of Marshall was performed in all the patients.
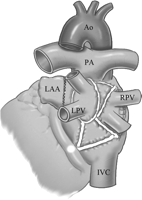
Left atrial ablation line includes isolation of the pulmonary veins and a connecting line performed between both islands of pulmonary veins on the roof, and a line from the left pulmonary veins to the posterior mitral annulus. The left atrial appendage was either amputated or sutured. Ao, aorta; PA, pulmonary artery; LPV, left pulmonary artery; RPV, right pulmonary artery; LAA, left atrial appendage; IVC, inferior vena cava.
In the patients who underwent biatrial ablation, the following lesions, other than cavotricuspid isthmus ablation, were added in the right atrium: excision of right atrial appendage; superior vena cava to inferior vena cava; lateral free-wall lesion complete to anterior-medial tricuspid valve annulus; medial free-wall lesion complete to anterior-medial tricuspid valve annulus (Fig. 2 ).

Right atrial ablation line includes superior vena cava to inferior vena cava; lateral free-wall lesion complete to anterior-medial tricuspid valve annulus; medial free-wall lesion complete to anterior-medial tricuspid valve annulus and cavotricuspid isthmus ablation with excision of right atrial appendage. Ao, aorta; SVC, superior vena cava; TV, tricuspid valve; CS, coronary sinus; FO, foramen ovale; IVC, inferior vena cava; CT, cavotricuspid isthmus.
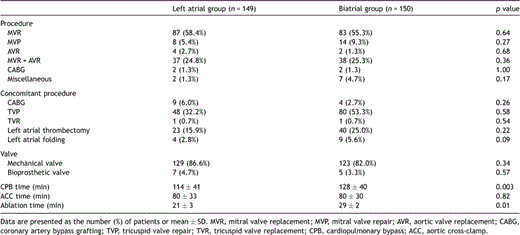
The data related to the operative procedures are given in Table 2 .
2.2 Postoperative care and follow-up
Administration of amiodarone was started with an intravenous bolus of 300 mg before the end of cardiopulmonary bypass and followed by an infusion of 900 mg/day for 3 days. Meanwhile oral administration of 200–400 mg/day was taken and was maintained for 6 months. In cases of thyroid disease, amiodarone incompatibility or other contraindication for amiodarone administration, sotalol was given alternatively (oral administration of 80 mg/day for 6 months). Early recurrence of AF was electrically cardioverted after saturation with amiodarone/sotalol and after exclusion of intracardiac thrombosis by transesophageal echocardiography. In all patients, oral anticoagulation was maintained and discontinued in the patients undergoing valve repair or replacement with bioprosthesis who were in stable sinus rhythm documented by 24 h Holter monitoring after 6 months postoperatively, and then switched to low dose aspirin, at 100 mg/day. Patients with mechanical valves were on lifelong anticoagulation.
After discharge, the patients were followed up with a clinical examination, chest X-ray, electrocardiogram, echocardiogram, and Holter monitoring. A standard 12-lead electrocardiogram, was checked during the hospitalization, 3, 6 and 12 months after discharge when followed up, and annually thereafter. All patients had a minimum of 12 months’ of follow-up, which was achieved in 96.6% (142/147) of the patients in the left atrial group and 96.6% (140/145) in the biatrial group by outpatient clinic visit or telephone and letter interviews. Complete data were available for 282 patients at the latest follow-up. The mean duration of the follow-up was 28 ± 5 months for all patients (range 12–46 months).
2.3 Statistical analysis
Continuous data were presented as mean ± standard deviation and categorical variables were expressed as the number of cases and percentage. The significance of the differences between the groups was assessed by the Mann–Whitney test for continuous variables and chi-square test for categorical variables. The actuarial survivals and freedom from stroke and cardiac-related events were estimated using the Kaplan–Meier method, expressed as mean ± standard error of the estimate and compared with the log-rank test. To assess the risk for late recurrence of AF, both univariate and multivariate analyses with the logistic regression model were used. The results were considered significant at a 95% confidence interval, p ≪ 0.05. The data were analyzed with the SPSS for Windows version 12.0 (SPSS Inc., Chicago, IL, USA).
3 Results
3.1 Intraoperative data
The intraoperative data of the patients are described in Table 2. The mean cardiopulmonary bypass time was significantly longer in the biatrial group (128 ± 40 min) than in the left atrial group (114 ± 41 min) (p = 0.003), and the mean ablation time was also longer in the biatrial group (29 ± 2 min) than in the left atrial group (21 ± 3 min) (p = 0.01).
3.2 Mortality and morbidity
There were seven in-hospital deaths (2.3%). Two patients died in the left atrial group (1.3%). One patient died from low cardiac output syndrome, and the other died from sudden death of unknown reasons. Another five patients were in the biatrial group (3.3%). One patient died from arrhythmia, one patient died from left ventricle rupture, one patient died from multisystem and organ failure, and the other two patients died from low cardiac output syndrome.
Reexploration for bleeding was required in nine patients (3.0%), including five (3.4%) in the left atrial group and four (2.7%) in the biatrial group. The other postoperative complications included a low cardiac output syndrome requiring intra-aortic balloon pump (IABP) or extracorporeal membrane oxygenation (ECMO) in six patients (2.0%), including two (1.3%) in the left atrial group and four (2.7%) in the biatrial group, respiratory failure requiring prolonged mechanical ventilation in two (0.7%), wound infection in two (0.7%), and acute renal failure requiring dialysis in four (1.3%). During the follow-up, there were two deaths in the biatrial group. One patient died from encephalorrhagia, and the other died from an unknown etiology. The 4-year actuarial survival was 98.7 ± 1.1% in the biatrial group and 100% in the left atrial group (p = 0.50). There were no differences in the incidence of the mortality and complications during the postoperative and follow-up periods between the groups.
3.3 Stroke rate
No stroke occurred in the two groups postoperatively. During the follow-up, cerebrovascular accidents occurred in two patients (1.4%) in the biatrial group. One patient, taking an oral anticoagulant therapy for mechanical mitral valve and AF had a cerebral hemorrhage 18 months postoperatively, and died from the encephalorrhagia. The other patient maintained sinus rhythm after mechanical mitral valve replacement, had a cerebral infarction 30 months postoperatively and underwent thrombolytic therapy but recovered without any sequelae. There were no significant differences in the stroke rate during the postoperative and follow-up periods between the groups.
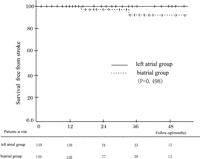
The 1- and 3-year actuarial survival free from stroke rates were both 100% in the left atrial group, and 98.7 ± 3.5% and 93.6 ± 5.7% in the biatrial group, respectively (p = 0.50) (Fig. 3 ).
3.4 Cardiac rhythm changes
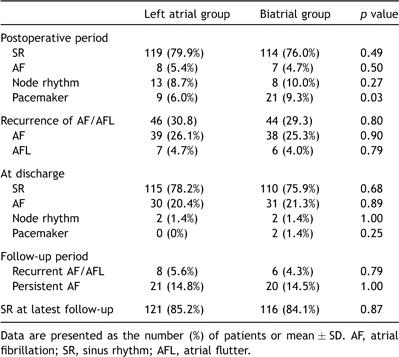
The changes in the cardiac rhythm after the procedure are described in Table 3 . Immediately after the operation, sinus rhythm was restored in 79.9% of the left atrial group patients and 76.0% of the biatrial group patients (p = 0. 49). AF was documented in eight patients (5.4%) in the left atrial group, and seven patients (4.7%) in the biatrial group (p = 0.50). Many patients had atrial tachyarrhythmias within the first or second week after the procedure. During hospitalization, AF and flutter were documented in 39 (26.3%) and 7 (4.7%) patients in the left atrial group, respectively, and 38 (25.3%) and 6 (4.0%) patients in the biatrial group, respectively (p = 0.90 and p = 0.79). Among those patients, 16 in the left atrial group and 10 in the biatrial group reverted from AF to sinus rhythm; other 13 patients with flutter all reverted to sinus rhythm. Two patients having intermittent sinus arrest in the biatrial group required a pacemaker before discharge. At discharge from the hospital, sinus rhythm was maintained in 77.1% of the patients, including 78.2% of those in the left atrial group and 75.9% in the biatrial group (p = 0.68).
Changes in the cardiac rhythm during the postoperative and follow-up periods.
During the follow-up, recurrent AF was documented in eight (5.6%) in the left atrial group, and in six (4.3%) in the biatrial group. Of those patients, six from the left atrial group and five from the biatrial group reverted to sinus rhythm by synchronized cardioversion. A permanent pacemaker was placed in two patients in the biatrial group and one patient in the left atrial group because of sinus node dysfunction. At the latest follow-up, 237 (85.0%) of the patients were in sinus rhythm, which was maintained in 85.2% (121/142) of the left atrial group and 84.1% (116/138) of the biatrial group patients (p = 0.87).
3.5 Predictors of a late recurrence of atrial fibrillation
According to the restoration of sinus rhythm at the last follow-up, the 280 survivors were divided into the two groups: sinus rhythm was not restored in 43 patients and restored in 237. Using the univariate and multivariate logistic analysis for the variables, a left atrial diameter of ≥80 mm (p = 0.02) was an independent predictor for a late recurrence of AF.
3.6 Postoperative clinical and echocardiographic findings
The NYHA functional class had improved by the last follow-up as compared with the preoperative class in all patients (1.1 ± 0.3 vs 2.6 ± 0.6, p = 0.003). Preoperatively, 160 patients (54%) were in functional class III or IV, but 238 (85%) were in functional class I and the remaining were in class II at the last follow-up. Echocardiographic evaluations before and after the operation were performed in all patients. The postoperative left atrial dimensions were similar between the left atrial group and biatrial group (49.8 ± 14.4 mm vs 50.8 ± 12.9 mm, p = 0.59), but were significantly reduced within each group after the operation (p ≪ 0.0001). At the latest follow-up, among 47 patients who did not need lifelong warfarin therapy, three patients (6.4%) were completely off warfarin.
4 Discussion
In this study, we investigated the effects of the procedure using radiofrequency ablation in treating permanent AF, by comparing the mid-term outcomes of the left atrial combined with cavotricuspid isthmus ablation, with that of a biatrial procedure.
The maze procedure cures AF by the isolation of the posterior left atrium including all four pulmonary veins and multiple incisions placed on the right and left atria. Although the maze procedure cures AF in the majority of patients, there has been no definitive evidence that all the incisions are required to cure AF, and not all the incisions are necessarily essential for terminating AF in every single patient. Sueda et al. simply isolated the posterior left atrium without any incisions on the right atrium in patients with permanent AF, and found that 74% of the patients were cured of AF during a follow-up period of up to 3 years [7]. Melo et al. simplified the procedure to an isolation of the right and left pulmonary veins and applied it to patients with permanent AF. During a 3–6-month follow-up period, 64% of the patients were in sinus rhythm [8]. Guden et al. adopt the biatrial approach in patients with a history of atrial flutter and where the right atrium has to be opened. Otherwise the procedure is restricted to the left atrial side. During the 2–24-month follow-up period, sinus rhythm was maintained in 79.6% of cases in biatrial group, while this rate was 75.6% in left atrial group [9]. Although the success rate for AF after those simplified procedures was lower than the maze procedure, they were advantageous in reducing operative blood loss and decreasing the cardiac arrest and operation times. Recently, Benussi et al. demonstrated a complete left atrial ablation with a single bipolar radiofrequency device yielding excellent clinical mid-term results. Freedom from AF was 84% at 6 months and 81% at 1 year [10]. In our study, the overall sinus restoration rate of 85.0% at about 4 years of follow-up, was acceptable in comparison to those of the previous series.
Ideally, the operation should be tailored to the pathogenesis of AF in each patient. Current understanding of AF and electroanatomic mapping tools do not enable this analysis in the operating room. The surgeon can discern the temporal pattern of AF, and this information can guide the choice of lesion set. However, the pathogenesis of permanent AF is not well understood. Intraoperative mapping studies in those patients suggest that the left atrium is usually the electrical driving chamber [11]. Clinical reports support this by demonstrating that permanent AF is frequently treated successfully by left atrial lesion sets that include isolation of the pulmonary veins and connecting lesions [5,12]. Our results suggest that the procedure should include separate encircled isolation of the right and left pulmonary veins, a connection between the right and left pulmonary veins on the roof, and a connection to the mitral annulus. The lesion to the mitral annulus enhanced results; reasons for this are not fully understood, but may include further remodeling of the left atrial substrate and block of common forms of left atrial macro-reentry [13]. An essential requirement for this lesion is that it incorporates a contiguous line of conduction block; an incomplete lesion at this location may actually potentiate macro-reentry, leading to recurrent atrial arrhythmias [13,14]. Addition of cavotricuspid isthmus lesions conferred additional benefit in these patients. It is possible that cavotricuspid isthmus lesions have reduced the incidence of atrial flutter and favored its spontaneous termination. The left atrial appendage should also be excised or excluded which improves results, particularly in patients with permanent AF. In patients with left atrial enlargement, we recommend left atrial reduction, as this may increase restoration of sinus rhythm [15]. Gillinov et al. examined the effectiveness of pulmonary vein isolation alone, pulmonary vein isolation with left atrium connecting lesion, and the maze procedure in patients with paroxysmal AF associated with mitral valve disease. There was no significant difference between the three different procedures in terms of the prevalence of AF 1 year postoperatively. They also examined the effectiveness of the different lesion sets on the cure of permanent AF and found that pulmonary vein isolation alone or lesion sets that did not include a lesion extended to the mitral annulus were less effective as compared with the maze procedure and a lesion set including a connecting lesion between the pulmonary vein isolation and the mitral annulus [16,17].
It is regretable that the numbers of our study were not larger, since it may be that the mortality between the two groups might become statistically significant. It is suggested that the biatrial maze procedure prolongs the cardiac ischemic time, and has the potential risk of complications, such as bleeding, sick sinus syndrome, and myocardial dysfunction [18]. However, left atrial combined with cavotricuspid isthmus ablation is advantageous in reducing the postoperative complications by minimizing the incisions and shortening the operative time.
The incidence of sick sinus syndrome has been reported as being 6–23% in patients after the conventional maze procedure, requiring a pacemaker placement [18,19]. In our study, temporary pacemaker implantation rate was significantly higher in the biatrial group postoperatively. Only two patients required a permanent pacemaker before discharge in the biatrial group, and during the follow-up, a permanent pacemaker was placed in two patients in the biatrial group and one patient in the left atrial group. It suggested that biatrial ablation increased the pacemaker implantation rate.
Furthermore, the sinus rhythm rate at the last follow-up was the same for both the left atrial and biatrial groups and was 85.2% and 84.1%, respectively, and there were no differences in the morbidity and mortality rate during the postoperative or follow-up periods in either group. These effects have helped us to determine to continue with the left atrial plus cavotricuspid isthmus ablation procedure.
The impact of the maze procedure on strokes in patients with AF was proven by other reports [18,20,21]. At a follow-up of about 4 years, only two patients (1.4%) in our study had cerebrovascular accidents, which was cerebral hemorrhage in one patient and the other had a cerebral infarction. These results suggest that the restoration of sinus rhythm after the ablation could play an important role in reducing the strokes in patients with AF.
An advanced age, the duration of AF, increased cardiothoracic ratio, low f-wave voltage, and severely enlarged left atrium are well known risk factors for late recurrence of AF [19,22]. In our study the significant predictors of late recurrences of AF was the left atrial diameter of ≥80 mm. Considering the results from other reports, there was a finding in our study in that the diameter of ≥80 mm as an independent predictor for late recurrence of AF was larger than others [23]. This was related to one of the patient characteristics in which there were a larger percentage of patients with a larger left atrial diameter in our study.
The current study has several limitations that should be considered. First, recurrence of atrial fibrillation may occur at intervals between clinical follow-up and may not be identified with surveillance electrocardiography. The true incidence of recurrence of atrial fibrillation in this patient cohort may therefore be underestimated. Furthermore, many of our patients continued antiarrhythmic drugs (including amiodarone). This should be considered when the patient desires AF catheter ablation to stop antiarrhythmic drugs. Second, the exact cause of recurrence of atrial fibrillation in each patient is not known, as few patients had postoperative electrophysiologic evaluation. Without such evaluation, the source and location of such failures, such as conduction gap or failed interruption of reentry, is unknown. Also, the restoration of the atrial transport function is an important goal of the maze procedure. However, unfortunately we did not investigate that issue in this study.
In summary, the maze procedure by using radiofrequency ablation is safe and effective in treating patients with permanent AF, with an acceptable sinus conversion rate, mortality and morbidity. For the selection of the maze procedure, both the left atrial combined with cavotricuspid isthmus ablation and biatrial procedure had similar outcomes despite the shorter cardiopulmonary bypass time in the left atrial group. A left atrial dimension of ≥80 mm was a significant predictor for a late recurrence of AF. For these reasons we recommend left atrial combined with cavotricuspid isthmus ablation as a modified maze procedure for treatment of AF. Also if dilatation of the left atrium develops, more aggressive intervention should be considered.




