-
PDF
- Split View
-
Views
-
Cite
Cite
Paul E. Van Schil, Michèle De Waele, A second mediastinoscopy: how to decide and how to do it?, European Journal of Cardio-Thoracic Surgery, Volume 33, Issue 4, April 2008, Pages 703–706, https://doi.org/10.1016/j.ejcts.2008.01.016
Close - Share Icon Share
Summary
Specific indications for a second or remediastinoscopy include an inadequate first procedure, metachronous second primary or recurrent lung cancer, lung cancer after unrelated disease, and restaging after induction therapy. Nowadays, restaging is the most frequent indication for remediastinoscopy. Only patients with proven mediastinal downstaging will benefit from a subsequent surgical resection. In contrast to imaging or functional studies, remediastinoscopy provides pathological evidence of response after induction therapy. Although technically more challenging than a first procedure, remediastinoscopy can select patients for subsequent thoracotomy and provides prognostic information. Technically, mediastinal dissection is usually started at the left paratracheal side to avoid the innominate artery. Under the aortic arch, dissection proceeds in the pretracheal plane until the subcarinal nodes are reached. Sensitivity of a second mediastinoscopy is lower than a first procedure but in the most recent series it is higher than 70% with an accuracy around 85%. Survival also depends on the findings of remediastinoscopy, patients with persisting mediastinal involvement having a poor prognosis. An alternative approach consists of the use of minimally invasive staging procedures as endobronchial or endoscopic esophageal ultrasound to obtain an initial proof of mediastinal nodal involvement. Mediastinoscopy is subsequently performed after induction therapy to evaluate response. In this way, a technically more difficult remediastinoscopy can be avoided.
1 Introduction
Mediastinal downstaging after induction therapy for locally advanced non-small cell lung cancer (NSCLC) is an important prognostic factor for long-term survival. Patients with persisting mediastinal involvement have a poor prognosis and will not benefit from surgical resection [1–3]. So, precise restaging after induction chemo or chemoradiotherapy is of utmost importance to determine subsequent treatment and prognosis. Different restaging methods are available. Repeat mediastinoscopy is an invasive technique providing pathological evidence of response. However, it is technically more demanding resulting in a lower accuracy compared to a first procedure. In this review we focus on how to decide on a second or repeat mediastinoscopy or its specific indications, how to do it or the precise operative technique, and general techniques or treatment strategies to restage the mediastinum.
2 A second or repeat mediastinoscopy: how to decide?
There are different indications for a second, redo or repeat mediastinoscopy (reMS) and these are listed in Table 1 . In every case the principal aim is to obtain a pathological proof of mediastinal nodal involvement or downstaging.

Currently, reMS is mostly performed for restaging of non-small cell lung cancer after induction chemo or chemoradiotherapy. Important prognostic factors after induction therapy for locally advanced NSCLC include complete surgical resection and downstaging of mediastinal lymph nodes [1]. Only patients with mediastinal downstaging will benefit from a subsequent surgical resection, especially when a lobectomy can be performed. In contrast to imaging or functional studies, reMS offers the advantage of providing pathological evidence of response after induction therapy. In this way, it is a valuable tool to select patients for surgical resection.
3 A second mediastinoscopy: how to do it?
Shortly after the introduction of mediastinoscopy, redo-procedures were considered to be technically impossible due to the scar tissue developing after the first intervention. However, in subsequent years reMS was found to be technically feasible, also after induction therapy [4–6]. To perform a reMS we prefer a classical, regular mediastinoscope for its small size and preservation of a three-dimensional view when looking through it [7]. The procedure is performed under general anaesthesia with endotracheal intubation. The transverse scar of the initial mediastinoscopy is reopened. Usually the strap muscles are fibrotic and sharp dissection is necessary to reach the pretracheal plane. Due to dense fibrosis, the innominate artery can be very adherent to the trachea, which represents a major danger point. For this reason the pretracheal plane usually cannot be safely developed. In most cases, a tunnel can be created by blunt dissection on the left paratracheal side [4]. Underneath the aortic arch, dissection proceeds in the pretracheal plane until the subcarinal nodes are reached. In the first place biopsies of initially involved lymph node stations are carefully taken. Bleeding is usually treated by packing or electrocoagulation. In severe cases, sternotomy or thoracotomy are indicated depending on the precise site of hemorrhage.
4 Techniques for mediastinal restaging
Recently, the role of reMS has focussed on restaging after induction therapy for locally advanced NSCLC as the pathologic response can be precisely evaluated. The different techniques for mediastinal restaging are listed in Table 2 and these can be subdivided into noninvasive, invasive and minimally invasive or alternative techniques.
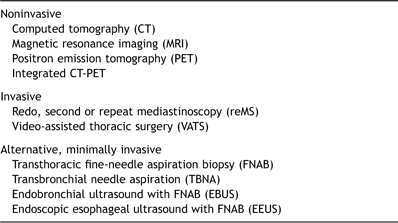
Noninvasive imaging techniques such as computed tomography (CT) and also magnetic resonance imaging are not accurate enough for mediastinal restaging. In a study of 24 patients who underwent restaging by reMS, sensitivity of chest CT was found to be only 41% with an accuracy of 58% [5]. Positron emission tomography (PET) provides additional metabolic information, but there are conflicting data regarding its use. PET has been shown to be more accurate in predicting the T factor than the N status [8]. In a prospective study including 25 patients with NSCLC treated by induction chemotherapy, positive predictive value of PET to detect persisting nodal disease was 73%, but below 20% for residual N2 disease [9]. Unfortunately, downstaging of the latter nodes is of major interest to determine subsequent treatment and prognosis.
Although experience is rather limited, integrated CT-PET combining precise anatomical and functional information seems to be more accurate for restaging. In a prospective study of 30 patients the accuracy of CT-PET was 83% that was significantly better than reMS [10]. However, sensitivity of reMS in this study was much lower than reported in other series [7]. In a larger prospective study of 93 patients who were restaged by chest CT and integrated CT-PET after induction chemoradiotherapy, repeat CT-PET was found to be more accurate than CT alone for all pathological stages [11]. However, there were 20% false-negative and 25% false-positive cases with repeat CT-PET. In case of suspicion of residual mediastinal disease, nodal biopsies are still required [11]. This underscores the continuous role of minimally invasive and invasive restaging procedures.
Results of recent series of reMS after induction therapy are summarized in Table 3[3,6,10,12–14]. In all series sensitivity of reMS was 70% or higher, except for the prospective study of De Leyn et al. comparing reMS to integrated CT-PET scanning [10]. This low sensitivity is largely explained by the fact that in 20 patients (67%) no adequate biopsies of the subcarinal lymph node station no. 7 could be taken. A possible explanation for this discrepancy with similar reports could be the use of a video mediastinoscope for the initial and redo-procedure [7]. The video mediastinoscope is larger than the classic mediastinoscope, the latter being more easily introduced into a narrow space as the subcarinal region. Moreover, when looking at the monitor screen during video mediastinoscopy, the three-dimensional view is lost.
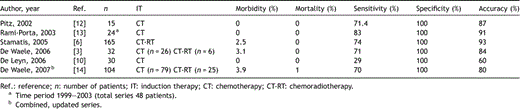
Recently, we were able to show that survival depends on the findings of reMS, for patients with a positive reMS having a poor prognosis compared to those with a negative reMS [3]. In a combined, updated series of 104 patients identical survival results were found with nodal status being the only significant factor in multivariate analysis [14].
The largest series of reMS was reported by Stamatis et al. in 2005, which described a total of 279 reMS, of which 165 were performed after induction chemoradiotherapy [6]. Results were very similar to other series; so, even combined induction chemo and radiotherapy does not give rise to increased difficulties or a lower accuracy of reMS compared to chemotherapy alone. In this study patients with a positive reMS also had a poor survival rate of only 5% after 5 years [6].
Video-assisted thoracic surgery (VATS) has been used for restaging but experience is limited. In a phase II study by the Cancer and Leukemia group B (CALGB), 70 patients with stage IIIA-N2 NSCLC underwent restaging by VATS [15]. Sensitivity in this series was 75%, specificity 100% and negative predictive value 76%.
Minimally invasive techniques comprise promising staging modalities and are increasingly used for lung cancer staging and also restaging (Table 2). However, false-negative rates between 20% and 30% have been reported. Mediastinal restaging was performed by endoscopic esophageal ultrasound (EEUS) after induction chemotherapy in 19 patients with proven N2 NSCLC [16]. There were no complications and patients with N0 disease underwent resection. Accuracy of EEUS in this setting was 83%; so, EEUS with needle aspiration might play a role in mediastinal restaging [16]. In a series of 83 patients with proven stage IIIA-N2 NSCLC treated by induction chemotherapy, mediastinal restaging with endobronchial ultrasound (EBUS) and transbronchial needle aspiration (TBNA) yielded a sensitivity of 70% and an accuracy of 75% [17].
Recent experience with CT-PET, EEUS and EBUS is summarized in Table 4 .
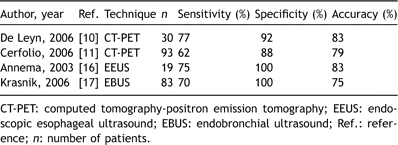
Recent experience with CT-PET, EEUS and EBUS after induction therapy
Also, TBNA without systematic use of ultrasound or EBUS might provide accurate results. In a smaller series, 17 lymph nodes were sampled in 14 patients who had undergone induction therapy for stage IIIA-N2 NSCLC [18]. A correct diagnosis was obtained in 71% of patients and more invasive procedures could be avoided in 35%.
For thoracic surgeons having no experience with reMS, an alternative approach consists of the initial use of minimally invasive staging procedures as TBNA, EBUS or EEUS to obtain a cytological proof of mediastinal nodal involvement [19]. After induction therapy, patients are subsequently restaged by mediastinoscopy. In this way, a technically more demanding reMS can be avoided.
Recently, the European Society of Thoracic Surgeons (ESTS) published guidelines for preoperative lymph node staging in NSCLC [20]. Regarding restaging after induction therapy, an invasive technique providing cytohistological information is still recommended at the present time. Endoscopic or surgical invasive procedures may be utilized, the precise choice depending on the availability of the technique and expertise of the centre [20].
Acknowledgement
The authors wish to thank Mrs. Gina Clerx for her excellent editorial assistance.
References
Author notes
Presented at the Safer-Repeat Symposium of the European Association for Cardio-thoracic Surgery, Geneva, Switzerland, September 19, 2007.




